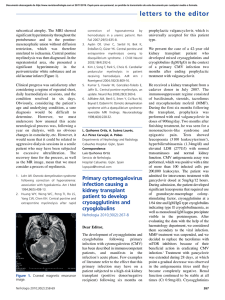
Nephrol Dial Transplant (2020) 1–8 doi: 10.1093/ndt/gfaa284 Heart failure in chronic kidney disease: the emerging role of myocardial fibrosis and 1 Department of Nephrology, University of Navarra Clinic, Pamplona, Spain, 2Program of Cardiovascular Diseases, CIMA Universidad de Navarra, Pamplona, Spain, 3Institute of Medical Research of Navarra, IDISNA, Pamplona, Spain, 4Center of Network Biomedical Research in Cardiovascular Diseases (CIBERCV), Carlos III Institute of Health, Madrid, Spain and 5Department of Cardiology and Cardiac Surgery, University of Navarra Clinic, Pamplona, Spain Correspondence to: Javier Dı́ez; E-mail: jadimar@unav.es Heart failure (HF) is one of the main causes of morbidity and mortality in patients with chronic kidney disease (CKD). Decreased glomerular filtration rate is associated with diffuse deposition of fibrotic tissue in the myocardial interstitium [i.e. myocardial interstitial fibrosis (MIF)] and loss of cardiac function. MIF results from cardiac fibroblast-mediated alterations in the turnover of fibrillary collagen that lead to the excessive synthesis and deposition of collagen fibres. The accumulation of stiff fibrotic tissue alters the mechanical properties of the myocardium, thus contributing to the development of HF. Accumulating evidence suggests that several mechanisms are operative along the different stages of CKD that may converge to alter fibroblasts and collagen turnover in the heart. Therefore, focusing on MIF might enable the identification of fibrosis-related biomarkers and targets that could potentially lead to a new strategy for the prevention and treatment of HF in patients with CKD. This article summarizes current knowledge on the mechanisms and detrimental consequences of MIF in CKD and discusses the validity and usefulness of available biomarkers to recognize the clinical–pathological variability of MIF and track its clinical evolution in CKD patients. Finally, the currently available and potential future therapeutic strategies aimed at personalizing prevention and reversal of MIF in CKD patients, especially those with HF, will be also discussed. Patients with the dual burden of CKD and HF experience unacceptably high rates of symptom load, hospitalization and mortality [2], thus representing a growing health, economic and societal problem as the ageing population leads to higher numbers of affected individuals. In this regard, remarkable interest has been placed recently on processes through which the diseased kidney facilitates HF. Beyond macroscopic left ventricular (LV) hypertrophy, the microscopic lesions constitutive of the structural remodelling of the myocardium that impairs cardiac function [e.g. cardiomyocyte hypertrophy, myocardial interstitial fibrosis (MIF) and coronary microvascular disease] are frequent findings in endomyocardial biopsies (EMBs) and necropsy studies in patients with CKD [3], namely in those with kidney failure [4]. In particular, recent scientific work has identified causal connections between CKD and MIF [5]. Furthermore, it has been proposed that MIF may play a key role in the pathophysiology of HF in CKD patients [6]. Therefore, focusing on MIF might enable development of new strategies for the prevention and treatment of HF in CKD. This article summarizes available knowledge on the mechanisms and detrimental consequences of MIF in CKD, discusses the validity and usefulness of non-invasive biomarkers to detect MIF in CKD patients with HF and analyses the currently existing and potential future therapeutic tools aimed at reversing MIF in these patients. Keywords: biomarkers, chronic kidney disease, fibrosis, heart failure HISTOPATHOLOGICAL ASPECTS ABSTRACT INTRODUCTION Cardiac disease in patients with chronic kidney disease (CKD) is more frequent and more severe compared with the non-CKD population [1]. Either ischaemic or non-ischaemic in origin, cardiac diseases present in CKD patients evolve to chronic congestive heart failure (HF) (i.e. type 4 cardiorenal syndrome) [2]. MIF is characterized by the diffuse deposition of an excess of collagen fibres within the myocardial interstitium that expands the interstitial space. The extent of MIF is quantified as the percentage of total myocardial tissue occupied by collagen fibres, denoted as collagen volume fraction (CVF) and determined in myocardial samples by collagen-specific stain. Microscopically, three patterns of fibrous deposits can be distinguished in MIF (Figure 1) [7]: microscars, thick sheaths located in the C The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA. All rights reserved. V REVIEW 2,3,4 Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 Gregorio Romero-González1, Arantxa González2,3,4, Bego~ na López2,3,4, Susana Ravassa Javier Dı́ez1,2,3,4,5 Perivascular fibrosis Peri- and intercardiomyocyte bands FIGURE 1: Endomyocardial biopsy of a patient with CKD Stage 3a and HF. Sections were stained with picrosirius red and collagen deposits were identified in red as perivascular, microscars and periand intercardiomyocytic bands (magnification 400). With permission from Lopez B et al. Kidney Int 2008; 74(Suppl 111): S19-S23. perivascular space around intramural coronary arteries and arterioles and bands surrounding the cardiac muscle bundles and individual cardiomyocytes. Recent evidence suggests that beyond the quantity of collagen fibres, its structural quality (i.e. the degree of cross-linking among its constitutive fibrils) is relevant in MIF present in the failing human heart [8]. Although the expansion of the myocardial interstitium in CKD was described in the middle of the past century [9, 10], the presence of MIF in patients with CKD and patients submitted to kidney replacement therapy (KRT) was for the first time demonstrated and characterized in detail in post-mortem studies performed at the end of the century [11, 12]. Later clinical and population-based post-mortem studies have shown that patients with CKD have significantly more interstitial fibrotic tissue (as assessed by CVF) in the heart compared with subjects without CKD, that the stage of CKD directly correlates with the extent of MIF, thus being the more severe fibrosis found in patients with kidney failure and patients on KRT, and that CKD is associated with MIF independent of other potential confounding variables [13, 14]. MIF can become an extensive injury in the failing heart of CKD patients, as demonstrated in a study with EMB performed in patients on haemodialysis with HF showing that CVF represented >20%, with the three patterns of fibrotic deposits previously mentioned coexisting in the biopsy samples from most patients [15]. FIBROTIC MECHANISMS From a general point of view, the pathogenesis of MIF is characterized by the activation of resident cardiac fibroblasts, the differentiation of fibroblasts to myofibroblasts and the transition of other cardiac cell types to myofibroblasts [16]. Both activated fibroblasts and myofibroblasts produce a pro-fibrotic secretome 2 G. Romero-González et al. Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 Microscopic scar that in turn leads to alterations in the extracellular turnover of fibrillary collagen types I and III with synthesis predominating over degradation, thus resulting in the excessive deposition of collagen fibres within the interstitium [7, 8, 16] (Figure 2). In particular, the combination of excessive synthesis of mature collagen type I molecules by procollagen proteinases and excessive cross-linking of collagen-type fibrils plus reduced or unchanged collagen type I fibre degradation by matrix metalloproteinase-1 are the key alteration of collagen type I turnover involved in MIF (Figure 2). Biomechanical stress– induced alarmins, cytokines and chemokines participate in the activation of cardiac fibroblasts and the generation of myofibroblasts through the stimulation of a number of pro-fibrotic signalling pathways, among which the transforming growth factor (TGF)-b-regulated pathway stands out [16]. It is known that advanced stages of CKD are characterized by stimulation of fibrogenesis in several organs and tissues, including the heart and the aortic and large arteries vessel wall, leading to LV dysfunction and arterial stiffness, respectively [17, 18]. Clinical data obtained in patients with CKD Stages 2 and 3 suggest that the two processes occur in parallel, thus lending support to the possibility of the interaction of their responsible mechanisms. [19]. As regards the heart, it has been demonstrated in surgical and non-surgical experimental models of short- and long-term kidney function impairment in animals that the development of MIF is characterized by stimulation of pro-fibrotic genes and signalling pathways linked to TGF-b and by the predominant deposition of collagen type I fibres [20–22]. Of note, in these models, MIF is associated with LV diastolic and systolic dysfunction [20–22]. Thus it has been proposed that in CKD, several groups of pro-fibrotic factors can induce biomechanical stress of cardiac cells and thus trigger MIF [5, 17] (Table 1). These hypothetical factors include haemodynamic factors (e.g. pressure overload due to coexisting hypertension and volume overload related to the progressive decline of diuresis) and nonhaemodynamic factors associated with the progressive loss of kidney function fe.g. neuro-hormonal activation, including activation of the renin–angiotensin–aldosterone system [RAAS]; chronic inflammation [mediated by activation of the nucleotide-binding oligomerization domain (NOD), leucinerich repeat (LRR) and pyrin domain-containing protein 3 (NLRP3) inflammasome], oxidative stress, endothelial dysfunction, anaemia and mineral metabolism disorders leading to excess parathyroid hormone and/or vitamin D deficiencyg. In contrast, as a result of decreasing glomerular filtration rate (GFR), increased synthesis of pro-fibrotic substances by various organs occurs, which may also promote MIF [e.g. cystatin C, fibroblast growth factor-23, advanced glycation end-products, nitric oxide inhibitors, trimethylamine N-oxide (TMAO) and endogenous cardiotonic steroids]. Finally, several uraemic retention solutes (e.g. indoxyl sulphate, p-cresol sulphate, b2 microglobulin) are pro-fibrotic factors that can also be involved in MIF in kidney failure. Of interest, some of these factors may share CKD-specific pro-fibrotic mediators. For instance, recent findings suggest that microRNA-29b can play an important role in the mechanism of MIF related to vitamin D deficiency Procollagen type I precursor secreted to the extracellular space by activated fibroblasts PINP Collagen type I Procollagen N-proteinase PINP PICP Procollagen C-proteinase Mature collagen type I molecule PICP Termino-terminal self-assembly of collagen type I molecules = collagen type I microfibril Mature collagen type I molecule Latero-lateral self-assembly of collagen type I microfibrils = collagen type I fibril Cysteine proteases Lysyl oxidases Cross-linking of collagen type I fibrils = collagen type I fibre Intramolecular and intermolecular covalent bonds, cross-links, between lysine residues in the molecules of two collagen type I fibrils MMP-1 First step degradation of collagen type I fibres Big N-terminal collagen type I telopeptide MMP-9 and MMP-10 CITP Second step degradation of collagen type I fibres Degradation of collagen type I fibres Matrikines FIGURE 2: Schematic representation of the extracellular steps involved in the synthesis and degradation of collagen type I fibres. In MIF, the excessive deposition of collagen type I fibres is the result of the predominance of their synthesis over their degradation (see text). All the enzymes intervening in this process are tightly regulated by stimulatory (e.g. procollagen C-proteinase enhancer) and inhibitory factors (e.g. tissue inhibitors of MMPs). PINP: N-terminal pro-peptide of procollagen type I; PICP: C-terminal pro-peptide of procollagen type I; CITP: Cterminal telo-peptide of collagen type I. [23] and excess endogenous cardiotonic steroids in experimental CKD [24, 25]. CLINICAL ASPECTS MIF is involved in LV dysfunction as well as in arrhythmias and alterations of myocardial perfusion in patients with HF (Figure 3), thus contributing to poor outcomes in this population [7]. Indeed, as demonstrated in clinical–pathological studies, MIF is associated with LV dysfunction, both diastolic (i.e. increased myocardial stiffness hampers passive inflow and causes a backward flow that further impairs early filling) and systolic (i.e. altered alignment of collagen fibres relative to cardiomyocytes impairs transmission of force generated by cardiomyocytes to the ventricular chamber, detrimentally affecting contractility), in HF patients with either preserved or reduced ejection fraction (HFpEF and HFrEF, respectively) [26, 27]. HF in CKD In addition, MIF is independently associated with outcomes (i.e. all-cause and cardiovascular mortality and hospitalization for HF) in HF patients [28, 29]. As the presence of CKD is associated with both more severe LV stiffness and dysfunction [30] and worst prognosis [31] in HF patients, the possibility exists that MIF may play a significant role in the progression and outcomes of HF in CKD patients [6]. In support of this possibility, it has been reported that in patients on haemodialysis with severe dilated cardiomyopathy and HF, the extent of MIF was a strong and independent predictor of cardiac death [15]. There is abundant evidence on MIF being an important pathophysiological contributor to ventricular arrhythmias as well as to atrial fibrillation (AF) [7]. CKD patients, especially those undergoing dialysis treatment, are more prone to develop arrhythmias, particularly ventricular arrhythmias and AF [1], and half of cardiovascular deaths in CKD, especially 3 Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 Synthesis of collagen type I fibres Table 1. Pro-fibrotic factors present in CKD and potentially involved in the development of MIF Mechanism Haemodynamic Neurohormonal Inflammatory Pro-oxidant CKD-related oversynthesized molecules Uraemic retention solutes Arterial hypertension Volume overload Norepinephrine Angiotensin II and aldosterone Galectin-3 Interleukins 1b, 18 and 6 Superoxide anion Serum uric acid Serum phosphate Parathyroid hormone excess Vitamin D deficiency Anaemia Cystatin C Fibroblast growth factor-23 Advanced glycation end-products Endogenous cardiotonic steroids Nitric oxide inhibitors Trimethylamine N-oxide Indoxyl sulphate p-Cresol sulphate b2 microglobulin Adapted from Hulshoff et al. [5], González et al. [7], Mutsaers et al. [17] and Dı́ez et al. [49]. in the population with kidney failure, are related to cardiac arrhythmia and sudden cardiac death [1]. Therefore the possibility exists that MIF may be the structural substrate invoed in the genesis of ventricular and atrial arrhythmias in CKD patients. In support of this possibility are experimental studies showing that the development of MIF that accompanies decreasing GFR is associated with the appearance of ventricular arrhythmias [32] and AF [33]. In the clinic, it was recently reported that patients with CKD Stages 1–3 with elevated serum indoxyl sulphate levels showed a higher AF recurrence rate after successful catheter ablation, with serum indoxyl sulphate being a significant predictor of AF recurrence [34]. Of note, a positive correlation between CVF and circulating levels of indoxyl sulphate has been found in subtotal-nephrectomized rats with MIF [35]. In HF patients, MIF, i.e. perivascular fibrosis, is associated with the severity of coronary microvascular disease and is inversely correlated with coronary flow reserve [36], thus facilitating myocardial ischaemia. Additionally, perivascular deposition of fibrotic tissue increases oxygen diffusion distance, leading to the impairment of oxygen supply to the cardiomyocytes [37]. Coronary microvascular disease (i.e. endothelial dysfunction of intramyocardial arteries, remodelling of the wall of arterioles and reduced capillarization) is common in CKD patients, particularly in those with kidney failure in dialysis treatment, and has been invoed also in reduced coronary flow reserve, which in turn is associated with cardiovascular mortality in this population [38]. It has been proposed that in patients with kidney failure, MIF, particularly perivascular fibrosis, may contribute to coronary microvascular disease through extrinsic compression of intramyocardial vessels [39]. 4 Although direct microscopic examination of myocardial tissue obtained by EMB is the most accurate procedure to assess MIF, it remains an underutilized tool due to the paucity of human tissue samples, which is caused by the perceived risk associated with EMB, namely in CKD patients. An additional limitation to obtaining tissue samples is sampling error due to the patchy distribution of fibrotic deposits, restricting the diagnostic accuracy. Alternatively, MIF can be assessed non-invasively in HF patients by imaging and/or circulating biomarkers. Although cardiac magnetic resonance (CMR) techniques are widely used for imaging of cardiac fibrosis, measuring myocardial extracellular volume fraction (ECV) using gadolinium as a contrast agent, the severe consequences of gadolinium administration in CKD patients (i.e. nephrogenic systemic fibrosis) has driven new gadolinium-free CMR methodologies, allowing the characterization of MIF in these patients using the native T1 as a readout [40]. Indeed, recent studies using these approaches have identified alterations in native T1 attributable to MIF in nonHF patients with CKD Stages 1–4 [41], patients with kidney failure [42] and patients on haemodialysis [43]. However, a recent study showed that MIF quantification by ECV but not by native T1 was an independent risk factor for HF, providing incremental prognostic value and risk stratification for cardiac events in haemodialysis patients [44]. Therefore, additional studies are required to ascertain the more accurate and safe procedure to diagnose MIF in CKD patients using CMR. In this regard, using macrocyclic gadolinium-based contrast agents (e.g. gadoterate meglumine and gadobutrol) appears to be safe even in patients receiving haemodialysis, with no significant reduction in image quality [45]. Speckle tracking echocardiography (STE) assesses myocardial deformation at both the segmental and global levels, thus allowing myocardial tissue characterization [46]. In this regard, the extent of MIF has been linked to the degree of reduction in myocardial global longitudinal strain (GLS) in patients with HF [46]. While a reduced myocardial GLS has been reported in all stages of CKD and in patients on KRT [47], no data are yet available on this parameter in these populations when presenting with HF. Molecular imaging methods (e.g. single-photon emission computed tomography and positron emission tomography) alone or in combination with CMR hold great potential in efficiently identifying and quantifying collagen as a hallmark of MIF [48]. However, there are still no data on their employment to assess MIF in CKD patients. Some circulating biomarkers related to the extracellular metabolism of collagen type I and associated with MIF have been characterized in clinical–pathological studies performed in HF patients [49]. For instance, serum levels of the carboxy-terminal propeptide of procollagen type I (PICP), released during the synthesis of mature collagen type I molecules from their procollagen type I precursors by procollagen proteinases, directly correlate with the amount of collagen type I deposition in the myocardium of HF patients. On the other hand, the serum carboxy-terminal telopeptide of collagen type I (generated during the degradation of collagen type I fibres) to matrix metalloproteinase-1 ratio (CITP:MMP-1) inversely correlates G. Romero-González et al. Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 Endocrine and metabolic Main representative factors NON-INVASIVE DIAGNOSIS Myocardial interstitial fibrosis Altered electrical activity of cardiomyocytes Altered alignment of cardiomyocytes Increased passive stiffness Compression of small arteries and arterioles Increased O2 diffusion distance Reduced V propagation and re-entry mechanisms After-depolarizations and ectopic automaticity Reduced transmission of generated force Reduced chamber compliance Reduced coronary flow reserve Reduced O2 availability Impaired ventricular contraction Impaired ventricular filling Systolic dysfunction Diastolic dysfunction Arrhythmogenic substrate Cardiac dysfunction Hypoperfusion and hypoxia Ventricular and atrial arrhythmias Heart failure Intramyocardial ischemia FIGURE 3: Pathophysiological pathways involved in the major clinical complications of MIF that negatively influence the cardiovascular outcomes and prognosis of patients with CKD (V, membrane potential; O2, oxygen). with myocardial collagen type I cross-linking in HF patients. Recently it was reported that in patients with HF, a combination of high serum PICP and low serum CITP:MMP-1 ratio identifies a subgroup of patients with a high risk of HF hospitalization or death from cardiovascular causes [50], suggesting that these patients may present a high clinical risk pattern for MIF (i.e. malignant MIF). Of interest, the prevalence of this biomarker combination in HFpEF is higher in patients with CKD Stages 1–4 than in patients without CKD [51] (Figure 4). In addition, there is an effect modification of CKD on the association of this biomarker combination with LV diastolic dysfunction in HFpEF patients [51]. There is a necessity to investigate the usefulness of the biomarker combination in patients with kidney failure, as CITP is filtered by the kidney and may accumulate in blood when the GFR is <30 mL/min/1.73 m2. ANTI-FIBROTIC THERAPY There is currently no fully effective anti-fibrotic therapy. However, there is clinical and experimental evidence that MIF can be reversed with treatment. From this perspective, a HF in CKD strategy based on three therapeutic approaches can be proposed to reverse MIF in patients in different stages of CKD and patients submitted to KRT (Figure 5). First, treatment of HF patients with drugs interfering with the RAAS [particularly the angiotensin-converting enzyme inhibitor (ACEI) lisinopril, the angiotensin receptor blocker (ARB) losartan and the mineralocorticoid receptor antagonist (MRA) spironolactone] has been shown to reduce CVF in EMB [7, 49]. Of note, as the use of steroidal MRA is limited in patients with advanced stages of CKD because of a high sideeffect burden (overall hyperkalaemia), the use of non-steroidal MRA may be considered. Indeed, Phase 2 randomized clinical trials have demonstrated that the non-steroidal MRA finerenone has a superior safety profile to spironolactone and eplerenone in patients with CKD Stage 5 and exhibits potent in vitro and in vivo experimental anti-fibrotic effects [52]. On the other hand, it has been shown that the loop diuretic torasemide reduces MIF (both CVF and the degree of collagen crosslinking) in the myocardium of HF patients with CKD Stages 1–3 [53]. The anti-fibrotic effect was associated with the reduction of LV stiffness and the amelioration of HF clinical and 5 Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 Disrupted electrophysiological homogeneity hormonal parameters, as well as the circulating level of PICP in torasemide-treated patients [53]. As the effects on MIF were not observed in furosemide-treated patients, the possibility exists that torasemide exerts diuretic-independent anti-fibrotic actions [i.e. inhibition of the enzymes procollagen C-proteinase that synthesizes mature collagen type I molecules from their precursors and lysyl oxidases that mediate the cross-linking between collagen type I fibrils to form collagen type I fibres (Figure 2)] [53]. In this regard, it can be hypothesized that P=0.001 P < 0.001 Patients with biomarker combination of high clinical risk MIF (%) 60 50 40 P=0.017 30 20 10 0 HF– HF + HF– CKD– HF + CKD+ FIGURE 4: Frequency distribution of the combination of bio- markers of high clinical risk of MIF (see text) in patients without and with Stages 1–4 (CKD and CKDþ groups, respectively), classified in subgroups according to the absence or presence of HF with preserved ejection fraction (HF and HFþ, respectively). Adapted with permission from Eiros et al. [51]. CKD stage 1 CKD stage 2 CKD stage 3 Type of evidence Direct clinical CKD stage 4 CKD stage 5 KRT Readout parameters ACEI or ARB Histopathological parameters and collagen-related circulating biomarkers Steroidal MRA Torasemide Direct clinical Successful kidney transplantation CMR- and STEderived biomarkers Indirect clinical Cardioprotective hemodialysis CMR-derived biomarkers Non-steroidal MRA Experimental Ferric citrate Vitamin D receptor activators Hydroxylase inhibitors Histopathological parameters NLRP3 inflammasome inhibitors Anti-TMAO agents Anti-indoxylsulfate measures FIGURE 5: Proposed approach to treat MIF in HF patients along the different Kidney Disease: Improving Global Outcomes stages of CKD and during KRT. The proposal is based on both clinical and experimental studies in which different types of readout parameters were used to assess the effects of treatment on MIF. 6 G. Romero-González et al. Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 patients with HFpEF and CKD Stages 1–4 presenting with the biomarker combination of high serum PICP (thus reflecting high collagen type I synthesis) and a low serum CITP:MMP-1 ratio (thus reflecting high collagen type I cross-linking) may be a subsidiary of a tailored treatment with torasemide on top of other HF medications. Additional studies are required to evaluate this possibility, as well as studies aimed at ascertaining the cardiac anti-fibrotic usefulness of torasemide in patients with kidney failure and patients on dialysis. As CMR-derived parameters assessing MIF (e.g. ECV and native T1) worsen with haemodialysis vintage [54], it can be suggested that targeting interdialysis volume and pressure overload in haemodialysis patients may be one of the ways to act on MIF in these patients, as blood pressure and volume control have substantial impacts on cardiac complications, especially HF [55]. Additionally, avoiding intradialysis haemodynamic instability that causes myocardial ischaemia and injury may also prevent the reparative response leading to MIF [56]. In this framework, one of the potential benefits of individualized cardioprotective dialysis may be the reversal of MIF. Finally, although scarce, there is evidence that successful kidney transplantation is associated with mid- and long-term improvement in the CMR parameters ECV and native T1 [57] and in the STE parameter GLS [58], which may be associated with the regression of MIF. Clearly, more studies are required to delineate the role of recovery of renal function and/or immunosuppressive treatment in kidney transplantation on MIF. Second, in the setting of CKD there is experimental evidence that some pharmacological agents aimed at treating the complications of the loss of kidney function (i.e. anaemia and disturbances of mineral metabolism) may also be useful for treating MIF, as their use in animal models of CKD is associated with reductions in CVF among other histomolecular parameters associated with fibrosis. For instance, ferric citrate [59], vitamin D receptor activators [23] and prolyl hydroxylase inhibitors [60] CONCLUSIONS AND PERSPECTIVES The development of HF in patients with CKD represents a huge and urgent unmet medical need and, as such, a better understanding of the link between both conditions is mandatory. The notion is emerging that the progressive deterioration of cardiac function in CKD can be related in part to the fact that the progressive loss of renal function stimulates several mechanisms that facilitate MIF. In this conceptual framework, limited data and a large potential for impact make it necessary to develop optimal strategies for the diagnosis of MIF in CKD patients, as well as strategies aimed at individualized prevention and management in these patients, namely in those with kidney failure and those on KRT. This means that the time has come for nephrologists, cardiologists and other specialists with a background in the implementation of HF medicine to work together, as patients with CKD and HF are cardiorenal patients with systemic involvement and challenging requirements that must be faced from a multidisciplinary clinical vision and practice. FUNDING This project was funded by the Spanish Ministry of Science, Innovation and Universities [Instituto de Salud Carlos III: CIBERCV CB16/11/00483, the ERA-CVD Joint Transnational Call 2016 LYMIT-DIS (AC16/00020) and LIPCAR-HF (AC16/00016), the European Commission H2020 Programme (CRUCIAL project 848109) and the Dirección General de Industria, Energı́a e Innovación, Gobierno de Navarra, Spain (MINERVA project 0011-1411-2018-000053)]. CONFLICT OF INTEREST STATEMENT None declared. REFERENCES 1. US Renal Data System. US Renal Data System 2018 annual data report. Chapter 4: cardiovascular diseases in patients with CKD. Am J Kidney Dis 2019; 73(Suppl 1): S79–S98 HF in CKD 2. House AA, Wanner C, Sarnak MJ et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2019; 95: 1304–1317 3. Kaesler N, Babler A, Floege J et al. Cardiac remodeling in chronic kidney disease. Toxins (Basel) 2020; 12: 161 4. Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol 2019; 15: 159–175 5. Hulshoff MS, Rath S, Xu X et al. Causal connections from chronic kidney disease to cardiac fibrosis. Semin Nephrol 2018; 38: 629–636 6. Zannad P, Rossignol P. Cardiorenal syndrome revisited. Circulation 2018; 138: 929–944 7. González A, Schelbert EB, Dı́ez J et al. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol 2018; 71: 1696–1706 8. González A, López B, Ravassa S et al. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim Biophys Acta Mol Cell Res 2019; 1866: 1421–1432 9. Rössle R. Über die seröse Entzündung der Organe. Virchows Arch 1943; 311: 252–284 10. Langendorf R, Pirani CL. The heart in uremia. Am Heart J 1947; 33: 282–307 11. Mall G, Huther W, Schneider J et al. Diffuse intercadiomyocytic fibrosis in uraemic patients. Nephrol Dial Transplant 1990; 5: 39–44 12. Ansari A, Kaupke CJ, Vaziri ND et al. Cardiac pathology in patients with end-stage renal disease maintained on hemodialysis. Int J Artif Organs 1993; 16: 31–36 13. Charytan DM, Padera R, Helfand A et al. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol 2014; 176: 99–109 14. Izumaru K, Hata J, Nakano T et al. Reduced estimated GFR and cardiac remodeling: a population-based autopsy study. Am J Kidney Dis 2019; 74: 373–381 15. Aoki J, Ikari Y, Nakajima H et al. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int 2005; 67: 333–340 16. Frangogiannis NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med 2019; 65: 70–79 17. Mutsaers HA, Stribos EG, Glorieux G et al. Chronic kidney disease and fibrosis: the role of uremic retention solutes. Front Med (Lausanne) 2015; 2: 60 18. Chue CD, Townend JN, Steeds RP et al. Arterial stiffness in chronic kidney disease: causes and consequences. Heart 2010; 96: 817–823 19. Edwards NC, Ferro CJ, Townend JN et al. Aortic distensibility and arterialventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart 2008; 94: 1038–1043 20. Martin FL, McKie PM, Cataliotti A et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol 2012; 302: R292–R299 21. Liu S, Kompa AR, Kumfu S et al. Subtotal nephrectomy accelerates pathological cardiac remodeling postmyocardial infarction: implications for cardiorenal syndrome. Int J Cardiol 2013; 168: 1866–1880 22. Kieswich JE, Chen J, Alliouachene S et al. A novel model of reno-cardiac syndrome in the C57BL/6 mouse strain. BMC Nephrol 2018; 19: 346 23. Panizo S, Carrillo-López N, Naves-Dı́az M et al. Regulation of miR-29b and miR-30c by vitamin D receptor activators contributes to attenuate uraemiainduced cardiac fibrosis. Nephrol Dial Transplant 2017; 32: 1831–1840 24. Drummond CA, Hill MC, Shi H et al. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol Genomics 2016; 48: 220–229 25. Drummond CA, Fan X, Haller ST et al. Na/K-ATPase signaling mediates miR-29b-3p regulation and cardiac fibrosis formation in mice with chronic kidney disease. PLoS One 2018; 13: e0197688 26. Zile M, Baicu CF, Ikonomidis J et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015; 131: 1247–1259 27. Querejeta R, López B, González A et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 2004; 110: 1263–1268 7 Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 attenuate MIF and improve cardiac function in different models of kidney failure in rodents. Third, the manifold fibrogenic mediators and pathways that are activated in CKD offer a multitude of potential targets for novel anti-fibrotic interventions useful in this condition. Some experimental examples may illustrate this approach. Suppression of NLRP3 inflammasome activation with the polyphenolic compound theracurmin ameliorates MIF and LV diastolic dysfunction in rats with kidney failure secondary to subtotal nephrectomy [61]. Reduction of plasma levels of TMAO with the guanylate cyclase C agonist linaclotide is associated with a reduction of MIF in mice with adenine-induced kidney failure [62]. A decrease of serum indoxyl sulphate levels using the oral charcoal adsorbent AST120 was associated with a reduction of MIF and improvement of LV diastolic dysfunction in subtotal-nephrectomized rats [35]. Clearly these promising preclinical studies need to be transferred to the clinic and therapeutic approaches that survive Phase 1 and 2 clinical trials must be identified. 8 46. Moharram MA, Lamberts RR, Whalley G et al. Myocardial tissue characterisation using echocardiographic deformation imaging. Cardiovasc Ultrasound 2019; 17: 27 47. Ravera M, Rosa GM, Fontanive P et al. Impaired left ventricular global longitudinal strain among patients with chronic kidney disease and end-stage renal disease and renal transplant recipients. Cardiorenal Med 2019; 9: 61–68 48. Hassan S, Barrett CJ, Crossman DJ. Imaging tools for assessment of myocardial fibrosis in humans: the need for greater detail. Biophys Rev 2020; 12: 969–987 49. Dı́ez J, González A, Kovacic JC. Myocardial interstitial fibrosis in nonischemic heart disease, part 3/4: JACC focus seminar. J Am Coll Cardiol 2020; 75: 2204–2218 50. Ravassa S, López B, Querejeta R et al. Phenotyping of myocardial fibrosis in hypertensive patients with heart failure. Influence on clinical outcome. J Hypertens 2017; 35: 853–861 51. Eiros R, Romero-González G, Gavira JJ et al. Does chronic kidney disease facilitate malignant myocardial fibrosis in heart failure with preserved ejection fraction of hypertensive origin? J Clin Med 2020; 9: 404 52. Capelli I, Gasperoni L, Ruggeri M et al. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J Nephrol 2020; 33: 37–48 53. López B, Querejeta R, González A et al. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension 2009; 53: 236–242 54. Graham-Brown MP, Patel AS, Stensel DJ et al. Imaging of myocardial fibrosis in patients with end-stage renal disease: current limitations and future possibilities. Biomed Res Int 2017; 2017: 5453606 55. Flythe JE, Chang TI, Gallagher MP et al. Blood pressure and volume management in dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2020; 97: 861–876 56. Chirakarnjanakorn S, Navaneethan SD, Francis GS et al. Cardiovascular impact in patients undergoing maintenance hemodialysis: clinical management considerations. Int J Cardiol 2017; 232: 12–23 57. Moraes M, Fregonesi M, Villanueva ADC et al. Kidney transplantation is associated with reduced myocardial fibrosis. A cardiovascular magnetic resonance study with native T1 mapping. J Cardiovasc Magn Reson 2019; 21: 21 58. Hewing B, Dehn AM, Staeck O et al. Improved left ventricular structure and function after successful kidney transplantation. Kidney Blood Press Res 2016; 41: 701–709 59. Francis C, Courbon G, Gerber C et al. Ferric citrate reduces fibroblast growth factor 23 levels and improves renal and cardiac function in a mouse model of chronic kidney disease. Kidney Int 2019; 96: 1346–1358 60. Uchida L, Tanaka T, Saito H et al. Effects of a prolyl hydroxylase inhibitor on kidney and cardiovascular complications in a rat model of chronic kidney disease. Am J Physiol Renal Physiol 2020; 318: F388–F401 61. Bugyei-Twum A, Abadeh A, Thai K et al. Suppression of NLRP3 inflammasome activation ameliorates chronic kidney disease-induced cardiac fibrosis and diastolic dysfunction. Sci Rep 2016; 6: 39551 62. Nanto-Hara F, Kanemitsu Y, Fukuda S et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant 2020; 35: 250–264 Received: 21.7.2020; Editorial decision: 11.9.2020 G. Romero-González et al. Downloaded from https://academic.oup.com/ndt/advance-article/doi/10.1093/ndt/gfaa284/6032823 by guest on 06 January 2021 28. Aoki T, Fukumoto Y, Sugimura K et al. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. Comparison between preserved and reduced ejection fraction heart failure. Circ J 2011; 75: 2605–2613 29. López B, Ravassa S, González A et al. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol 2016; 67: 251–260 30. Unger ED, Dubin RF, Deo R et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2016; 18: 103–112 31. Damman K, Valente MA, Voors AA et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated metaanalysis. Eur Heart J 2014; 35: 455–469 32. Fontes MS, Papazova DA, van Koppen A et al. Arrhythmogenic remodeling in murine models of deoxycorticosterone acetate-salt-induced and 5/6-subtotal nephrectomy-salt-induced cardiorenal disease. Cardiorenal Med 2015; 5: 208–218 33. Qiu H, Ji C, Liu W et al. Chronic kidney disease increases atrial fibrillation inducibility: involvement of inflammation, atrial fibrosis, and connexins. Front Physiol 2018; 9: 1726 34. Yamagami F, Tajiri K, Doki K et al. Indoxyl sulphate is associated with atrial fibrillation recurrence after catheter ablation. Sci Rep 2018; 8: 17276 35. Lekawanvijit S, Kompa AR, Manabe M et al. Chronic kidney diseaseinduced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One 2012; 7: e41281 36. Dai Z, Aoki T, Fukumoto Y et al. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol 2012; 60: 416–421 37. Sabbah HN, Sharov VG, Lesch M et al. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem 1995; 147: 29–34 38. Shah NR, Charytan DM, Murthy VL et al. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 2016; 27: 1823–1829 39. Radhakrishnan A, Pickup LC, Price A et al. Coronary microvascular dysfunction: a key step in the development of uraemic cardiomyopathy? Heart 2019; 105: 1302–1309 40. Kis E, Ablonczy L, Reusz GS. Cardiac magnetic resonance imaging of the myocardium in chronic kidney disease. Kidney Blood Press Res 2018; 43: 134–142 41. Edwards NC, Moody WE, Yuan M et al. Diffuse interstitial fibrosis and myocardial dysfunction in early chronic kidney disease. Am J Cardiol 2015; 115: 1311–1317 42. Stromp TA, Spear TJ, Holtkamp RM et al. Quantitative gadolinium-free cardiac fibrosis imaging in end stage renal disease patients reveals a longitudinal correlation with structural and functional decline. Sci Rep 2018; 8: 16972 43. Thompson RB, Raggi P, Wiebe N et al. A cardiac magnetic resonance imaging study of long-term and incident hemodialysis patients. J Nephrol 2019; 32: 615–626 44. Xu HY, Yang ZG, Zhang Y et al. Prognostic value of heart failure in hemodialysis-dependent end-stage renal disease patients with myocardial fibrosis quantification by extracellular volume on cardiac magnetic resonance imaging. BMC Cardiovasc Disord 2020; 20: 12 45. Rahsepar AA, Ghasemiesfe A, Suwa K et al. Comprehensive evaluation of macroscopic and microscopic myocardial fibrosis by cardiac MR: intraindividual comparison of gadobutrol versus gadoterate meglumine. Eur Radiol 2019; 29: 4357–4367