TRAIL/Apo-2L death signaling pathway and molecular
Anuncio

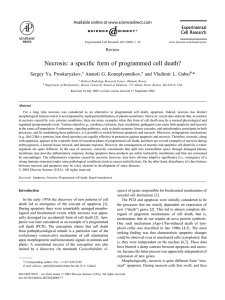
Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. REVISIONES TRAIL/Apo-2L death signaling pathway and molecular targeting agents: implications for chemoresistance Rafael Rosella, Hernán Cortés-Funesb, Mariano Monzó a, c , Enriqueta Felipd, Agustí Barnadasa and Miquel Taróna a Medical Oncology Service. Hospital Germans Trias i Pujol. Badalona (Barcelona). bMedical Oncology Service. Hospital 12 de Octubre. Madrid. c Department of Morphologic Sciences. School of Medicine. University of Barcelona. d Medical Oncology Service. Hospital Vall d’Hebron. Barcelona. TRAIL/ Apo-2L (tumor necrosis factor-related apoptosis-inducing ligand or Apo-2 ligand) was discovered by its sequence homology to tumor necrosis factor (TNF) and CD95 ligand (Fas ligand). Recombinant soluble human TRAIL/ Apo-2L is a candidate for clinical research in cancer therapy because it induces apoptosis in a broad spectrum of human cancer cell lines but not in many normal cells. It is now well-known that either ligands of death receptors or chemotherapeutic drugs can induce apoptosis in tumor cells through a common apoptotic machinery. Central to this process is a family of intracellular proteases, known as caspases. During apoptosis, they can act either as initiators in response to apoptotic signals or as effectors that finally cleave a number of vital proteins and lead to the demise of the cell. The activation of caspases is controlled via multiple signaling pathways that are described in this review. There are multiple kinases involved in survival signaling that may be targeted by novel agents. There are several compounds targeting the protein kinase Akt/ PKB that may inhibit apoptosis at several levels of the caspase cascade, which are also described in this review. Akt is the major kinase which phosphorylates the proapoptotic Bcl-2 member Bad and thereby converts Bad into an anti-apoptotic form that does not induce cytochrome c release. Chemotherapeutic drugs trigger the death pathway through the release of cytochrome c from damaged mitochondria. Besides TRAIL/ Apo-2L, several novel agents are described that can lead to extend the therapeutic threshold. Hopefully, clinical trials will be begun very soon to elucidate the possibility of enhancing the therapeu- Correspondence: Dr. R. Rosell. Medical Oncology Service. Hospital Germans Trias i Pujol. Ctra. Canyet, s/ n. 08916 Badalona (Barcelona). Correo electrónico: rrosell@ ns.hugtip.scs.es Recibido el 4-4-2001. Aceptado para su publicación el 9-7-2001. 284 tic effect in terms of response and, especially, survival. It is thus essential for clinical investigators to understand the distinct pathways of apoptosis and caspase activation when deciding to participate in these trials. Key words: TRAIL/ Apo-2L, IAP genes, caspase-8, caspase-9, XIAP, Akt, STI571. Rev Oncología 2001; 3: 284-291. TRAIL/ Apo-2L y nuevas dianas terapéuticas: implicaciones en quimiorresistencia El TRAIL/ Apo-2L (tumor necrosis factor-related apoptosis-inducing ligand o Apo-2 ligando) se descubrió por su homología de secuencia con el TNF y el CD95 (fas ligando). El TRAIL/ Apo-2L humano soluble recombinante es un candidato para su investigación clínica del tratamiento del cáncer, ya que induce apoptosis en un amplio espectro de líneas celulares derivadas de tumores humanos, pero no en la mayoría de líneas celulares normales no neoplásicas. Actualmente se conoce que los ligandos de los receptores inductores de muerte celular (death receptors) o los agentes de quimioterapia para inducir apoptosis en células tumorales a través de un mecanismo común. Un papel central de este proceso lo desarrolla una familia de proteasas intracelulares conocidas como caspasas. Durante la apoptosis pueden actuar tanto como iniciadores de la respuesta a las señales apoptóticas o como efectores que finalmente degradan un número elevado de proteínas esenciales conduciendo a la muerte celular. La activación de las caspasas está controlada por múltiples cascadas de señalización y que se describen en esta revisión. Existen múltiples kinasas involucradas en las vías de rescate o inhibidoras de la muerte celular programada que pueden ser dianas para nuevos fármacos. En la actualidad disponemos de múltiples compuestos que inhiben la proteín kinasa Akt/ PKB que a su vez puede ac- Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. R. ROSELL ET AL — TRAIL/APO-2L DEATH SIGNALING PATHWAY AND MOLECULAR TARGETING AGENTS: IMPLICATIONS FOR CHEMORESISTANCE tuar inhibiendo la respuesta apoptótica en distintos niveles de la cascada de caspasas, los cuales se describen igualmente a continuación. AKT es la principal kinasa que fosforila la proteína BAD (miembro de la familia de Bcl-2 que actúan como inductores de la apoptosis), convirtiendo a BAD en una forma antiapoptótica que impide la inducción de la liberación del citocromo c. Los agentes de quimioterapia inducen las vías de muerte celular a través de la liberación del citocromo c desde la mitocondria dañada. Juntamente con el TRAIL/ api-2L se han descrito multitud de nuevos agentes que pueden ampiar los umbrales de respuesta de los tratamientos actuales. De forma esperanzadora algunos ensayos clínicos se pondrán en marcha en un breve período de tiempo y permitirán clarificar la posibilidad de potenciar el efecto terapéutico en términos de respuesta y, especialmente, de supervivencia. Por tanto es esencial para los investigadores clínicos entender las diferentes vías de apoptosis y de activación de las caspasas en el momento de decidir su participación en este tipo de ensayos. Palabras clave: cTRAIL/Apo-2L, genes IAP, caspasa-8, caspasa-9, XIAP, Akt, STI571. In spite of the frequent success of surgical and chemotherapeutic measures in controlling primary tumor growth, metastatic disease is seldom amenable to surgery, displays resistance to chemotherapy, and is the major cause of terminal illness. Apoptosis, or programmed cell death, is a physiologic process in which cells die after exposure to normal or pathologic stimuli. There is mounting evidence that the ability of cancer cells to evade or subvert signals that lead to apoptosis plays a major role in tumor survival in the face of cytotoxic drug regimens. The anticancer action of cytotoxic drugs is in large part attributable to their ability to induce apoptosis of tumor cells and is therefore dependent on the integrity of the cellular signaling pathways that lead to apoptotic cell death. Defects in some of these pathways are common in cancer cells, especially of metastatic origin, and are therefore likely to underlie tumor resistance to chemotherapy. By outlining the relevance of new apoptotic signaling pathways, this review attempts to shed some light on the mechanism whereby cytotoxic agents cause apoptosis and, more importantly, to highlight novel therapeutic approaches that intervene in downstream signaling pathways. TRAIL/ Apo-2L surfaces as a new therapeutic agent that can overcome resistance to cytotoxic drugs in many tumors. Oncologists today need to be fully aware of the progress that is being made towards understanding these death signaling pathways. Many drugs are being developed to circumvent chemoresistance and enhance tumor killing, leading to a new therapeutic threshold. Figures 1 and 2 summarize the points dealt with here. Recent evidence has implicated the members of the tumor necrosis factor (TNF) receptor superfamily of cell surface proteins in tumor cell death. Because transduction of signals that induce apoptosis is the salient functional property of TNF-R1 and Fas (Apo-1/ CD95), they are frequently referred to as death receptors (DRs) and share cysteine-rich repeats in the ligand-binding extracellular region as well as a stretch of 80 amino acids in the cytoplasmic domain, termed the «death domain», that is essential for apoptosis signaling. Recently, another member of the TNF family, the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), also called Apo-2L, has been identified1. TRAIL/ Apo-2L interacts with 2 newly discovered DRs, DR4 and DR5. Transfection experi- Fig. 1. Apoptosis control by death and decoy receptors. TNF: tumor necrosis factor; DR: death receptor; FADD: fas associated death domain; TRADD: TNFR associated death domain; TNFR1: tumor necrosis factor receptor 1. 285 Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. REV ONCOLOGÍA, VOLUMEN 3, NÚMERO 6, NOVIEMBRE-DICIEMBRE 2001 Fig. 2. TRAIL/Apo-2L and PI3K/AKT survival signaling pathways. ments have shown that both DR4 and DR5 can initiate caspase-mediated apoptosis1,2. Tumor necrosis factor receptor 1 (TNFR1), Fas (Apo-1/ CD95), and the DRs for TRAIL/ Apo-2L regulate programmed cell death (apoptosis). Upon engagement by their respective ligands, TNFR1 and Fas recruit adaptor molecules and activate a cascade of cysteine proteases (caspases), the proteolytic activity of which induces apoptosis. In summary, the best-characterized DRs and their cognate ligands thus far include TNFR1/ TNF- α Fas (Apo-1/ CD95)/ FasL (Apo-1L or CD95L), DR4 and DR5/TRAIL/Apo-2L, and Apo-3 (DR3)/Apo-3L (table 1). We have reviewed 18 references concerning TRAIL/ Apo-2L. TRAIL/ Apo-2L is a 281-amino acid protein, related most closely to Fas/Apo-1 ligand1. Soluble TRAIL/ Apo-2L induces extensive apoptosis in lymphoid as well as non-lymphoid tumor cell lines. The effect of TRAIL/ Apo-2L is not inhibited by soluble Fas/ Apo-1 and TNF receptors. It was found that TRAIL/ Apo-2L acts via a receptor which is distinct from Fas/ Apo-1 and TNF receptors, and it was suggested that TRAIL/ Apo-2L could function as an extracellular signal that TABLE 1. Death receptors (DR) and their cognate ligands TNFR1/TNF-α Fas (CD95 or Apo-1)/FasL (CD95L or Apo-1L) DR5 and DR5/TRAIL (Apo-2L) Apo3 (DR3)/Apo-3L 286 triggers programmed cell death. The CD95 ligand (CD95L) and TNF are important extracellular activators of apoptosis in the mammalian immune system. The cognate receptors for these cytokines, Fas (CD95/ Apo-1) and TNFR1, contain cytoplasmic «death domains» (table 1 and fig. 1) that activate the cell’s apoptotic machinery through interaction with the death domains of the adapter proteins FADD (Fas associated death domain) and TRADD (TNFR associated death domain). Upon activation by a ligand, Fas (CD95/Apo-1) recruits FADD. FADD in turn activates the ced-3-related protease MACHα/ FLICE (caspase-8), thereby initiating a series of caspase-dependent events that lead to cell death2. TRAIL/ Apo-2L activates rapid apoptosis in tumor cell lines, acting independently of Fas (CD95/Apo-1), TNFR1, or FADD. A receptor for TRAIL, DR4 activates apoptosis independently of FADD. DR4 exhibits several mRNA transcripts that are expressed in multiple human tissues, including peripheral blood leukocytes. In 1997, 2 additional TRAIL/ Apo-2L receptors were identified: DR5, which contain a cytoplasmic death domain and induces apoptosis like DR4; and the receptor designated decoy receptor 1 (DcR1), which acts like a decoy receptor that inhibits TRAIL/Apo-2L signaling2. By cross-hybridization with DcR1, a fourth Apo-2L receptor was identified, named decoy receptor 2 (DcR2). The DcR2 gene mapped to human chromosome 8p21, as did the gene encoding DR4, DR5 and DcR1. Upon overexpression, DcR2 did not activate apoptosis or nuclear factor-KB; however, Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. R. ROSELL ET AL — TRAIL/APO-2L DEATH SIGNALING PATHWAY AND MOLECULAR TARGETING AGENTS: IMPLICATIONS FOR CHEMORESISTANCE it reduced cellular sensitivity to TRAIL/ Apo-2L-induced apoptosis3. In 1999, the crystal structure of the complex between Apo-2L and DR5 was reported4. Fas (CD95/Apo-1) and TNFR1 trigger apoptosis by recruiting the apoptosis initiator caspase-8 through the adaptor FADD. Fas binds FADD directly, whereas TNFR1 binds FADD indirectly, through TRADD. TRAIL/ Apo-2L initiates apoptosis through caspase-8 and FADD is also involved as a universal adaptor for DRs5,6 (fig. 1). In summary, apoptosis is controlled by death and decoy receptors. TRAIL/ Apo-2L can bind to five members of the TNFr family, DR4, DR5, DcR1, DcR2, and osteoprotogerin. Binding of TRAIL/ Apo-2L to DR4 and DR5 leads to the cleavage and activation of caspase-8 and caspase-10. Processed and actived caspase-8 can also cleave and activate the BH3 domain containing BID (a pro-apoptotic Bcl-2 member), which translocates to the mitochondria, triggering the cytosolic release of cytocrome c (fig. 2). In the cytosol, cytocrome c and dATP bind to Apaf-1 and cause its oligomerization. Apaf-1, processes procaspase-9 into and active caspase, which recruits, cleaves, and activates the executioner caspase-3. This can proteolytically cleave poly (ADP-ribose) polymerase, DNA fragmentation factor 45, resulting in the morphologial features and DNA fragmentation of apoptosis. The recombinant soluble human TRAIL/ Apo-2L molecule appears remarkably safe and non-inmunogenic, and it is now being tested in clinical trials. In vitro , soluble TRAIL/ Apo-2L exerted cytostatic or cytotoxic effects on a variety of tumor cell lines7, but not on several types of normal cells. In vivo, soluble TRAIL/ Apo-2L shows antitumor activity in xenograft models in colon carcinoma cell lines, and synergistic activity with 5-FU or CPT-11, causing marked regression or complete remission of tumors7. THE ROLE OF TRAIL/ APO-2L IN DIFFERENT CHEMORESISTANT TUMORS In contrast to DR4 and DR5, DcR1 (TRAIL-R3/ TrID) and DcR2 (TRAIL-R4/ TRUNDD) are unable to transduce death signals and may function as decoy receptors. They may also provide inhibitory signals, possibly through activation of nuclear factor-KB. The DR4, DR5, DcR1 and DcR2 genes are tightly clustered on human chromosome 8p22-21, they may have been derived from a common ancestor by gene duplication8. A fifth TRAIL/ Apo-2L receptor, osteoprotegerin, exists in a soluble form and inhibits apoptosis by interfering with the binding of TRAIL/ Apo-2L to the DR4 and DR5 receptors. In contrast to Fas ligand, the expression of which is limited to cells of the immune system and a few immune-privileged sites, TRAIL/ Apo-2L is expressed in a wide range of normal fetal and adult tissues and induces apoptosis only in transformed and malignant cells, thus constituting a promising new candidate for cancer treatment. Restricted DR5 expression, or lack thereof, may be an important mechanism by which Ewing’s sarcoma family tumors (ESFT) cells become resistant to TRAIL/ Apo-2L8. TRAIL/ Apo-2L-resistant tumors may also exhibit functional inactivation of the receptors, either by lack of expression on the cell surface, as reported for melanomas, or by the presence of structural abnormalities, as in the case of DR5 mutations in head and neck cancers and of the polymorphism at codon 441 in the death domain of DR4, which acts in a dominant-negative fashion (reviewed in Mitsiades et al) 8. DR5 is up-regulated by chemotherapeutic agents. Therefore, its absence may have implications in chemotherapy-related tumor cell responses. TRAIL/ Apo-2L effectively and specifically kills ESFT cells by engaging DR4 and DR5 and activating a cascade of caspases that begins at caspase-10. Caspase-8 is also activated, but it is not recruited to the TRASC (TRAIL-associated signaling complex) 8. Experimental data suggest that TRAIL/ Apo-2L has excellent therapeutic potential in ESFTs. In general, treatment with DNA damaging anticancer agents can induce p53 and/ or nuclear factor κB, which in turn can up-regulate DR5 and/ or DR4 expression, thereby enhancing TRAIL/ Apo-2L-induced apoptotic signaling. In contrast, DcR1 and DcR2 can act as inhibitors of TRAIL/ Apo-2L-induced apoptotic signaling. Flame-1 (also known as c-FLIP [FADD-like IL-1 β-converting enzyme]) has a dominant negative effect against caspase-8 and potentially inhibits TRAIL/ Apo-2L induced death signaling (fig. 2) 9. For example, pretreatment with paclitaxel or docetaxel enhances TRAIL/ Apo-2L-induced apoptosis of prostate cancer cells by inducing DR4 and DR5 protein levels9. The levels of IAP family members: cIAP1, cIAP2, X-linked XIAP, and survivin, may also inhibit TRAIL-induced apoptosis by specifically binding to and inhibiting the activities of caspase-3, caspase-9, and caspase-7 (fig. 2). Taxane-induced apoptosis is triggered by mitochondrial ∆ψm (permeability transition), release of cytocrome c into the cytosol, and induction of Apaf-1mediated caspase-9 and caspase-3 activities (fig. 1 and 2). TRAIL/ Apo-2L triggers the molecular events of both the extrinsic (DR) and intrinsic (mitochondrial) pathway of apoptosis9. Pretreatment with paclitaxel up-regulates DR4 and DR5 expression and enhances TRAIL/ Apo-2L-induced caspase-8, caspase-10, and Bid processing and the cytosolic accumulation of cytocrome c. It also down-regulates XIAP, cIAP1 and survivin levels9. TRAIL-sensitive ESFT cell lines carry p53 mutations or deletions suggesting that TRAIL-induced apoptosis is independent of p538. Wild-type p53 can induce transcriptional regulation of DR5 TRAIL/ Apo-2L receptor in cells undergoing apoptosis. Ectopic expres- 287 Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. REV ONCOLOGÍA, VOLUMEN 3, NÚMERO 6, NOVIEMBRE-DICIEMBRE 2001 sion of wild-type p53 can enhance TRAIL-induced apoptosis in malignant gliomas. Using the TRASC assay, it was shown that caspase-10 is the apical in the TRAIL-signaling pathway8. The TRAIL/ Apo-2L-resistant phenotype has also been investigated in brain tumors. Malignant glioma cells primarily express DR5. DR5 expression is increased by DNA-damaging chemotherapeutic agents, thus enhancing TRAIL/ Apo-2L killing of glioma cells. TRAIL/ Apo-2L killing of glioma cells is characterized by caspase-8 and caspase-3 activation, PARP (poly [ADP-ribose] polymerase) cleavage, and DNA fragmentation10. PED/ PEA-15 (phosphoprotein enriched in diabetes/ phosphoprotein enriched in astrocytes Mr 15,000) protein expression leads to resistance to TRAIL-induced apoptosis11. PED/ PEA-V is overexpressed in fibroblasts from type 2 diabetics compared with non-diabetic individuals, as well as in skeletal muscle and adipose tissues, 2 major sites of insulin resistance in type 2 diabetes. Overexpression of the PED/ PEA-15 gene may contribute to insulin resistance in glucose uptake in type 2 diabetes. PED cloning shows that it encodes a 15 KDa phosphoprotein identical to the protein kinase c (PKC) substrate PEA-1511. Transfection of PED antisense in TRAIL-resistant cell lines converted these lines into sensitive lines, whereas transfection of PED cDNA in TRAIL-sensitive cell lines rendered the cells resistant. Inhibition of PKC activity restored TRAIL-induced apoptosis in resistant cells, demonstrating that induction of apoptosis in TRAIL-resistant glioma cells is prevented by PKC phosphorylation. The critical PKC substrate is postulated to be PED/ PEA-1510. Apoptosis was induced by combination of CD437 and TRAIL. The synthetic retinoid CD437 is a potent inductor in a variety of cancer cell types through increased levels of DRs. Treatment of human lung cancer cells with a combination of suboptimal concentrations of CD437 and TRAIL/ Apo-2L enhanced induction of apoptosis in tumor cell lines but not in normal lung epithelial cells12. CD437 upregulates DR4 and DR5 expression. The CD437/ TRAIL/ Apo-2L combination enhances activation of caspase-3, caspase-7, caspase-8 and caspase-9 and the subsequent cleavage of PARP and DNA fragmentation factor 4512. This combination induces BID cleavage and increases cytocrome c release from mitochondria (fig. 2). Anticancer drugs sensitize tumor cells. Doxorubicin and cisplatin sensitize colon cancer cells to TRAILinduced cleavage of procaspase-8 and PARP and caspase activation. Anticancer drugs increase the ability of TRAIL/ Apo-2L to trigger caspase-dependent cell death, also described in breast cancer13. TRAIL/ Apo-2L-resistant neuroblastomas lack caspase-8. Semiquantitative RT-PCR reveals that DR5 (TRAIL-R2, KILLER) and DcR1 (TRAIL-R3, TRID) are the main TRAIL-receptors used by neuroblasto- 288 ma cells. Failure to express caspase-8 and/ or caspase-10 could be an important mechanism of resistance to chemotherapy in neuroblastoma cells. Demethylation by 5-ADC (5-aza-2’-deoxycytidine) restores caspase-8 and caspase-10 expression and TRAIL/ Apo-2L sensitivity14. TRAIL/Apo-2L is also active in pancreatic cancer. TRAIL/ Apo-2L caused a sustained profound cell death in several human pancreatic cancer cells lines but not in Aspc1 cells. The resistance of Aspc1 cells to TRAIL/ Apo-2L was not related to the lack of TRAIL/ Apo-2L receptors. The combination of actinomycin D and TRAIL/ Apo-2L induced an almost complete lysis of Aspc1 cells15. Actinomycin D alone had no effect but inhibited the expression of FLICE15. STI-571 AND TRAIL/ APO -2L IN BCR-ABL-POSITIVE ACUTE LEUKEM IAS Bcr-Abl tyrosine kinase inhibitor STI-571 produces a high rate of remission in chronic myeloid leukemia (CML), but remissions induced in patients with blast crisis of CML or Bcr-Abl-positive Abl are not durable. STI-571 and TRAIL/ Apo-2L significantly enhances TRAIL/ Apo-2L induced apoptosis and increases the processing of caspase-9, and -3 and XIAP, without affecting the levels of DR4, DR5, decoy receptor or C-FLIPL (fig. 2) 16. Interestingly, STI-571-mediates sensitization of Bcr-Abl-positive leukemia cells to TRAIL/ Apo-2L-induced apoptosis. By inhibiting Bcr-Abl tyrosine kinase, STI-571 inhibits constitutively active PI3 kinase, STAT5 proteins (Signal Transducers and Activators of Transcription) 17 and NFκB in Bcr-Abl positive leukemia blasts16. The result is the lowering of BclXL levels and its antiapoptotic activity. It also has the capacity to lower the levels of IAP and improve caspase-9 activity. These effects of STI-571 could promote TRAIL/Apo-2L-induced cytocrome c and SMAC/ DIABLO release from mitochondria and the subsequent activation of the effector caspase-3 and caspase-7, resulting in apoptosis (reviewed in Nimmanapalli et al) 16. M ETALLOPROTEINASES IN CANCER TNF and FasL have potent cytotoxic activity against many types of tumor cells, however, the application of these death ligands to cancer therapy has been restricted by their severe toxicity to normal tissues. Although we can not use FasL for treatment, intriguingly, FasL is observed to be proteolytically cleaved from the surface of some cell types by MMP-7 (matrix metalloproteinase) 18. Proteolytic cleavage of FasL could provide at least partial protection against Fasmediated cell deaths (also reviewed in Lacal) 19. MMPs are zinc-dependent enzymes that help regulate the turnover of ECM (extracellular matrix) components. They are believed to play an important role in Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. R. ROSELL ET AL — TRAIL/APO-2L DEATH SIGNALING PATHWAY AND MOLECULAR TARGETING AGENTS: IMPLICATIONS FOR CHEMORESISTANCE embryo development, morphogenesis, and tissue remodeling, as well as in tumor invasion and metastasis. MMPs are thought to induce ECM degradation, angiogenesis, and possibly, regulation of tumor growth itself. Invading and metastatic tumor cells typically secrete MMPs and induce MMP production by surrounding stromal cells that may overwhelm the local tissue capacity to maintain their proteolytic activity in check. It was recently found that the proteolytic activity of MMP-7, which is locally expressed in several tumors, may contribute to tumor resistance to cytotoxic agents. Specific inhibition of MMP-7 may provide a potentially effective approach toward increasing the efficacy of chemotherapy18. Development of MMP inhibitors is a very appealing novel anti-cancer approach20. OTHER MOLECULAR TARGETING AGENTS Recent evidence has suggested that X-linked inhibitors of apoptosis protein (XIAP) is a determinant in cisplatin sensitivity in ovarian cancer. Cell fate is determined by a balance between cell survival and apoptotic signaling. Cisplatin decreased XIAP protein levels and induced AKT cleavage and apoptosis in chemosensitive, but not in chemoresistant, ovarian cancer cells. It has been proven that XIAP down-regulation induced AKT cleavage and apoptosis21. In the presence of the PI3-K (phosphatidylinositol 3-kinase) inhibitor (LY294002), XIAP overexpression failed to block cisplatin-induced apoptosis and to induce AKT phosphorylation, suggesting that the site of action of XIAP is upstream of AKT in this cell survival pathway21. AKT (also known as protein kinase B) consists of a family of highly conserved serine/ threonine kinases. Activated AKT has been shown to mediate cell survival by phosphorylating several downstream targets, such as BAD and caspase-9 (fig. 2). In contrast, the tumor suppressor PTEN inhibits PI3K-dependent activation of AKT (reviewed in Tanno et al) 22. PI3K was discovered as an activity that phosphorylates phosphoinositols at the D-3’ position of the inositol ring and produces novel phosphoinositides23. The flavanoid derivative LY 294002, a potent PI3K inhibitor, is a competitive, reversible inhibitor of the ATP binding site of the enzyme (reviewed in Hidalgo, Rowinsky) 24. Rapamycin, a macrolide fungicide, and its analog CCI-779 have prominent anti-tumor activity. Rapamycin binds intracellularly to members of the immunophilin family of FK506 binding proteins (FKBPs) especially FKBP12. The resultant rapamycin-FKBP12 complex interacts with and inhibits the activity of a kinase named mammalian target of rapamycin (mTOR). Experimental data show that mTOR functions downstream of the PI3K/AKT pathway24. Another major signaling pathway is the MAPK (mitogenactivated protein kinase) pathway. Similarly to AKT, the phosphorylation of MAPK is also increased in some lung adenocarcinoma cells upon detachment, suggesting that both PI3K and MAPK are similarly regulated in the tumor cells25. The gene corral displayed by the PI3K-AKT pathway and TRAIL/ Apo-2L open the gates to novel therapeutic approaches. For instance, the 26s proteasome is the universal multi-catalytic protease, responsible for the bulk of protein turnover in all cells. A selective inhibitor PS-341 has been developed, inhibiting the activation of NF- κB26. Finally, inhibitors of histone deacetylase (HDAC) like depsipeptide are associated with apoptosis through suppression of IAP genes. Interestingly, the activity of HDAC inhibitors is enhanced in the presence of the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine (DAC) 27. Hence, the effect of HDAC inhibitors may be intimately linked to the methylation pattern of tumors, at least in non-smallcell lung cancers28. It is well known that apoptotic and necrotic cells are a major source for plasma DNA in cancer patients29. Also biochips can be used to test RNA array expression in blood. cDNA arrays of 170 genes were analyzed in blood samples from 26 breast cancer patients and 22 healthy controls. Cluster analysis identified a group of 12 genes that were elevated in the blood of cancer patients. Hierarchical clustering identified 5 core genes: CD44, maspin, gro-alpha, ß-tubulin and N30,31. A bloodbased assay could be a tool to define personalized chemotherapy or at least classified patterns of chemoresistance by hierarchical cluster analysis. A cDNA microarray containing 7,075 genes was used to compare gene expression profiles of 3 non-small-cell lung cancer cell lines and to examine changes after exposure to paclitaxel. There were 27 differentially expressed genes in the chemoresistant line in comparison to the parental line. Of these, 12 genes were increased whereas 15 were repressed. We can foresee an array analysis of gene expression in peripheral blood (table 2) in patients with stage IV before starting chemotherapy and after 3 cycles at the time of radiographic assessment of clinical remission. A hierarchical set of apoptotic-resistant genes to selective chemotherapy combinations could then be identified, and this TABLE 2. Chemotherapy-mediated regulation of some genes related to apoptosis DR4 DR5 BID Caspase-10 Caspase-8 Caspase-3 c-IAP1 XIAP Survivin Ribonucleotide reductase DcR1 DcR2 Osteoprotegerin PI3K AKT NF-κB Bcl-XL BAD β-tubulin STAT5 Caspase-9 SMAC/DIABLO Hsp70 Hsp90 MMP-7 289 Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. REV ONCOLOGÍA, VOLUMEN 3, NÚMERO 6, NOVIEMBRE-DICIEMBRE 2001 TABLE 3. Targeted therapies Target Activation of caspase-8, caspase-3 Inhibits IAP genes Upregulates DR4 and DR5 New drugs TRAIL/Apo-2L (Genentech Inc) Retinoid CD437 Targretin? (Ligand Pharmaceuticals) Inhibits Abl tirosine kinase acti- STI571 (for signal-transduction vity, PDGF receptor and cinhibitor) (Novartis) kit tyrosine kinases Inhibits SCF-mediated kit activation Blocks the phosphorylation of Akt Inhibits XIAP through P13K LY294002 (flavonoid derivative) inhibition (Lilly) Inhibits mTOR Rapamycine (Wyeth) Inhibits IAP genes Inhibitors of HDAC, CI-994 (Pfizer) EGF-receptor inhibitor ZD1839 (Iressa) (AstraZeneca) Inhibits the M2 subunit of ribo- Triapine (Vion) nucleotide reductase chemoresistance could —at least in some instances— be overcome by the addition of very promising novel therapeutic agents like TRAIL/ Apo-2L. Table 3 summarizes novel therapeutic approaches based on exploiting new crucial targets in several apoptotic pathways as well as on inhibiting tyrosine kinases. In addition to TRAIL/ Apo-2L STI571 stands out because of its intricate action. STI571 is a phenylaminopyrimidine derivative that selectively inhibits the enzymatic activity of several tyrosine kinases: ABL; the BCR-ABL fusion protein of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia; platelet-derived growth factor receptor; and the product of the c-kit gene. It has demonstrated excellent activity and tolerability in patients with chronic myeloid leukemia in whom treatment with interferon alfa had failed32. Responses have also been observed in 55% of patients with a myeloid-blast crisis33. At least 70% of small-cell lung cancers express the kit receptor tyrosine kinase and its ligand, stem cell factor (SCF). This coexpression constitutes a functional autocrine loop, suggesting that inhibitors of kit tyrosine kinase activity could have therapeutic efficacy34. It was demonstrated that STI571 efficiently blocked SCF-mediated activation of mitogen-activated protein kinase and Akt but did not affect insulin-like growth factor 135. Aberrant expression of c-kit and/ or SCF has been reported in several breast, lung, prostate, and colorectal tumors. In gastrointestinal tissues, c-kit expression has been observed in interstitial cells of Cajal in the small intestine. It has been suggested that c-kit activation plays a role in gastrointestinal stromal tumors derived from Cajal cells either through overexpression or mutations of 290 c-kit35. Recently it has been shown that gastrointestinal stromal tumors are highly responsive to STI571 because they uniformly express c-kit36. Also data indicates that STI571 blocks the phosphorylation of Akt, which is downstream from c-kit37. It is possible that STI571 can be active in other tumors such as non-small cell lung cancer and melanoma. Clinical trials have been started in lung, prostate and brain tumors38. CONCLUSIONS Physicians caring for cancer patients need to become aware of the rapid development of science today, and medical oncologists faced with a plethora of clinical trials with new agents must be able to discern which is the best combination for their patients. The Spanish Lung Cancer Group is committed to developing pharmacogenetic studies to elucidate which genetic markers can help to distinguish different subsets of patients that may be more responsive to specific therapeutic approaches. By including combinations with some of the new drugs, we may finally be able to break through the ceiling of therapeutic results obtained with current chemotherapy regimens. Acknowledgments The authors thank Beatriz Rodrigo for technical assistance and Renée O’Brate for assistance with the manuscript. References 1. Pitti RM, Marsters SA, Ruppert S, et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271: 12.687-12.690. 2. Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-duced apoptosis by a family of signaling and decoy receptors. Science 1997; 277: 818-821. 3. Marsters SA, Sheridan JP, Pitti RM, et al. A novel receptor for Apo2L/ TRAIL contains a truncated death domain. Curr Biol 1997; 7: 1.003-1.006. 4. Hymowithz SG, Christinger HW, Fuh G, et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell 1999; 4: 563-571. 5. Kischkel FC, Lawrence DA, Chuntharapai A, et al. Apo-2L/ TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 ad 5. Immunity 2000; 12: 611-620. 6. Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 1999; 11: 255-260. 7. Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo-2 Ligand. J Clin Invest 1999; 104: 155-162. 8. Mitsiades N, Poulaki V, Mitsiades C, et al. Ewing’s sarcoma family tumors are sensitive to tumors necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res 2001; 61: 2.704-2.712. 9. Nimmanapalli R, Perkins CL, Orlando M, et al. Pretreatment with paclitaxel enhances Apo-2 Ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res 2001; 61: 759-763. Documento descargado de http://www.elsevier.es el 16/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. R. ROSELL ET AL — TRAIL/APO-2L DEATH SIGNALING PATHWAY AND MOLECULAR TARGETING AGENTS: IMPLICATIONS FOR CHEMORESISTANCE 10. Hao C, Beguinot F, Condorelli G, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res 2001; 61: 1.162-1.170. 11. Condorelli G, Vigliotta G, Iavarone C, et al. PED/ PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J 1998; 17: 3.858-3.866. 12. Sun S-Y, Yue P, Hon WK, et al. Augmentation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)induced apoptosis by the synthetic retinioid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphtalene carboxylic acid (CD437) through up-regulation of TRAIL receptors in human lung cancer cells. Cancer Res 2000; 60: 7.149-7.155. 13. Lacour S, Hammann A, Wotawa A, et al. Anticancer agents sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated caspase-8 activation and apoptosis. Cancer Res 2001; 61: 1.645-1.651. 14. Eggert A, Grotzer MA, Zuzak TJ, et al. Resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res 2001; 61: 1.314-1.319. 15. Matsuzaki H, Schmied BM, Ulrich A, et al. Combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and actinomycin D induces apoptosis even in TRAIL-resistant human pancreatic cancer cells. Clin Cancer Res 2001; 7: 407-414. 16. Nimmanapalli R, Porosnicu M, Nguyen D, et al. Cotreatment with STI-571 enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL or Apo-2L)-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Clin Cancer Res 2001; 7: 350-357. 17. Bromberg J, Darnell JE. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 2000; 19: 2.468-2.473. 18. Mitsiades N, Yu W-H, Poulaki V, et al. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 2001; 61: 577-581. 19. Lacal JC. Sistemas de transmisión de señales. In: Rosell R, Abad A, Monzó M, Barnadas A, eds. Oncología clínica y molecular. Madrid: Editorial Arán, 2000; 59-66. 20. Zucker S, Cao J, Chen W-T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 2000; 19: 6.642-6.650. 21. Asselin E, Mills GB, Tsang BK. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatininduced apoptosis in human ovarian epithelial cancer cells. Cancer Res 2001; 61: 1.862-1.868. 22. Tanno S, Tanno S, Mitsuuchi Y, et al. AKT activation upregulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res 2001; 61: 589-593. 23. Slupianek A, Nieborowska-Skorska M, Hoser G, et al. Role of phosphatidylinositol 3-kinase-akt pathway in nucleophosmin/ anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res 2001; 61: 2.194-2.199. 24. Hidalgo M, Rowinsky E. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene 2000; 19: 6.680-6.686. 25. Wei L, Yang Y, Yu Q. Tyrosine kinase-dependent, phosphtidylinositol 3’-kinase, and mitogen-activated protein kinase-independent signaling pathways prevent lung adenocarcinoma cells from anoikis. Cancer Res 2001; 61: 2.439-2.444. 26. Adams J, Elliott PJ. New agents in cancer clinical trials. Oncogene 2000; 19: 6.687-6.692. 27. Zhu W-G, Lakshmanan RR, Beal MD, et al. DNA methyltransferase inhibiton enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res 2001; 61: 1.327-1.333. 28. Zöchbauer-Müller S, Fong KM, Virmani AK, et al. Aberrant promoter methylation of multiple genes in nonsmall cell lung cancers. Cancer Res 2001; 61: 249-255. 29. Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001; 61: 1.659-1.665. 30. Martin KJ, Graner E, Li Y, et al. High-sensitivity array analysis of gene expression for the early detection of disseminated breast tumor cells in peripheral blood. PNAS 2001; 98: 2.646-2.651. 31. Volas-Redd G, Guo Y, Zeng H, et al. The use of cDNA microarray to characterize gene expression profiles in paclitaxel-treated and paclitaxel-resistant non-small cell lung carcinoma cell lines. 7th IASLC Workshop. Barcelona, April 30-May 3, 2001 (abstr). 32. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the Bcr-Abl tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1.031-1.037. 33. Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the Bcr-Abl tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 2001; 344: 1.038-1.042. 34. Krystal GW, Honsawek S, Litz J, Buchdunger E. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res 2000; 6: 3.319-3.326. 35. Bellone G, Carbone A, Sibona N, et al. Aberrant activation of c-kit protects colon carcinoma cells against apoptosis and enhances their invasive potential. Cancer Res 2001; 61: 2.200-2.206. 36. Oensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001; 344:1.052-1.056. 37. Wang W-L, Healy ME, Sattler M, et al. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI571. Oncogene 2000; 19: 3.521-3.528. 38. Goldman JM, Melo JV. Targetin the Bcr-Abl tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1.084-1.086. 291