ExamView - Unit 19 Practice Test.tst
Anuncio
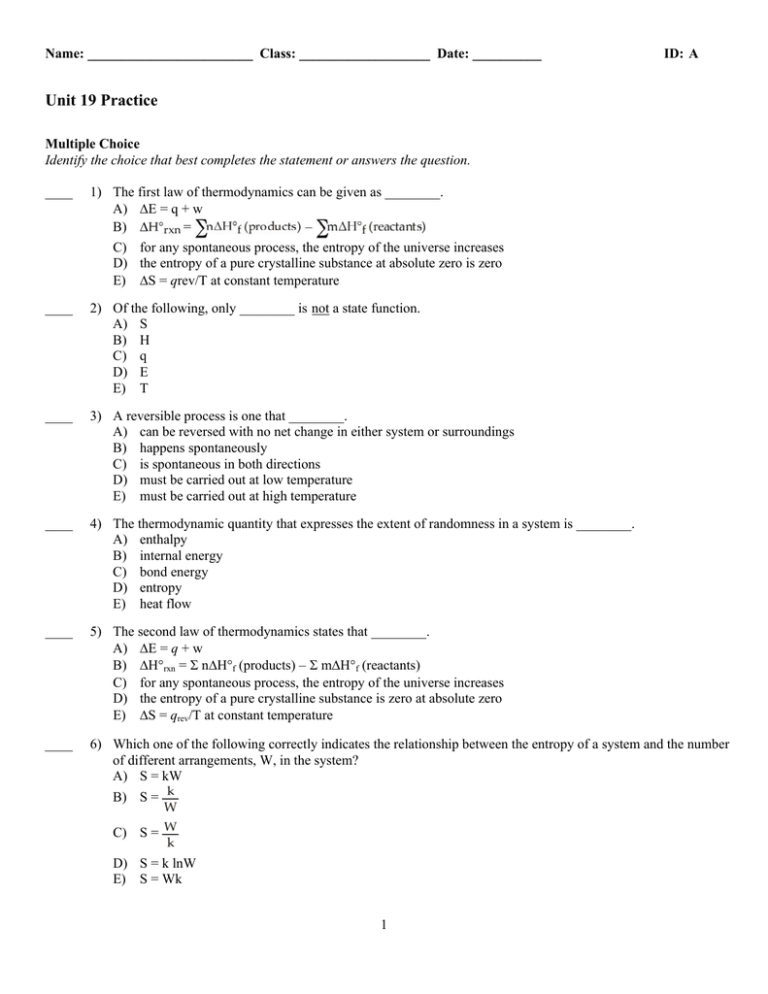
Name: ________________________ Class: ___________________ Date: __________ ID: A Unit 19 Practice Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1) The first law of thermodynamics can be given as ________. A) E = q + w = – B) C) for any spontaneous process, the entropy of the universe increases D) the entropy of a pure crystalline substance at absolute zero is zero E) S = qrev/T at constant temperature ____ 2) Of the following, only ________ is not a state function. A) S B) H C) q D) E E) T ____ 3) A reversible process is one that ________. A) can be reversed with no net change in either system or surroundings B) happens spontaneously C) is spontaneous in both directions D) must be carried out at low temperature E) must be carried out at high temperature ____ 4) The thermodynamic quantity that expresses the extent of randomness in a system is ________. A) enthalpy B) internal energy C) bond energy D) entropy E) heat flow ____ 5) The second law of thermodynamics states that ________. A) E = q + w B) H°rxn = nH°f (products) – mH°f (reactants) C) for any spontaneous process, the entropy of the universe increases D) the entropy of a pure crystalline substance is zero at absolute zero E) S = qrev/T at constant temperature ____ 6) Which one of the following correctly indicates the relationship between the entropy of a system and the number of different arrangements, W, in the system? A) S = kW B) S = C) S = D) S = k lnW E) S = Wk 1 Name: ________________________ ID: A ____ 7) S is positive for the reaction ________. A) CaO (s) + CO2 (g) CaCO3 (s) B) N2 (g) + 3H2 (g) 2NH3 (g) C) 2SO3 (g) 2SO2 (g) + O2 (g) D) Ag+ (aq) + Cl– (aq) AgCl (s) E) H2O (l) H2O (s) ____ 8) Which reaction produces an increase in the entropy of the system? A) Ag+ (aq) + Cl– (aq) AgCl (s) B) CO2 (s) CO2 (g) C) H2 (g) + Cl2 (g) 2 HCl (g) D) N2 (g) + 3 H2 (g) 2 NH3 (g) E) H2O (l) H2O (s) ____ 9) The equilibrium position corresponds to which letter on the graph of G vs. f (course of reaction) below? A) B) C) D) E) A B C D E ____ 10) Given the following table of thermodynamic data, complete the following sentence. The vaporization of TiCl 4 is ________. A) spontaneous at all temperatures B) spontaneous at low temperature and nonspontaneous at high temperature C) nonspontaneous at low temperature and spontaneous at high temperature D) nonspontaneous at all temperatures E) not enough information given to draw a conclusion ____ 11) With thermodynamics, one cannot determine ________. A) the speed of a reaction B) the direction of a spontaneous reaction C) the extent of a reaction D) the value of the equilibrium constant E) the temperature at which a reaction will be spontaneous 2 Name: ________________________ ID: A Use the table below to answer the questions that follow. Thermodynamic Quantities for Selected Substances at 298.15 K (25 °C) Substance H°f (kJ/mol) G°f (kJ/mol) S (J/K-mol) Carbon C (s, diamond) C (s, graphite) C2H2 (g) C2H4 (g) C2H6 (g) CO (g) CO2 (g) 1.88 0 226.7 52.30 –84.68 –110.5 –393.5 2.84 0 209.2 68.11 –32.89 –137.2 –394.4 2.43 5.69 200.8 219.4 229.5 197.9 213.6 Hydrogen H2( g) 0 0 130.58 Oxygen O2 (g) H2O (l) 0 –285.83 0 –237.13 205.0 69.91 ____ 12) The value of S° for the reaction 2C (s, diamond) + O2 (g) 2CO (g) is ________ J/K · mol. A) –185.9 B) +185.9 C) –9.5 D) +9.5 E) –195.7 ____ 13) The value of S° for the oxidation of carbon to carbon dioxide, C (s, graphite) + O2 (g) CO2(g) is ________ J/K · mol. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide. A) +424.3 B) +205.0 C) –205.0 D) –2.9 E) +2.9 3 Name: ________________________ ID: A ____ 14) The value of S° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic) + 3O2(g) 2SO3 (g) is ________ J/K · mol. A) +19.3 B) –19.3 C) +493.1 D) –166.4 E) –493.1 ____ 15) The value of S° for the formation of POCl3 from its constituent elements, P2 (g) + O2 (g) + 3Cl2 (g) 2POCl3 (g) is ________ J/K · mol. A) –442.0 B) +771.0 C) –321.0 D) –771.0 E) +321.0 ____ 16) The value of S° for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3Cl2 (g) 2PCl3 (g) is ________ J/K · mol. A) –311.7 B) +311.7 C) –263.6 D) +129.4 E) –129.4 ____ 17) The value of H° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, S (s, rhombic) + O2 (g) SO2 (g) is ________ kJ/mol. A) +269.9 B) –269.9 C) +0.00 D) –11.6 E) +11.6 4 Name: ________________________ ID: A ____ 18) The value of H° for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3Cl2 (g) 2PCl3 (g) is ________ kJ/mol A) –288.1 B) +432.4 C) –720.5 D) +720.5 E) –432.4 ____ 19) The value of G° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic) + 3O2 (g) 2SO3 (g) is ________ kJ/mol. A) +740.8 B) –370.4 C) +370.4 D) –740.8 E) +185.2 ____ 20) The value of G° at 25 °C for the formation of POCl3 from its constituent elements, P2 (g) + O2 (g) + 3Cl2 (g) 2POCl3 (g) is ________ kJ/mol. A) –1108.7 B) +1108.7 C) –606.2 D) +606.2 E) –1,005 ____ 21) The value of G° at 25 °C for the formation of calcium chloride from its constituent elements, Ca (s) + Cl2 (g) CaCl2 (s) is ________ kJ/mol. A) –795.8 B) +795.8 C) +763.7 D) +748.1 E) –748.1 5 Name: ________________________ ID: A ____ 22) Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g) A) B) C) D) E) 2 SO3 (g) 2.40 1024 1.06 1.95 3.82 1023 More data are needed. ____ 23) Consider the reaction: FeO (s) + Fe (s) + O2(g) Fe2O3 (s) Given the following table of thermodynamic data at 298 K: The value K for the reaction at 25 °C is ________. A) 370 B) 5.9 104 C) 3.8 10–14 D) 7.1 1085 E) 8.1 1019 ____ 24) The normal boiling point of C 2Cl3F3 is 47.6 °C and its molar enthalpy of vaporization is 27.49 kJ/mol. What is the change in entropy in the system in J/K when 28.6 grams of C 2Cl3F3 vaporizes to a gas at the normal boiling point? A) –13.1 B) –4.19 C) 4.19 D) 13.1 E) 27.5 ____ 25) Of the following, the entropy of ________ is the largest. A) HI (g) B) HBr (g) C) HCl (g) D) HCl (s) E) HCl (l) 6 Name: ________________________ ID: A ____ 26) The standard Gibbs free energy of formation of ________ is zero. A) B) C) D) E) (a) H2O (l) (b) O (g) (c) Cl2 (g) (a) only (b) only (c) only (b) and (c) (a), (b), and (c) ____ 27) The value of G° at 181.0 °C for the formation of calcium chloride from its constituent elements: Ca (s) + Cl2 (g) CaCl2 (s) is ________ kJ/mol. At 25.0 °C for this reaction, ÄH° is –795.8 kJ/mol, G° is –748.1 kJ/mol, and S° is –159.8 J/K. A) –868.4 B) –723.2 C) 7.18 104 D) –766.9 E) 2.81 104 ____ 28) For a given reaction, H = –24.2 kJ/mol and S = –55.5 J/K-mol. The reaction will have G = 0 at ________K. Assume that H and S do not vary with temperature. A) 436 B) 2293 C) 298 D) 2.29 E) 0.436 ____ 29) For a given reaction with H = –28.1 kJ/mol, the G = 0 at 372 K. The value of S must be ________ J/K-mol, assuming that H and S do not vary with temperature. A) –75.5 B) 75.5 C) –7.55 10–5 D) 7.55 10–5 E) –1.32 10–2 7 Name: ________________________ ID: A ____ 30) In the Haber process, ammonia is synthesized from nitrogen and hydrogen: N2 (g) + 3H2 (g) 2NH3 (g) G° at 298 K for this reaction is –33.3 kJ/mol. The value of G at 298 K for a reaction mixture that consists of 1.9 atm N2, 2.3 atm H2, and 0.85 atm NH3 is ________. A) –1.0 B) –4.09 103 C) –8.62 103 D) –118.0 E) –41.9 ____ 31) The equilibrium constant for a reaction is 0.38 at 25 °C. What is the value of G° (kJ/mol) at this temperature? A) 2.4 B) –4.2 C) 200 D) 4.2 E) More information is needed. 8 ID: A Unit 19 Practice Answer Section MULTIPLE CHOICE 1) ANS: OBJ: 2) ANS: OBJ: 3) ANS: OBJ: 4) ANS: OBJ: 5) ANS: OBJ: 6) ANS: OBJ: 7) ANS: OBJ: 8) ANS: OBJ: 9) ANS: OBJ: 10) ANS: OBJ: 11) ANS: OBJ: 12) ANS: OBJ: 13) ANS: OBJ: 14) ANS: OBJ: 15) ANS: OBJ: 16) ANS: OBJ: 17) ANS: OBJ: 18) ANS: OBJ: 19) ANS: OBJ: 20) ANS: OBJ: 21) ANS: OBJ: A 19.1; G2 C 19.1; G2 A 19.1; G2 D 19.2; G2 C 19.2; G2 D 19.3; G2 C 19.4; G2 B 19.4; G2 C 19.5; G3 C 19.6; G3 A 19.6; G2 B 19.4; G3 E 19.4; G3 D 19.4; G3 A 19.4; G3 C 19.4; G3 B 19.5; G3 C 19.5; G3 D 19.5; G3 A 19.5; G3 E 19.5; G3 PTS: 1 DIF: 1 REF: Page Ref: 19.1 PTS: 1 DIF: 2 REF: Page Ref: 19.1 PTS: 1 DIF: 2 REF: Page Ref: 19.1 PTS: 1 DIF: 1 REF: Page Ref: 19.2 PTS: 1 DIF: 1 REF: Page Ref: 19.2 PTS: 1 DIF: 3 REF: Page Ref: 19.3 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.5 PTS: 1 DIF: 3 REF: Page Ref: 19.6 PTS: 1 DIF: 2 REF: Page Ref: 19.7 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 2 REF: Page Ref: 19.5 PTS: 1 DIF: 2 REF: Page Ref: 19.5 PTS: 1 DIF: 2 REF: Page Ref: 19.5 PTS: 1 DIF: 2 REF: Page Ref: 19.5 PTS: 1 DIF: 2 REF: Page Ref: 19.5 1 ID: A 22) ANS: OBJ: 23) ANS: OBJ: 24) ANS: OBJ: 25) ANS: OBJ: 26) ANS: OBJ: 27) ANS: OBJ: 28) ANS: OBJ: 29) ANS: OBJ: 30) ANS: OBJ: 31) ANS: OBJ: A 19.7; G3 D 19.7; G3 D 19.2; G4 A 19.4; G2 C 19.5; G2 B 19.5; G4 A 19.6; G4 A 19.6; G4 E 19.7; G4 A 19.7; G4 PTS: 1 DIF: 4 REF: Page Ref: 19.7 PTS: 1 DIF: 5 REF: Page Ref: 19.7 PTS: 1 DIF: 3 REF: Page Ref: 19.2 PTS: 1 DIF: 2 REF: Page Ref: 19.4 PTS: 1 DIF: 1 REF: Page Ref: 19.5 PTS: 1 DIF: 2 REF: Page Ref: 19.5 PTS: 1 DIF: 2 REF: Page Ref: 19.6 PTS: 1 DIF: 4 REF: Page Ref: 19.6 PTS: 1 DIF: 3 REF: Page Ref: 19.7 PTS: 1 DIF: 3 REF: Page Ref: 19.7 2 Unit 19 Practice [Answer Strip] ID: A C _____ 7) D 14) _____ C 18) _____ A 15) _____ D 19) _____ C 16) _____ A 20) _____ B 17) _____ E 21) _____ A _____ 1) B _____ 8) C _____ 2) C _____ 9) A _____ 3) B 12) _____ D _____ 4) C 10) _____ C _____ 5) D _____ 6) E 13) _____ A 11) _____ Unit 19 Practice [Answer Strip] A 22) _____ C 26) _____ ID: A E 30) _____ B 27) _____ A 31) _____ D 23) _____ A 28) _____ A 29) _____ D 24) _____ A 25) _____
