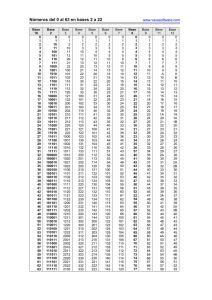
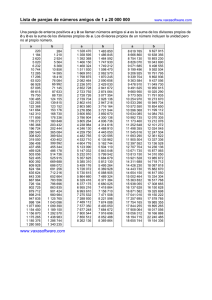
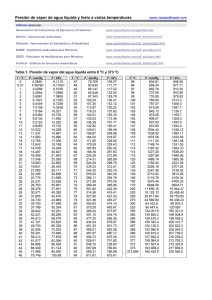
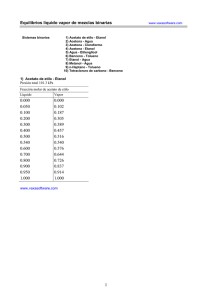
Periodic table of elements
Anuncio
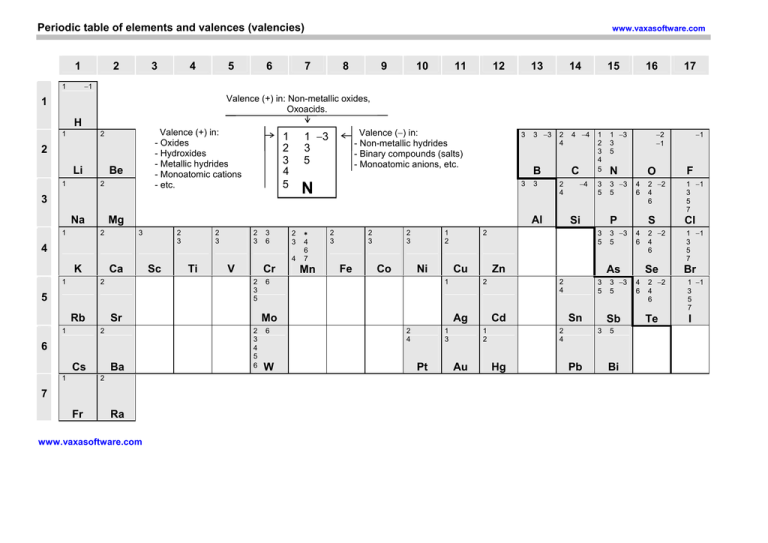
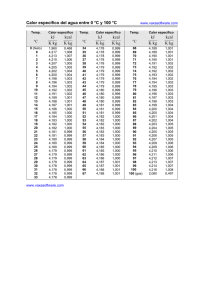
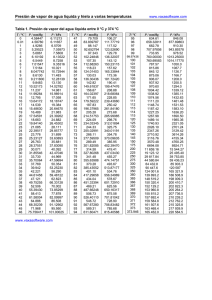
Periodic table of elements and valences (valencies) 1 2 3 4 5 6 www.vaxasoftware.com 7 8 9 10 11 12 13 14 15 16 17 −1 1 Valence (+) in: Non-metallic oxides, Oxoacids. 1 H 1 Valence (+) in: - Oxides - Hydroxides - Metallic hydrides - Monoatomic cations - etc. 2 2 Li 1 Be 2 1 −3 3 5 1 2 3 4 5 Na 2 3 2 3 2 3 3 6 2 3 4 Ca 2 Rb 6 Cs Ba 2 7 Fr Ti V Cr Sr 2 1 Sc 2 3 5 5 1 2 4 3 3 Ra www.vaxasoftware.com ∗ 4 6 7 Mn 2 3 2 3 Fe 2 3 Co 1 2 Ni 6 2 4 6 Cu W 1 3 Pt Au N 3 5 3 −3 5 3 5 3 −3 5 Si Cd Sn Sb 3 Pb 3 −3 5 5 Bi −1 O F 4 6 2 −2 4 6 1 −1 3 5 7 S Cl 4 6 2 −2 4 6 1 −1 3 5 7 P 3 5 2 4 Hg −2 −1 As 2 4 1 2 1 −3 3 5 1 2 3 4 5 Zn 2 Ag 2 4 −4 2 1 Mo 2 3 4 5 6 4 −4 C Al 3 4 K 3 −3 B Mg 2 1 3 N 3 1 Valence (−) in: - Non-metallic hydrides - Binary compounds (salts) - Monoatomic anions, etc. Se 4 6 2 −2 4 6 Te Br 1 −1 3 5 7 I