View presentation
Anuncio
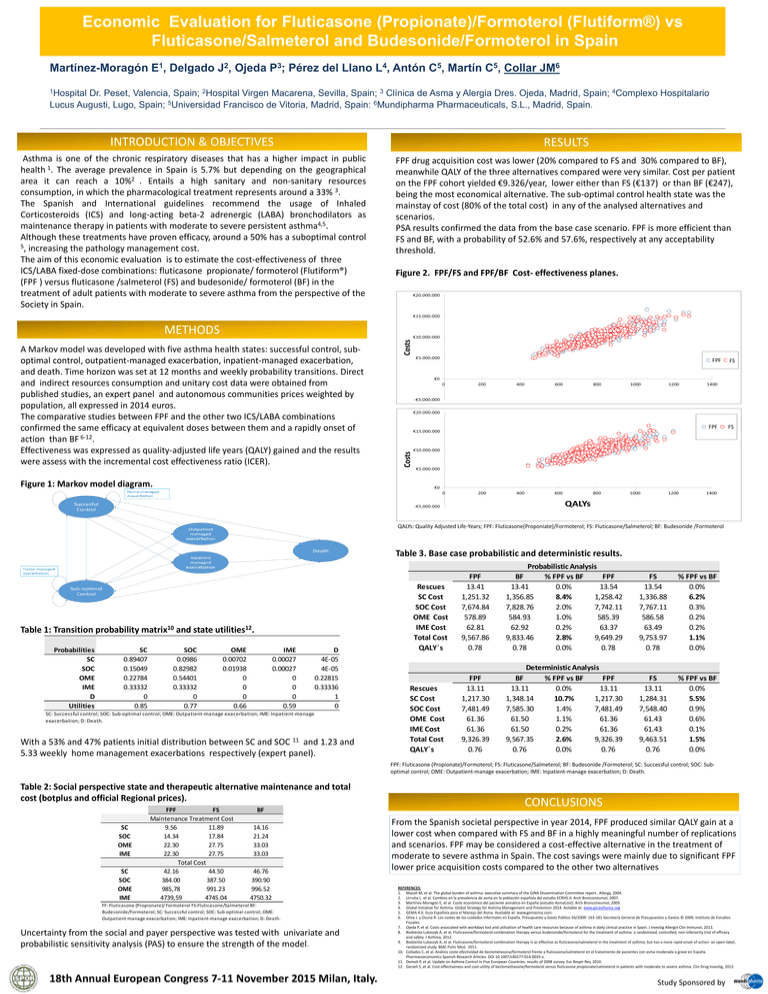
Economic Evaluation for Fluticasone (Propionate)/Formoterol (Flutiform®) vs Fluticasone/Salmeterol and Budesonide/Formoterol in Spain Martínez-Moragón E1, Delgado J2, Ojeda P3; Pérez del Llano L4, Antón C5, Martín C5, Collar JM6 1Hospital Dr. Peset, Valencia, Spain; 2Hospital Virgen Macarena, Sevilla, Spain; 3 Clínica de Asma y Alergia Dres. Ojeda, Madrid, Spain; 4Complexo Hospitalario Lucus Augusti, Lugo, Spain; 5Universidad Francisco de Vitoria, Madrid, Spain: 6Mundipharma Pharmaceuticals, S.L., Madrid, Spain. INTRODUCTION & OBJECTIVES RESULTS Asthma is one of the chronic respiratory diseases that has a higher impact in public health 1. The average prevalence in Spain is 5.7% but depending on the geographical area it can reach a 10%2 . Entails a high sanitary and non-sanitary resources consumption, in which the pharmacological treatment represents around a 33% 3. The Spanish and International guidelines recommend the usage of Inhaled Corticosteroids (ICS) and long-acting beta-2 adrenergic (LABA) bronchodilators as maintenance therapy in patients with moderate to severe persistent asthma4,5. Although these treatments have proven efficacy, around a 50% has a suboptimal control 5, increasing the pathology management cost. The aim of this economic evaluation is to estimate the cost-effectiveness of three ICS/LABA fixed-dose combinations: fluticasone propionate/ formoterol (Flutiform®) (FPF ) versus fluticasone /salmeterol (FS) and budesonide/ formoterol (BF) in the treatment of adult patients with moderate to severe asthma from the perspective of the Society in Spain. FPF drug acquisition cost was lower (20% compared to FS and 30% compared to BF), meanwhile QALY of the three alternatives compared were very similar. Cost per patient on the FPF cohort yielded €9.326/year, lower either than FS (€137) or than BF (€247), being the most economical alternative. The sub-optimal control health state was the mainstay of cost (80% of the total cost) in any of the analysed alternatives and scenarios. PSA results confirmed the data from the base case scenario. FPF is more efficient than FS and BF, with a probability of 52.6% and 57.6%, respectively at any acceptability threshold. Figure 2. FPF/FS and FPF/BF Cost- effectiveness planes. METHODS A Markov model was developed with five asthma health states: successful control, suboptimal control, outpatient-managed exacerbation, inpatient-managed exacerbation, and death. Time horizon was set at 12 months and weekly probability transitions. Direct and indirect resources consumption and unitary cost data were obtained from published studies, an expert panel and autonomous communities prices weighted by population, all expressed in 2014 euros. The comparative studies between FPF and the other two ICS/LABA combinations confirmed the same efficacy at equivalent doses between them and a rapidly onset of action than BF 6-12. Effectiveness was expressed as quality-adjusted life years (QALY) gained and the results were assess with the incremental cost effectiveness ratio (ICER). FPF FPF FS FS Figure 1: Markov model diagram. QALYs: Quality Adjusted Life-Years; FPF: Fluticasone(Proponiate)/Formoterol; FS: Fluticasone/Salmeterol; BF: Budesonide /Formoterol Table 3. Base case probabilistic and deterministic results. Table 1: Transition probability matrix10 and state utilities12. Probabilities SC SOC OME IME D Utilities SC 0.89407 0.15049 0.22784 0.33332 0 0.85 SOC 0.0986 0.82982 0.54401 0.33332 0 0.77 OME 0.00702 0.01938 0 0 0 0.66 IME 0.00027 0.00027 0 0 0 0.59 D 4E-05 4E-05 0.22815 0.33336 1 0 SC: Successful control; SOC: Sub-optimal control; OME: Outpatient-manage exacerbation; IME: Inpatient-manage exacerbation; D: Death. With a 53% and 47% patients initial distribution between SC and SOC 11 and 1.23 and 5.33 weekly home management exacerbations respectively (expert panel). Rescues SC Cost SOC Cost OME Cost IME Cost Total Cost QALY´s Rescues SC Cost SOC Cost OME Cost IME Cost Total Cost QALY´s FPF 13.41 1,251.32 7,674.84 578.89 62.81 9,567.86 0.78 Probabilistic Analysis BF % FPF vs BF FPF 13.41 0.0% 13.54 1,356.85 8.4% 1,258.42 7,828.76 2.0% 7,742.11 584.93 1.0% 585.39 62.92 0.2% 63.37 9,833.46 2.8% 9,649.29 0.78 0.0% 0.78 FS 13.54 1,336.88 7,767.11 586.58 63.49 9,753.97 0.78 % FPF vs BF 0.0% 6.2% 0.3% 0.2% 0.2% 1.1% 0.0% FPF 13.11 1,217.30 7,481.49 61.36 61.36 9,326.39 0.76 Deterministic Analysis BF % FPF vs BF FPF 13.11 0.0% 13.11 1,348.14 10.7% 1,217.30 7,585.30 1.4% 7,481.49 61.50 1.1% 61.36 61.50 0.2% 61.36 9,567.35 2.6% 9,326.39 0.76 0.0% 0.76 FS 13.11 1,284.31 7,548.40 61.43 61.43 9,463.51 0.76 % FPF vs BF 0.0% 5.5% 0.9% 0.6% 0.1% 1.5% 0.0% FPF: Fluticasone (Propionate)/Formoterol; FS: Fluticasone/Salmeterol; BF: Budesonide /Formoterol; SC: Successful control; SOC: Suboptimal control; OME: Outpatient-manage exacerbation; IME: Inpatient-manage exacerbation; D: Death. Table 2: Social perspective state and therapeutic alternative maintenance and total cost (botplus and official Regional prices). SC SOC OME IME SC SOC OME IME FPF FS Maintenance Treatment Cost 9.56 11.89 14.34 17.84 22.30 27.75 22.30 27.75 Total Cost 42.16 44.50 384.00 387.50 985,78 991.23 4739,59 4745.04 BF 14.16 21.24 33.03 33.03 46.76 390.90 996.52 4750.32 FF: Fluticasone (Propionate)/ Formoterol FS:Fluticasone/Salmeterol BF: Budesonide/Formoterol; SC: Successful control; SOC: Sub-optimal control; OME: Outpatient-manage exacerbation; IME: Inpatient-manage exacerbation; D: Death. Uncertainty from the social and payer perspective was tested with univariate and probabilistic sensitivity analysis (PAS) to ensure the strength of the model. 18th Annual European Congress 7-11 November 2015 Milan, Italy. CONCLUSIONS From the Spanish societal perspective in year 2014, FPF produced similar QALY gain at a lower cost when compared with FS and BF in a highly meaningful number of replications and scenarios. FPF may be considered a cost-effective alternative in the treatment of moderate to severe asthma in Spain. The cost savings were mainly due to significant FPF lower price acquisition costs compared to the other two alternatives REFERENCES: 1. Masoli M, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report.. Allergy, 2004. 2. Urrutia I, et al. Cambios en la prevalencia de asma en la población española del estudio ECRHS-II. Arch Bronconeumol, 2007. 3. Martínez-Moragón E, et al. Coste económico del paciente asmático en España (estudio AsmaCost). Arch Bronconeumol, 2009. 4. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2014. Avilable at: www.ginasthama.org 5. GEMA 4.0. Guía Española para el Manejo del Asma. Available at: www,gemasma.com. 6. Oliva J. y Osuna R. Los costes de los cuidados informales en España. Presupuesto y Gasto Público 56/2009: 163-181 Secretaría General de Presupuestos y Gastos © 2009, Instituto de Estudios Fiscales. 7. Ojeda P, et al. Costs associated with workdays lost and utilization of health care resources because of asthma in daily clinical practice in Spain. J Investig Allergol Clin Immunol, 2013. 8. Bodzenta-Lukaszyk A, et al. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. J Asthma, 2012. 9. Bodzenta-Lukaszyk A, et al. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulm Med, 2011. 10. Collados C, et al. Análisis coste-efectividad de beclometasona/formoterol frente a fluticasona/salmeterol en el tratamiento de pacientes con asma moderada a grave en España. Pharmacoeconomics Spanish Research Articles. DOI 10.1007/s40277-014-0035-x. 11. Demoli P, et al. Update on Asthma Control in Five European Countries: results of 2008 survey. Eur Respir Rev, 2010. 12. Gerzeli S, et al. Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig, 2012. Study Sponsored by





