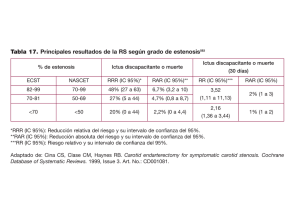
Comments, Opinions, and Reviews
Anuncio

Comments, Opinions, and Reviews Systematic Review of the Perioperative Risks of Stroke or Death After Carotid Angioplasty and Stenting Emmanuel Touzé, PhD; Ludovic Trinquart, MSc; Gilles Chatellier, PhD; Jean-Louis Mas, MD Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Background and Purpose—Carotid angioplasty and stenting (CAS) has not been shown to be as safe as carotid endarterectomy (CEA) with regard to the risks of periprocedural complications, but beyond the perioperative period, the risks are comparable, suggesting that CAS may be an acceptable option in selected patients. However, risk factors for perioperative stroke and death have not been clearly established. We aimed to estimate the 30-day absolute risks of stroke or death after CAS and investigate sources of heterogeneity. Methods—We sought articles published between January 1990 and June 2008 by using MEDLINE, EMBASE, the COCHRANE databases, hand-searching, abstract books from conferences, and official websites. Two reviewers independently and in duplicate selected articles on the risks of CAS, irrespective of the type of treatment, study design, setting, or language. The 2 reviewers abstracted data and assessed the quality of the studies. Results—Two hundred six independent studies (with 54 713 patients) were included. The overall 30-day risk of stroke or death was 4.7% (95% CI, 4.1 to 5.2) with substantial heterogeneity across studies. Symptomatic patients were about twice as likely as those with asymptomatic stenoses to have complications. The 30-day risk of stroke or death was 7.6% (3.6 to 9.1) in symptomatic and 3.3% (2.6 to 4.1) in asymptomatic patients. Risks increased with age, hypertension, and history of coronary artery disease; were unrelated to sex and the presence of contralateral carotid occlusion; and were lower in patients who had carotid restenosis after CEA and in those treated with the use of a cerebral protection device. Risks have also decreased over time. Conclusions—Risks of CAS vary substantially across studies. Risks are overall higher than those of CEA in symptomatic patients. Some factors are likely to help select good candidates for CAS. (Stroke. 2009;40:e683-e693.) Key Words: stroke 䡲 carotid disease 䡲 stenting 䡲 angioplasty 䡲 atherosclerosis 䡲 systematic review E xtracranial internal carotid artery stenosis accounts for 15% to 20% of ischemic strokes, depending on the population studied.1 The efficacy of carotid endarterectomy (CEA) to prevent stroke in patients with carotid stenosis is well established, particularly in those who have symptomatic stenosis.1–3 Carotid angioplasty and stenting (CAS), a potential alternative treatment to CEA, has been evaluated in a few randomized trials and many nonrandomized studies and involving many specialists, including neurologists, radiologists, cardiologists, vascular surgeons, and neurosurgeons, most of whom have already implemented the technique in their clinical practice.4 However, recent randomized trials and meta-analyses failed to demonstrate that CAS is as safe as CEA with regard to the risks of periprocedural complications,4 –12 and current guidelines recommend that CAS should not be used in good surgical candidates.2,3,13 Nevertheless, the clinical trials have also shown that beyond the perioperative period, the risk of ipsilateral stroke is very low and comparable in CAS and CEA patients,10,14,15 suggesting that CAS may be an acceptable option in selected patients who have a low risk of periprocedural complications. However, there is currently no means to select good candidates for CAS. Moreover, although CAS is widely used in some centers, it is unknown whether the absolute risk of CAS observed in randomized trials can be generalized to everyday clinical practice. The risks of complications after CEA and their relations with different subgroups have been estimated in several studies and meta-analyses,16 –20 but no similar data exist for CAS. Some studies have shown that the risk of complications after CAS is likely to be related to some patient characteristics and technical aspects. However, the number of complications observed in individual studies was usually small, precluding any reliable conclusion. Moreover, many studies have focused on patients with a perceived high surgical risk, hypothesising that those patients should be good candidates for CAS.4,21 However, it is possible that comorbidities associated with a greater perioperative risk with CEA also increase the periprocedural risk with CAS. Received July 8, 2009; final revision received August 25, 2009; accepted September 4, 2009. From the Université Paris Descartes, INSERM U894 (E.T., J.-L.M.), Hôpital Sainte-Anne, Service de Neurologie, and INSERM CIE4 (L.T., G.C.), Assistance Publique–Hôpitaux de Paris, Unité de recherche clinique, Hôpital Européen Georges Pompidou, Paris, France. The first 2 authors contributed equally to the data collection, analysis, and writing of the article. Correspondence to Emmanuel Touzé, MD, PhD, Université Paris Descartes, INSERM U894, Department of Neurology, Hôpital Sainte-Anne, 1 rue Cabanis, 75014 Paris, France. E-mail e.touze@ch-sainte-anne.fr © 2009 American Heart Association, Inc. Stroke is available at http://stroke.ahajournals.org DOI: 10.1161/STROKEAHA.109.562041 e683 e684 Stroke December 2009 Table 1. Search Strategy in MEDLINE and EMBASE PubMed search strategy (“Carotid stenosis”关Mesh兴 AND (“stents”关Mesh兴 OR “angioplasty”关Mesh兴 OR “angioplasty, balloon”关Mesh兴) AND (“treatment outcome”关Mesh兴 OR “postoperative complications”关Mesh兴 OR “myocardial infarction”关Mesh兴 OR “stroke”关Mesh兴 OR “brain ischemia”关Mesh兴 OR “death”关Mesh兴 OR “death, sudden, cardiac”关Mesh兴 OR “mortality”关Mesh兴)) OR (“carotid stenosis” AND (“carotid angioplasty” OR “stent”*) AND (“stroke” OR “myocardial infarction” OR “death” OR “mortality”) AND 关(“1990”关PDat兴:”2008/06”关PDat兴) AND (“humans”关Mesh兴)兴) EMBASE search strategy (“Carotid artery obstruction”/exp AND (“stent”/exp OR “angioplasty”/exp OR “percutaneous transluminal angioplasty”/exp) AND (“treatment outcome”/exp OR “postoperative complication”/exp OR “heart infarction”/exp OR “cerebrovascular accident”/exp OR “stroke”/exp OR “brain ischemia”/exp OR “death”/exp OR “sudden death”/exp) NOT 关“review”兴/lim AND 关“humans”兴/lim AND 关1990 –2008兴/py) Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 We therefore systematically reviewed studies reporting the risk of stroke, death, and myocardial infarction (MI) after CAS to estimate the absolute risks and investigate any relation between observed risks and study design, population, clinical factors, and technical aspects. Methods Before conducting the review, we developed a protocol containing the background and objectives, along with an outline of the proposed search methods and plans for collecting and analyzing data. The manuscript was prepared in accordance with the MOOSE guidelines.22 Selection Criteria and Search Strategy Studies were eligible for review if: (1) they enrolled patients with symptomatic and/or asymptomatic stenoses located in the region of the carotid bifurcation; (2) patients were treated by angioplasty irrespective of the type of treatment (balloon angioplasty without stenting or stenting), arterial route, and the use of cerebral protection; and (3) the number of events (stroke, MI, or death) could be extracted. Studies that enrolled a specific population group only (restenosis after CEA, postradiotherapy stenosis, fibromuscular dysplasia, carotid dissection, and patients treated in an emergency context) were excluded. We sought articles published between January 1990 and June 2008 on the risks of stroke, death, or MI after CAS, irrespective of the study design, setting, or language. Electronic searches were performed using MEDLINE, and EMBASE with both medical subject heading terms and text words (Table 1) and the COCHRANE Library database (CENTRAL and DARE). We hand-searched the reference lists of all included articles, any relevant review articles, our personal files, and the contents pages of the 3 journals from which most eligible articles were identified in the electronic search (Journal of Vascular Surgery, Journal of Endovascular Therapy, and Catheter Cardiovascular Interventions). To identify recent studies not yet published as complete articles, we also searched books of abstracts from recent conferences (Joint World Congress on Stroke 2006, American Heart Association International Stroke Conferences 2007 and 2008, the European Stroke Conferences 2007 and 2008, the Cardiovascular and Interventional Radiological Society of Europe meetings 2006 and 2007, the Transcatheter Cardiovascular Therapeutics meetings 2006 and 2007, the American College of Cardiology Scientific sessions 2007 and 2008, and the Society of Interventional Radiology meetings 2007 and 2008) and the clinical trial registration (www.clinicaltrials.gov), the US Food and Drug Admin- istration (www.fda.gov), and the European Medicines Agency (www. emea.europa.eu) websites. Selection of Studies and Data Collection Two reviewers independently and in duplicate assessed the eligibility of citations identified by the search strategy from titles and then from abstracts. At each step, discrepancies were resolved by discussion. Final selection was made after reviewing full-text articles that were retrieved for all studies either that met the selection criteria or for which there was uncertainty regarding selection as per the abstract. In cases of multiple publications pertaining to the same population, that which reported the largest number of patients was chosen for the absolute risk analysis. Additional subgroup data of the SPACE trial were obtained from the authors.8 The 2 reviewers extracted the data by means of a standardized form (available from the authors). Study quality was assessed according to an existing scheme23 that we adapted to our context and that included the following list of criteria: (1) design (randomized trial vs cohorts/registries), (2) setting (singlecenter vs multicenter study), (3) patient enrollment (prospective vs retrospective and consecutive vs nonconsecutive), (4) description of the population (adequate vs inadequate), and (5) outcome measurement (systematic assessment by a neurologist after the procedure, yes vs no). The description of the population was deemed adequate when the sampling frame, recruitment, inclusion and exclusion criteria, and the baseline study sample characteristics were adequately described. Systematic assessment by a neurologist referred to every patient being seen by a neurologist at 30 days (30-day outcomes) or before discharge (periprocedural and in-hospital events), whether the patient had an event or not. Data Synthesis and Data Analysis The primary outcomes were the 30-day risks of stroke; stroke or death; and stroke, MI, or death. The secondary outcomes were in-hospital and periprocedural (within 24 hours) risks. When the timing of assessment of complications was unclear without systematic follow-up at 30 days, we considered events as periprocedural complications. Combined estimates of risk were calculated separately for the different outcomes. Each individual proportion was first transformed into a quantity with the Freeman-Tukey variancestabilizing transformation.24 A weighted mean of the transformed proportions was computed by using a DerSimonian-Laird randomeffects model.25 The combined proportion was calculated as the back-transform of this weighted mean.26 To explore sources of heterogeneity, we first performed subgroup comparisons according to the following factors: clinical presentation (symptomatic vs asymptomatic; stroke vs transient ischemic attack; cerebral vs ocular event), age (⬎75 to 80 vs ⬍75 to 80 years), sex, diabetes mellitus, coronary artery disease (CAD), peripheral arterial disease (PAD), contralateral carotid occlusion, restenosis after CEA versus de novo lesion, plaque structure (ulcerated vs smooth, presence of calcification), timing for CAS (⬍14 days vs ⬎14 days after cerebral ischemic event), side of the treated lesion, and cerebral protection device use. Those comparisons were done within studies. We calculated combined relative risks (RRs) for all studies by using a fixed-effect meta-analysis, according to the Mantel-Haenszel method, or by using a DerSimonian-Laird random-effects meta-analysis, where appropriate. Then we performed indirect comparisons of pooled absolute risks according to clinical presentation and the study quality items defined earlier and assessed potential changes of risk over time from a meta-regression analysis. We used a logisticnormal model that specified the binomial distribution of the dependent variable (30-day risk of stroke or death) and a random effect to account for shared variance within the study.27 The midcohort year, defined as the midpoint of the inclusion period, was calculated for each study if the inclusion period was available and considered as a covariate. We assessed publications biases by means of a simple visual analysis of funnel plots in the meta-analysis of absolute risks, as there is no validated statistical test to detect asymmetry, and used funnel plots and Egger’s test in the subgroup comparisons.28 In all Touzé et al Risks After Carotid Angioplasty and Stenting e685 Figure 1. Flow chart for selection of studies. Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 analyses, inconsistency of findings across studies was assessed with Cochran’s Q statistic and the I2 statistic with associated 95% CI, the latter being the percentage of variability that is due to between-study heterogeneity rather than sampling error (chance).29,30 According to the Cochrane handbook, heterogeneity was classified as moderate (I2ⱖ30%), substantial (I2ⱖ50%), or considerable (I2ⱖ75%).31 We considered a 2-sided probability value ⬍0.05 as significant. Statistical analysis was performed with SAS version 9.1 and MIX (http://mix-for-meta-analysis.info). Results Of the 1796 articles identified from our electronic search in MEDLINE and EMBASE, 605 abstracts were screened and 457 articles were retrieved for assessment in full text (Figure 1). We identified 53 additional articles or abstracts from other sources. Among the 510 references analyzed in detail, 206 independent studies were eligible (133 for absolute risk only, 62 for absolute risk and subgroups, and 11 for subgroups only). Because of multiple publications of some registries with different subgroup analyses, the 206 independent studies resulted in 234 reports (212 full articles, 19 abstracts, 2 US Food and Drug Administration documents, and 1 web publication) that were finally relevant to our analysis. Summarized characteristics of the included studies are given in Table 2, and the list of references and the characteristics of individual reports are available in supplemental Table I, available online at http://stroke.ahajournals.org. Among the 206 independent studies (54 713 patients), there were 10 randomized clinical trials (RCTs) comparing CAS with CEA (1613 patients),5,7,8,21,32–37 3 RCTs comparing different strategies in patients treated by CAS (144 patients),38 – 40 and 193 registries (52 956 patients). There were 32 studies (2922 patients) in which 95% or more of patients had symptomatic stenosis, 2 studies (136 patients) in which 95% or more of patients had asymptomatic carotid stenosis, and 172 studies (51 655 patients) with both symptomatic and asymptomatic patients. Roughly half (51%) of the studies started enrolling patients before 2000. As shown in Table 2, 83% of studies had a single-center setting and 40% were stated as prospective. The description of the population was adequate in 46%, and assessment of outcomes was done by an independent neurologist in 40% of studies published as a full article. Both a fully described population and neurologic assessment of outcomes were found in 49 (26%) of studies published as a full article. Among the 172 studies that enrolled symptomatic and asymptomatic patients, 36 (21%) reported the risks of CAS stratified according to clinical presentation. Among the 173 studies that clearly reported the type of treatment performed, there were 161 (93%) studies in which ⬎90% of patients were treated with stenting. The main characteristics of RCTs and registries were very similar with regard to age (median, 69 vs 70 years), proportion of men (median, 71% vs 71%), proportion of patients with contralateral carotid occlusion (median, 10% vs 10%), and proportion of patients with CAD (median, 57% vs 61%). The proportion of symptomatic patients was higher in RCTs than in registries (median, 81% vs 49%). Conversely, registries were more likely than RCTs to have enrolled patients with restenosis after CEA (median, 15% vs 8%), to have patients with diabetes (median, 32% vs 24%), and to have treated patients with the use of cerebral protection devices (median, 83% vs 42%). Table 3 shows the pooled estimates of the absolute risks according to the timing of the outcome assessment. The 30-day risk of stroke was 3.9% (95% CI, 3.4 to 4.4; 118 studies; 27 186 patients); that of stroke or death was 4.7% (95% CI, 4.1 to 5.2; 113 studies; 25 237 patients); and that of stroke, death, or MI was 5.3% (95% CI, 4.6 to 6.0; 63 studies; 17 291 patients). Respective in-hospital and periprocedural risks were slightly lower. There was, however, substantial heterogeneity across studies. Exclusion of the abstracts or postmarketing studies that might have included patients also published in individual studies did not change the estimates (data not shown). Regarding study quality, meta-regression analyses showed that the 30-day risk of stroke or death was unrelated to study setting (multicenter 4.8% vs single-center, 4.6%, P⫽0.77), and consecutive enrollment (yes 4.6% vs no 4.8%, P⫽0.89). However, the risk was higher when there was an adequate description of the population (yes 5.2% vs no 4.0%, P⫽0.04), e686 Stroke December 2009 Table 2. Quality, Population Characteristics, and Technical Aspects of the Included Studies No. of Studies (%), Total⫽206* No. of Patients (No. of Procedures) Study quality Single center RCT (other)† 34 898 (35 502) No. of studies included 118 113 63 19 815 (20 684) 10 (5) 1613 (1613) No. of patients 27 186 25 237 17 291 28 149 26 145 17 858 3 (1) 144 (144) 52 956 (54 430) No. of procedures Prospective 83 (40) 24 878 (25260) Pooled risk (95% CI) Retrospective 42 (20) 8580 (8881) Not clear 81 (40) 21 255 (22045) Patient enrollment Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Consecutive Stroke, MI, Death 36 (17) 193 (94) Registry Stroke, Death 170 (83) Design RCT (CEA vs CAS) Stroke 30-day events Setting Multicenter Table 3. Pooled Estimates of the Absolute Risks of Stroke, Death, or MI According to the Timing of the Outcome Assessment After CAS 3.9% (3.4 to 4.4) 4.7% (4.1 to 5.2) 5.3% (4.6 to 6.0) ⬍0.0001 ⬍0.0001 ⬍0.0001 67% (60 to 73) 69% (62 to 74) 64% (52 to 72) No. of studies included 53 48 19 P(het) I2 (95% CI) 119 (58) 29 485 (30250) 87 (42) 25 228 (25936) Sampling frame described 154 (75) 38 056 (39 354) Inclusion criteria described 140 (68) 36 487 (37 429) Baseline characteristics described 132 (64) 34832 (3552) No. of patients 11 694 7912 1723 Assessment by a neurologist 79 (39) 26 286 (26 835) No. of procedures 12 073 8243 1806 Definition of stroke outcome given 87 (43) 37 499 (38 292) At least 1 neurologist in the list of authors 66 (32) 9075 (9410) Not clear Description of population Outcome assessment No. of Studies With Data Available In-hospital events Pooled risk (95% CI) Median Value (IQR) No. of patients 206 90 (41 to 204) No. of procedures 206 94 (43 to 215) Percent men 179 71 (65 to 80) Mean age, y 180 70 (67 to 71) Percent symptomatic 180 50 (33 to 78) Percent diabetic patients 128 31 (24 to 38) Percent patients with carotid restenosis 95 14 (7 to 24) Percent patients with postradiation carotid stenosis 47 5 (2 to 9) Percent patients with CAD 104 60 (40 to 71) Percent patients with PAD 44 28 (20 to 37) Percent patients with contralateral carotid occlusion 79 10 (6 to 14) No patient treated with cerebral protection, n studies (%) 201 54 (27) All patients treated with cerebral protection, n studies (%) 201 71 (35) Percent successful procedures, median (IQR) 109 98 (97–100) IQR indicates interquartile range. *Also include abstracts. †RCTs comparing different strategies in patients treated by CAS. 4.6% (3.5 to 5.9) ⬍0.0001 ⬍0.0001 54% (36 to 67) 30% (0 to 60) No. of studies included 53 40 13 No. of patients 9003 3893 979 No. of procedures 9413 4199 1006 0.11 Periprocedural events* Pooled risk (95% CI) P(het) Technical aspects 4.1% (3.3 to 5.0) 56% (41 to 68) P(het) I2 (95% CI) Population characteristics 3.9% (3.2 to 4.6) I2 (95% CI) 3.5% (2.7 to 4.4) 3.7% (2.6 to 5.0) ⬍0.0001 ⬍0.0001 71% (61 to 78) 66% (53 to 76) 4.0% (2.6 to 5.7) 0.23 21% (0 to 59) P(het) indicates the P value associated with the 2 test for heterogeneity; I2, percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). Combined estimates of risk were calculated separately for the different outcomes. Each individual proportion was first transformed into a quantity with the Freeman-Tukey variance-stabilizing transformation.24 A weighted mean of the transformed proportions was computed with a DerSimonian-Laird random-effects model.25 The combined proportion was calculated as the back-transform of this weighted mean. *Also includes events of uncertain timing. in the case of prospective enrollment (yes 5.2% vs no 4.2%, P⫽0.07), and when the assessment was done by a neurologist (yes 5.4% vs no 4.1%, P⫽0.02). We did not find evidence for publication bias in the visual analysis of the funnel plots of the study sample size against the absolute risk of stroke or Touzé et al Risks After Carotid Angioplasty and Stenting e687 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure 2. Pooled RRs of stroke and stroke or death for different subgroups. P(het) indicates the probability value associated with the Cochran 2 statistical test for heterogeneity; I2, percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance); NA, not assessable; TIA, transient ischemic attack; CABG, coronary artery bypass graft; and P(sig), P value for significance. We used a fixed-effect model to calculate the pooled estimates, except where P(het)⬍0.10 or I2⬎30%, in which case a random-effects model was used. See supplemental Figures II and III for each individual meta-analysis. Outcomes assessed at 30 days, at discharge, during the procedure, or when timing was unknown were pooled. The comparison is yes vs no unless indicated otherwise. A, Pooled RR (95% CI) for stroke or death (see also supplemental Figure II). B, Pooled RR (95% CI) for stroke (see also online-only Figure III). death, as there were as many smaller studies with high and low risks of complications (supplemental Figure I, available online at http://stroke.ahajournals.org). Findings were similar with stroke and with stroke, MI, and death (data not shown). Figure 2 shows a summary of the pooled RRs of stroke and stroke or death resulting from CAS for the different prespecified subgroup analyses separated in terms of clinical symp- toms, patient characteristics (including vascular risk factors and past medical history), stenosis features, and technical factors. (Forest plots of the corresponding analyses are available in supplemental Figures II and III.) Symptomatic stenosis (RR⫽1.86; 95% CI, 1.61 to 2.14), cerebral event versus ocular event (RR⫽2.28; 95% CI, 1.08 to 4.77), age ⬎75 to 80 years (RR⫽1.93; 95% CI, 1.66 to 2.24), CAD e688 Stroke December 2009 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure 3. Absolute 30-day risk of stroke or death (%) after CAS in 91 studies (18 538 patients) according to the midcohort year, together with a summary random-effects meta-regression. The area of each circle is inversely proportional to the variance of the absolute risk. (RR⫽1.41; 95% CI, 0.97 to 2.06), history of coronary artery bypass graft (RR⫽2.21; 95% CI, 1.03 to 4.72), and PAD (RR⫽2.04; 95% CI, 0.92 to 4.52) were associated with a higher risk of stroke or death after CAS. There was also a trend for a higher risk of complications in patients who had calcified plaque. Conversely, the risk of stroke or death after CAS was lower in patients treated for carotid disease due to restenosis after CEA than in those treated for atherosclerotic carotid stenosis (RR⫽0.45; 95% CI, 0.28 to 0.71). The use of cerebral protection systems was associated with a lower risk of stroke or death (RR⫽0.57; 95% CI, 0.43 to 0.76). The risk of stroke or death was not related to sex, contralateral carotid occlusion, diabetes mellitus, plaque ulceration, timing for CAS, and side of the treated lesion. Similar results were found for stroke outcome, except that hypertension was significantly associated with a higher risk of complications (RR⫽1.86; 95% CI, 1.30 to 2.68). In contrast to the substantial heterogeneity found in the pooled estimates of absolute risks, there was either no or only moderate heterogeneity in these combined estimates of the RRs. We did not find evidence for publication biases in these analyses on the funnel plots or with the Egger test (data not shown). The pooled 30-day risk of stroke was 6.3% (95% CI, 4.8 to 8.0) in studies with a midcohort year before 1998, 5.0% (95% CI, 4.1 to 5.9) in those with a midcohort year between 1998 and 2002, and 3.9% (95% CI, 3.0 to 4.9) in those with a midcohort year after 2002. Meta-regression analysis with midcohort year as the covariate showed a significant decrease in the 30-day risk of stroke or death over time, corresponding to a RR reduction of ⬇6% per year (91 studies, P⬍0.0001; Figure 3). A similar result was found when publication year was used instead of midcohort year (P⬍0.0001) or when stroke was considered instead of stroke or death (data not shown). Figure 4 shows the pooled 30-day absolute risks of stroke or death stratified according to clinical indication and further stratified according to study design and whether outcomes were assessed by a neurologist or not. In patients with symptomatic stenosis, the overall absolute 30-day risk of stroke or death was 7.6% (95% CI, 6.3 to 9.1; 42 studies; 4910 patients). That risk was higher in RCTs (10.8%; 95% CI, 6.8 to 15.5) than in registries that enrolled symptomatic patients only (7.3%; 95% CI, 5.3 to 9.6; P⫽0.16) and than in subgroups of symptomatic patients enrolled in other registries (7.0%; 95% CI, 5.2 to 9.0; P⫽0.04). The absolute 30-day risk of stroke or death was nonsignificantly higher in studies where there was an independent neurologic assessment than in those where the method of assessment of outcomes was not clearly performed by an independent neurologist. For patients with asymptomatic stenosis, there was only 1 RCT in which the 30-day risk of stroke or death was not clearly assessed,34 and 1 registry enrolled asymptomatic patients only.41 The overall absolute risk of stroke or death was 3.3% (95% CI, 2.6 to 4.1; 23 studies; 8504 patients). As for symptomatic stenosis, risks were nonsignificantly higher in studies where there was an independent neurologic Touzé et al Risks After Carotid Angioplasty and Stenting e689 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure 4. Pooled 30-day risk of stroke or death after CAS stratified according to clinical indication and further stratified according to study design and whether outcomes were assessed by a neurologist or not. The dotted line corresponds to the 30-day risk combined across RCTs and all registry data. There was an independent neurologic assessment in all RCTs. Some RCTs could not be included in this analysis because the 30-day stroke or death outcome was unavailable. assessment. All of these findings were similar when we used the presence of at least 1 neurologist in the list of authors as a marker of quality instead of independent neurologic assessment (data not shown). Discussion First, we have shown that the overall 30-day risk of stroke or death after CAS is ⬇5%, but it varies substantially across studies. Those variations may result from differences in case mix, design, quality of the study, and skill of the interventionists. Second, the risks of CAS depend on the clinical indication, with symptomatic patients being about twice as likely as those with asymptomatic stenosis to have complications and on patient characteristics that are also high surgical risk factors, including age, hypertension, and history of CAD (including coronary artery bypass graft). Conversely, other established high surgical risk factors either did not seem to influence the risk of complications due to CAS (female sex and contralateral carotid occlusion) or were even associated with a lower risk (carotid restenosis after CEA). Therefore, our results strongly suggest that there are simple clinical factors that are likely to help in selecting good candidates for CAS in future clinical trials comparing that technique with CEA and eventually in clinical practice. Finally, our results suggest that risks have decreased over time, and the use of a cerebral protection device is associated with a lower risk of complications. We identified 206 studies that reported risks of CAS on 54 713 patients. It should be noticed that RCT patients accounted for only 3% of the overall population, underlining how much the technique has been implemented in clinical practice despite a low level of evidence. Because of substantial heterogeneity between studies, our pooled estimates of absolute operative risks cannot be interpreted in a straightforward manner. However, the 95% CI obtained from a random-effect meta-analysis well describes uncertainty in the average risk. For instance, for all studies, the 30-day risk of stroke or death was, with 95% confidence, at least equal to 4.1% and as high as 5.2%. Interestingly, a similar heterogeneity was found in previous systematic reviews for the risks of CEA.16 –18 e690 Stroke December 2009 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 There are several sources of heterogeneity across studies that we were able to identify. It is well established that the benefit of CEA strongly depends on the clinical indication, with greater benefit observed in symptomatic compared with asymptomatic patients, and that there is a higher risk of perioperative complications in symptomatic patients.17,18,42,43 A previous systematic review showed that only ⬇25% of studies on CEA stratified their results according to whether the patients were asymptomatic or symptomatic.16 Similarly, we found that only 21% of CAS studies reported risks stratified according to clinical indication. We have shown that, as for CEA, symptomatic patients are about twice as likely as asymptomatic patients to have complications after CAS. This result is based on subgroup analyses, ie, comparing symptomatic and asymptomatic patients within the same studies, and we did not find heterogeneity across studies. For symptomatic patients, we found that the 30-day risk of stroke or death of CAS was 7.6%, with the lower limit of the 95% CI being 6.3%, which is higher than the 30-day risk resulting from CEA found in a previous systematic review (5.1%; 95% CI 4.6 to 5.6).16 That level of risk is also higher than the risk threshold established by the ad hoc committees of the American Heart Association Stroke Council guidelines, recommending that the combined risk of stroke and death resulting from CEA should be no more than 5% for patients with transient ischemic attack and 7% for those with stroke.2,3 Although questionable, this comparison of pooled absolute risks is in full agreement with the meta-analysis of RCTs comparing CAS with CEA in symptomatic patients and showing that CAS is associated with a 40% increase in risk of stroke or death within 30 days.12 We also found that the risk was higher in RCTs than in registries. It is likely that the definition of symptomatic stenosis differed across studies, although this information cannot be easily extracted from publications (eg, some registries considered stroke that occurred in any territory or at any time lapse). In addition, as for CEA,18 our results suggest that the quality of neurologic assessment partly accounts for the observed differences. Although most patients enrolled in registries had asymptomatic stenosis, we found very limited specific data on the risks of CAS in those patients. The overall 30-day risk of stroke or death with CAS was 3.3%, with the lower limit of the 95% CI being 2.6%. That level of risk is close to that resulting from CEA (2.8%; 95% CI, 2.4 to 3.2),16 and the 3% risk threshold established in guidelines for asymptomatic stenosis.2,3 Many registries have focused on patients with a high surgical risk according to a set of criteria, varying in number and type, hypothesising that those patients should be good candidates for CAS.4 Commonly cited factors associated with higher surgical risk are anatomic factors such as surgically inaccessible lesions, prior CEA or neck irradiation, old age, contralateral carotid occlusion, and medical comorbidities. However, there is no evidence that high surgical risk patients benefit from any revascularization strategy in comparison with medical treatment alone.44 Moreover, it is possible that comorbidities associated with a greater perioperative risk with CEA also increase the periprocedural risk with CAS. Studies that addressed whether factors that identify high surgical risk patients have any impact on the risks of CAS usually had low statistical power to draw any reliable conclusion. Although previous meta-analyses of RCTs and registries have consistently shown that age has only a small impact on the risk of complications after CEA,45 elderly patients are considered a high surgical risk and potential good candidates for CAS. In fact, we found that age was associated with an ⬇2-fold increase in risk of complications after CAS, suggesting that age has more impact on risks of CAS than on risks of CEA. Interestingly, several studies have shown that older patients are more likely to have tortuous, severely calcified vessels that probably increase the risk of embolization during wire manipulation and catheter exchanges at some stage in CAS.46,47 Another important finding of our analysis is that, in contrast to CEA, where women have a higher risk of complications than do men, the risks of CAS are not related to sex. However, although both very close to 1 and nonsignificant, the pooled RR of women versus men for stroke and that for stroke or death are on either side of 1. In fact, results for stroke were strongly driven by those of the CAPTURE registry, in which women had a slightly higher risk of stroke in univariate analysis but not in multivariate analyses.48 The lack of a sex effect on the risk of CAS has also been demonstrated in 2 recent studies, published outside the inclusion period defined in our systematic review.49,50 Inclusion of those results would have not changed our estimates [stroke pooled RR⫽1.02; 95% CI, 0.87 to 1.27; P(het)⫽0.87; stroke or death pooled RR⫽0.90; 95% CI, 0.74 to 1.10; P(het)⫽0.84]. Our findings that the risks of CAS do not depend on contralateral carotid occlusion and are lower in patients with restenosis after CEA are also important because they identify a potential target population in whom CAS could be compared with CEA. Finally, although obtained from more limited data and in line with previous data on CEA, our results suggest that the risks of CAS are higher in patients who had a cerebral compared with an ocular event and that a previous history of CAD may not be helpful to select good CAS candidates. There are potential reasons why risk factors for complications of CEA and CAS may differ. The higher risk of complications after CEA in women is usually explained by the fact that the internal carotid artery is smaller than in men, predisposing to technical errors or early postoperative thrombosis, although this hypothesis has been questioned.51,52 Surgery of carotid restenosis is associated with a high risk of complications, probably because of important postoperative fibrotic modifications of the neck tissue and the fact that restenosis is commonly due to myointimal hyperplasia rather than atherosclerosis.53 Although in symptomatic patients with restenosis the long-term benefit of CEA still justifies the immediate surgical risk and outweighs the risk from medical therapy alone, the only randomized evidence suggests that asymptomatic patients with restenosis do slightly better with medical management.1 Because CAS does not require neck and arterial incision, sex- or post-CEA–related anatomic factors are likely to be overcome with CAS. Contralateral carotid occlusion may compromise compensatory mechanisms and consequently, cerebral perfusion during the clamping of the carotid artery required to perform CEA. The shorter duration of carotid occlusion during CAS compared with Touzé et al Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 CEA could explain the lack of increase in operative risk during CAS. By contrast, other factors such as age, hypertension, and history of CAD or PAD are strongly associated with the severity and the extent of atherosclerosis and are likely to be related to the risk of thromboembolic complications during arterial navigation through the aorta and carotid artery. Using meta-regression analysis, we found that the risks of CAS have decreased over time from 1993 to 2006. This may result from improvements in CAS technique, devices, or training and/or a better selection of CAS candidates over time. The development of devices to protect against embolism during the CAS procedure potentially constitutes an important advance. Previous systematic reviews of nonrandomized case series showed that the use of cerebral protection devices seems to reduce thromboembolic complications during CAS54 and to reduce the incidence of new, mostly asymptomatic, ischemic lesions on diffusion-weighted magnetic resonance imaging performed within 48 hours after CAS.55,56 Our results obtained from a larger number of studies are in line with those previous data. However, there was significant heterogeneity across studies in this analysis. In fact, the apparent advantage of cerebral protection devices may be illusory. Indeed, the use of such protection devices has increased over time, and the apparent protective effect of those devices may have been confounded by advances in stenting technique and patient selection over time. It could also reflect patient selection. In addition, there are still no data from randomized studies comparing CAS with or without cerebral protection, and because the protecting devices must pass through the arterial stenosis, they might themselves cause complications. Our study has several potential limitations. First, confounding is a major threat in meta-analyses of observational studies because subgroup analyses are based on univariate comparisons. Only a meta-analysis of individual data would deal with that issue. However, our subgroup analyses were highly consistent across studies and have plausible pathophysiologic explanations. Moreover, with the use of a similar approach for CEA, all risk factors for complications found in systematic reviews of registries were replicated in a pooled analysis of individual data from RCTs.16 –20,43 Our findings are therefore unlikely to be spurious. Second, although we included studies in any language and used multiple sources of data, publication biases might have distorted our findings because registries with a low risk of complications could be more likely to be published. However, using simple scatterplots, as there is no validated statistical test to assess publication bias in meta-analyses of absolute risks, we did not find evidence for publication biases. Moreover, publication biases are unlikely in the subgroup analyses because the chance of publication is unlikely to be related to the results of subgroup analyses, and we did not find evidence for such biases in the funnel plots. In addition, RRs usually do not depend on absolute risk. Third, potential inclusion of duplicated data might have also distorted our findings.57 However, we thoroughly examined the authors’ list and setting of each report to exclude redundant populations as far as possible. We also Risks After Carotid Angioplasty and Stenting e691 performed sensitivity analyses by excluding some large registries that might have enrolled patients whose data were also published in small, single-center studies and found similar results. Fourth, heterogeneity in the quality of data is another issue in meta-analyses of observational studies. Although the quality of outcome assessment varied across studies, our findings were not influenced by that parameter. Fifth, some subgroup analyses (eg, type of cerebrovascular event, plaque surface aspect, or history of coronary artery bypass graft) were based on relatively small numbers of studies and would require additional confirmatory data. Finally, there are other potential risk factors for complications that could not be assessed. For instance, the role of operator experience and the learning curve could not be assessed in our systematic review, because there was no standardized definition across studies. Arterial anatomic factors are also likely to influence the feasibility and risks of CAS.58 In conclusion, the risks of CAS are overall higher than those of CEA in symptomatic patients. Our findings support the current guidelines recommending that CAS should not be used in good surgical candidates. However, they also suggest that there are factors that are likely to help select good candidates for CAS in future trials and eventually in clinical practice. Acknowledgments We would to thank very much Peter A. Ringleb and the SPACE investigators for providing unpublished subgroup data from the trial. We thank Marta Pasquini, Enrico Flomann, Kaori Flomann, Hu Chau, Maria Koziak, Daniel Freddy, Didier Leys, Ghislain Nokam, Barish Turak, and Suzanne Vobecky for their help to extract the data from non–French-language and non–English-language articles. We also thank Bernard Beyssen and Olivier Naggara for advising us on technical aspects and Isabelle Laurent for technical support. Disclosures None. References 1. Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy–an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:794 – 801. 2. Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006; 37:1583–1633. 3. Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-Sponsored by the Council on Cardiovascular Radiology and e692 4. 5. 6. 7. 8. Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. Stroke December 2009 Intervention: The American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577– 617. Touzé E, Calvet D, Chatellier G, Mas JL. Carotid stenting. Curr Opin Neurol. 2008;21:56 – 63. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729 –1737. Coward LJ, Featherstone RL, Brown MM. Safety and efficacy of endovascular treatment of carotid artery stenosis compared with carotid endarterectomy: a Cochrane systematic review of the randomized evidence. Stroke. 2005;36:905–911. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660 –1671. Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239 –1247. Ringleb PA, Chatellier G, Hacke W, Favre JP, Bartoli JM, Eckstein HH, Mas JL. Safety of endovascular treatment of carotid artery stenosis compared with surgical treatment: a meta-analysis. J Vasc Surg. 2008; 47:350 –355. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579. Jeng JS, Liu HM, Tu YK. Carotid angioplasty with or without stenting versus carotid endarterectomy for carotid artery stenosis: a meta-analysis. J Neurol Sci. 2008;270:40 – 47. Ederle J, Featherstone RL, Brown MM. Randomized controlled trials comparing endarterectomy and endovascular treatment for carotid artery stenosis: a Cochrane systematic review. Stroke. 2009;40:1373–1380. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touze E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Henon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885– 892. Bond R, Rerkasem K, Rothwell PM. Systematic review of the risks of carotid endarterectomy in relation to the clinical indication for and timing of surgery. Stroke. 2003;34:2290 –2301. Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke. 1996;27:266 –269. Rothwell PM, Slattery J, Warlow CP. A systematic review of the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis. Stroke. 1996;27:260 –265. Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ. 1997;315:1571–1577. Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351: 1493–1501. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283:2008 –2012. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427– 437. Freeman M, Tukey J. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607– 611. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. Trinquart L, Touzé E. Pitfalls in meta-analysis of observational studies: lessons from a systematic review of the risks of stenting for intracranial atherosclerosis. Stroke. 2009;40:e586 – e587. Gao S. Combining binomial data using the logistic normal model. J Stat Comput. 2004;74:293. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629 – 634. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539 –1558. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Updated September 2008. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Alberts MJ. Results of the multicenter prospective randomized trial of carotid artery stenting vs. carotid endarterectomy. Stroke. 2001;32:325. Abstract. Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589 –1595. Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery. 2004;54:318 –324. Hoffman A, Engelter T, Taschner C, Mendellowitsch A, Merlo A, Radue EW, Lyrer P. Carotid artery stenting versus carotid endarterectomy–a prospective randomised controlled single-centre trial with long-term follow-up (BACASS). Schweizer Archiv Neurol Psychiatr. 2008;159: 84 – 89. Ling F, Jiao LQ. Preliminary report of trial of endarterectomy versus stenting for the treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chin J Cerebrovasc Dis. 2006;3:4 – 8. Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N, Lloyd AJ, London NJ, Bell PR. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;28:326 –334. Dalainas I, Nano G, Bianchi P, Stegher S, Malacrida G, Tealdi DG. Dual antiplatelet regime versus acetyl-acetic acid for carotid artery stenting. Cardiovasc Intervent Radiol. 2006;29:519 –521. Kaposzta Z, Clifton A, Molloy J, Martin JF, Markus HS. S-nitrosoglutathione reduces asymptomatic embolization after carotid angioplasty. Circulation. 2002;106:3057–3062. Jordan WD Jr, Roye GD, Fisher WS III, Redden D, McDowell HA. A cost comparison of balloon angioplasty and stenting versus endarterectomy for the treatment of carotid artery stenosis. J Vasc Surg. 1998;27: 16 –22. Marine LA, Rubin BG, Reddy R, Sanchez LA, Parodi JC, Sicard GA. Treatment of asymptomatic carotid artery disease: similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J Vasc Surg. 2006;43:953–958. Redgrave JN, Rothwell PM. Asymptomatic carotid stenosis: what to do. Curr Opin Neurol. 2007;20:58 – 64. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. Ederle J, Featherstone RL, Brown MM. Long-term outcome of endovascular treatment versus medical care for carotid artery stenosis in patients not suitable for surgery and randomised in the Carotid and Vertebral Artery Transluminal Angioplasty study (CAVATAS). Cerebrovasc Dis. 2009;28:1–7. Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20:69 –77. Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A. Increased aortic arch calcification in patients older than 75 years: implications Touzé et al 47. 48. 49. 50. 51. for carotid artery stenting in elderly patients. J Vasc Surg. 2007;46: 841– 845. Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007;45:875– 880. Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, Hopkins LN, Atkinson R, Raabe R, Barnwell S, Green R. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Cathet Cardiovasc Interv. 2007;69:341–348. Howard VJ, Voeks JH, Lutsep HL, Mackey A, Milot G, Sam AD, Tom M, Hughes SE, Sheffet AJ, Longbottom M, Avery JB, Hobson RW, Brott TG. Does sex matter? thirty-day stroke and death rates after carotid artery stenting in women versus men: results from the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) Lead-in Phase. Stroke. 2009;40:1140 –1147. Goldstein LJ, Khan HU, Sambol EB, Kent KC, Faries PL, Vouyouka AG. Carotid artery stenting is safe and associated with comparable outcomes in men and women. J Vasc Surg. 2009;49:315–323. Lee JW, Pomposelli F, Park KW. Association of sex with perioperative mortality and morbidity after carotid endarterectomy for asymptomatic carotid stenosis. J Cardiothorac Vasc Anesth. 2003;17:10 –16. Risks After Carotid Angioplasty and Stenting e693 52. Rockman CB, Castillo J, Adelman MA, Jacobowitz GR, Gagne PJ, Lamparello PJ, Landis R, Riles TS. Carotid endarterectomy in female patients: are the concerns of the Asymptomatic Carotid Atherosclerosis Study valid? J Vasc Surg. 2001;33:236 –240. 53. Lal BK. Recurrent carotid stenosis after CEA and CAS: diagnosis and management. Semin Vasc Surg. 2007;20:259 –266. 54. Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34:813– 819. 55. Kastrup A, Nagele T, Groschel K, Schmidt F, Vogler E, Schulz J, Ernemann U. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke. 2006;37:2312–2316. 56. Schnaudigel S, Groschel K, Pilgram SM, Kastrup A. New brain lesions after carotid stenting versus carotid endarterectomy: a systematic review of the literature. Stroke. 2008;39:1911–1919. 57. Senn SJ. Overstating the evidence– double counting in meta-analysis and related problems. BMC Med Res Methodol. 2009;9:10. 58. Silvestro A, Civelli P, Laffranchini G, Troianiello B, Graziani L. Influence of anatomical factors on the feasibility and safety of carotid stenting in a series of 154 consecutive procedures. J Cardiovasc Med (Hagerstown, Md). 2008;9:137–141. Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 e694 Stroke December 2009 Figure I. Scatterplot of the 30-day risk of stroke or death against the study sample size (logarithmic scale). The solid line represents the pooled absolute risk obtained from a random-effect meta-analysis (4.7%). Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Touzé et al Risks After Carotid Angioplasty and Stenting e695 Pooled relative risks of stroke or death according to different subgroups Page Subgroup 2 Symptomatic stenosis 3 Qualifying event (stroke vs TIA) 4 Qualifying event (cerebral vs ocular) 5 Delay between symptoms and CAS 6 Age 7 Sex 8 Diabetes 9 Hypertension 10 Coronary heart disease 11 History of CABG 12 Peripheral artery disease 13 Restenosis Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 14 Contralateral occlusion 15 Plaque calcification 16 Plaque ulceration 17 Stenosis side 18 Cerebral protection device Figure II. Pooled RRs of stroke or death for the different subgroups. Combined RRs were calculated with a fixed-effect meta-analysis, according to the Mantel-Haenszel method, or with a DerSimonian-Laird random-effects meta-analysis, where appropriate. Error bars correspond to the 95% CIs of individual RRs and pooled RR. December 2009 Stroke e696 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure II (Continued). Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure II (Continued). e697 December 2009 Stroke e698 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure II (Continued). Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure II (Continued). e699 December 2009 Stroke e700 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure II (Continued). Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure II (Continued). e701 e702 Stroke December 2009 Pooled relative risks of stroke according to different subgroups Page Subgroup 2 Symptomatic stenosis 3 Qualifying event (stroke vs TIA) 4 Qualifying event (cerebral vs ocular) 5 Delay between symptoms and CAS 6 Age 7 Sex 8 Diabetes 9 Hypertension 10 Coronaryy heart disease 11 History of CABG 12 Peripheral artery disease 13 Restenosis Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 14 Contralateral occlusion 15 Plaque calcification 16 Plaque ulceration 17 Stenosis side 18 Cerebral protection device Figure III. Pooled RRs of stroke for the different subgroups. Combined RRs were calculated with a fixed-effect meta-analysis, according to the Mantel-Haenszel method, or with a DerSimonian-Laird random-effects meta-analysis, where appropriate. Error bars correspond to the 95% CIs of individual RRs and pooled RR. Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). e703 December 2009 Stroke e704 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). e705 December 2009 Stroke e706 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). e707 December 2009 Stroke e708 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). Touzé et al Risks After Carotid Angioplasty and Stenting Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Figure III (Continued). e709 e710 Table I. Publication Year Stroke December 2009 Characteristics of the Studies Study 1992 Munari1 BERGAMO-NEURO-1 1996 Theron2 CAEN Eckert3 HAMBURG-1 AR Germany 1990–1995 Cohort 58 Diethrich4 PHOENIX AR USA 04/1993–09/1995 Cohort 110 Criado5 BALTIMORE AR USA 12/1997–03/2001 Cohort 33 Crawley6 LONDON-1 AR UK NA RCT 28 Vozzi ROSARIO AR Argentina 10/1995–03/1997 Cohort 22 Mendelsohn8 DURHAM-1 AR USA 06/1996–10/1997 Cohort 28 1997 7 1998 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 1999 2000 Accrual Period Design AR Italy 01/1986 – 01/1991 Cohort 40 AR & SG France 1984–1995 Cohort 229 LEICESTER AR UK 06/1996–09/1999 RCT Mathur10 LENNOX HILL, BIRMINGHAM SG USA NA Cohort Teitelbaum11 LOS ANGELES AR & SG USA NA Cohort 21 Henry12 NANCY-1 AR France 04/1995–04/1998 Cohort 163 Gross13 BERLIN AR Germany 02/1996–08/1998 Cohort 85 Sievert14 FRANKFURT-CVC AR Germany NA Cohort 71 Arakawa15 KURASHIKI AR Japan NA Cohort 6 Matushita16 OSAKA-TOKUSHUKAI-1 AR Japan 05/1996–10/1998 Cohort 17 7 231 Reifart17 BAD SODEN AR Germany 11/1996–03/1999 Cohort 50 Parodi18 BUENOS-AERES SG Argentina-USA 09/1998–09/1999 Cohort 46 Qureshi BUFFALO-1 AR & SG USA 06/1996–12/1998 Cohort 111 Jacksch20 ESSEN AR Germany NA Cohort 47 Griewing21 HANNOVER AR Germany 05/1996–11/1997 Cohort 20 Gupta22 MILWAUKEE AR & SG USA 1996–1999 Cohort 100 Kaul23 NEW DELHI AR India 07/1997–06/1998 Cohort 14 Link24 REGENSBURG AR Germany 08/1999–02/2000 Cohort 23 Malek25 SAN FRANCISCO-MALEK AR & SG USA 07/1996–07/1999 Cohort 28 Yoon26 SEOUL-YONSEI AR Korea 05/1996–07/1999 Cohort 36 27 Shawl TAKOMA PARK AR & SG USA 08/1995–06/1998 Cohort 170 Dangas28 WASHINGTON AR USA NA Cohort 133 Brown29 CAVATAS AR International 03/1992–07/1997 RCT 240 Dietz30 FRANKFURT-UNIVERSITY AR Germany 1997–2000 Cohort 43 31 Brooks 2002 Country Naylor9 19 2001 Analysis N Patients First Author KENTUCKY-1 AR USA NA RCT 53 Roubin32 LENNOX HILL, BIRMINGHAM AR & SG USA 09/1994–09/1999 Cohort 528 Pappada33 MILAN-NEUROSURGERY AR Italy 01/1997–07/2000 Cohort 27 Baudier34 ODENSE AR Denmark 05/1993–10/1999 Cohort 54 Kirsch35 PERTH AR & SG Australia 11/1996–10/1999 Cohort 53 Orlandi36 PISA-1 AR Italy 02/1998–07/2000 Cohort 38 Ahmadi37 VIENNA SG Austria 01/1997–11/2000 Cohort 303 Alberts 38 WALLSTENT AR USA NA RCT 107 39 BALTIMORE AR & SG USA 01/1994–06/1996 Cohort 132 Guimaraens40 Criado BARCELONA-SANT CUGAT AR Spain 04/1994–04/2000 Cohort 159 Wang41 BEEJING-WANG AR China NA Cohort 16 Bonaldi42 BERGAMO-NEURO-2 AR Italy 01/1997–10/2000 Cohort 70 43 Qureshi BUFFALO-2 AR USA 01/1999–12/1999 Cohort 70 Castriota44 COTIGNOLA SG Italy NA Cohort 275 Koch45 HAMBURG-2 AR & SG Germany 07/1997–12/2001 Cohort 161 Milosevic46 LJUBLJANA AR Slovenia NA Cohort 17 47 LONDON-2 AR UK NA RCT Kaposzta 16 (Continued) Touzé et al Table I. Risks After Carotid Angioplasty and Stenting e711 Continued Study N Attempted Procedures Mean Age, y Male (%) Diabetes (%) Symptomatic Carotid Stenosis (%) Prior CEA/CAS (%) Prior CAD (%) Prior PAD (%) Contralateral Occlusion (%) BERGAMO-NEURO-1 44 64 75 … 100 … … … … CAEN 229 … … … … 22 … … … HAMBURG-1 61 60 67 … 100 … … … … PHOENIX 117 72 79 17 28 17 54 34 … BALTIMORE 33 … 63 24 73 … 30 … … LONDON-1 28 68 … 4 … … 25 … … … ROSARIO 24 69 73 … 45 … 32 … DURHAM-1 19 69 61 28 78 6 … … … LEICESTER 7 68 71 … 100 … … … … 259 69 71 … 60 22 71 … 12 LOS ANGELES 26 63 59 … 68 … … … … NANCY-1 174 71 76 23 33 5 51 32 5 BERLIN 89 … 82 32 22 … 100 19 13 FRANKFURT-CVC 76 70 77 39 … 1 … 27 … LENNOX HILL, BIRMINGHAM Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 KURASHIKI 7 71 100 … 50 … … … … OSAKA-TOKUSHUKAI-1 18 … … … … … … … … BAD SODEN 50 68 86 34 … 12 84 … … BUENOS-AERES 46 67 76 28 39 15 41 … 7 BUFFALO-1 111 68 64 43 51 25 66 30 … ESSEN 48 68 67 … 67 … … … … HANNOVER 20 65 75 … 80 … 71 … 55 MILWAUKEE 100 76 76 32 85 30 80 … 3 NEW DELHI 15 61 86 43 100 0 29 … 14 REGENSBURG 23 67 87 … … … … … … SAN FRANCISCO-MALEK 28 71 64 29 100 18 61 21 … SEOUL-YONSEI 48 65 89 33 58 … … … 11 TAKOMA PARK 192 73 59 36 61 9 50 … 8 WASHINGTON 140 71 70 27 … 19 71 52 … CAVATAS 240 67 69 14 97 … 39 24 10 FRANKFURT-UNIVERSITY 43 67 71 63 100 0 54 32 … KENTUCKY-1 53 66 … 36 100 … 74 … 9 LENNOX HILL, BIRMINGHAM 604 69 67 32 44 15 71 … 10 MILAN-NEUROSURGERY 27 … … … … … … … … ODENSE 53 64 69 9 98 … 24 19 … PERTH 57 71 72 26 68 … 74 45 19 PISA-1 41 68 67 … 100 … … … … VIENNA 320 70 71 35 38 … 47 45 18 WALLSTENT 107 67 66 … 100 … … … … BALTIMORE 135 68 65 37 40 39 60 … … BARCELONA-SANT CUGAT 186 63 78 … 92 … … … … BEEJING-WANG 20 64 … … 100 … … … 13 BERGAMO-NEURO-2 73 68 60 … 100 13 … … … BUFFALO-2 70 70 54 37 39 11 53 … … COTIGNOLA 275 71 75 18 42 12 … … … HAMBURG-2 167 66 72 … 77 … … … … LJUBLJANA 17 … 71 … 100 … 41 … 12 LONDON-2 16 67 81 25 100 … … … … (Continued) e712 Table I. Publication Year Stroke December 2009 Continued First Author Study Stankovic48 MILAN-COLUMBUS Grego49 PADUA Gray 50 N Patients 01/1999 –12/2000 Cohort 100 09/1999–02/2001 Cohort 26 122 Accrual Period AR Italy AR Italy SEATTLE AR USA 03/1996–09/1998 Cohort STOCKTON AR USA NA Cohort 49 Kao52 TAPEI AR & SG Taiwan 04/1998–10/2000 Cohort 118 VIENNA SG Austria 03/2000–03/2001 Cohort 111 54 Moller-Hartmann AACHEN AR Germany 12/1999–03/2002 Cohort 41 McKinlay55 CAReSS AR USA 04/2001–12/2002 Cohort 143 Becquemin56 CRETEIL AR France 01/1995–07/2002 Cohort 107 Gable57 DALLAS AR USA 05/1998–01/2002 Cohort 29 58 Rath Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 HYDERABAD AR India 01/2002–01/2003 Cohort 22 LEUVEN-1 AR & SG Belgium 04/2000–04/2002 Cohort 51 Cernetti60 MIRANO AR & SG Italy 11/1999–03/2001 Cohort 100 Shevchenko61 MOSCOW-SHEVCHENKO AR & SG Russia 02/1998–09/2002 Cohort 39 Maleux59 62 OSAKA-POLICE AR Japan NA Cohort 41 Wholey63 PITTSBURGH AR USA 04/1994–12/2001 Cohort 550 Piske64 SAO PAULO-BENEFICIENCA AR Brazil 03/1998–06/2000 Cohort 42 Costa65 SAO PAULO-DO CORACAO AR Brazil 08/1996–04/2001 Cohort 86 Tan66 SHEFFIELD SG UK 10/1999–10/2002 Cohort 201 Cremonesi67 SIENNA-COTIGNOLA SG Italy 12/1999–06/2002 Cohort 442 Pucillo68 VALHALLA AR USA 2000–2003 Cohort 74 Causin69 VICENZA AR & SG Italy 06/1996–10/2002 Cohort 150 Dabrowski70 WARSAW AR & SG Poland NA Cohort 75 Zahn71 ALKK SG Germany 07/1996–03/2003 Cohort 1483 Koshimae 2004 Design Country Madyoon51 Ahmadi53 2003 Analysis Garcia-Sanchez72 BADALONA AR Spain 01/2002–10/2002 Cohort 10 Sganzerla73 BERGAMO-CARDIO AR & SG Italy NA Cohort 94 Linfante74 BOSTON-BETH AR USA 06/2001–07/2002 Cohort 23 Clark75 BOSTON-ST ELIZABETH AR & SG USA 06/1995–01/2001 Cohort 98 Kobayashi76 CHIBA AR Japan 03/1998–04/2002 Cohort 30 Hobson77 CREST AR & SG USA 01/2000–03/2004 Cohort 749 Biasi78 ICAROS AR & SG International 07/2000–12/2001 Cohort 418 Brooks79 KENTUCKY-2 AR USA NA RCT 43 Pieniazek80 KRAKOW AR Poland 01/2001–04/2006 Cohort 132 Sadato81 KYOTO AR & SG Japan 1999 Cohort 40 82 LEBANON SG USA 10/2000–09/2003 Cohort 69 Schmidt83 LEIPZIG AR Germany 03/2002–02/2003 Cohort 42 Henry84 NANCY-2 AR & SG France NA Cohort 242 Choi85 NEWARK AR USA 09/1996–03/2004 Cohort 177 753 Powell 86 Reimers REIMERS SG Germany-Italy 09/1999–09/2002 Cohort Kihara87 RIO DE JANEIRO AR & SG Brazil 02/1998–03/2003 Cohort 79 Yadav88 SAPPHIRE AR & SG USA 08/2000–07/2002 RCT 159 McKevitt89 SHEFFIELD AR & SG UK 01/1993–09/2002 Cohort 328 90 Chang Sztriha91 STANFORD SAN JOSE AR USA 08/2001–08/2003 Cohort 20 SZEGED AR & SG Hungary 01/2001–07/2003 Cohort 245 Sabeti92 VIENNA SG Austria 01/1997–12/2001 Cohort 471 Terada93 WAKAYAMA AR & SG Japan 08/1997–10/2003 Cohort 215 (Continued) Touzé et al Table I. Risks After Carotid Angioplasty and Stenting e713 Continued Mean Age, y Male (%) Diabetes (%) Symptomatic Carotid Stenosis (%) Prior CEA/ CAS (%) Prior CAD (%) Prior PAD (%) Contralateral Occlusion (%) Study N Attempted Procedures MILAN-COLUMBUS 102 67 71 17 28 14 49 … 9 PADUA 26 72 85 58 46 27 62 15 4 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 SEATTLE 136 73 57 25 31 12 56 … 6 STOCKTON 49 74 61 … … 18 … … 6 TAPEI 128 73 89 34 75 … 75 5 11 VIENNA 111 70 67 35 30 … 41 50 … AACHEN 43 … … … … … … … … CAReSS 143 71 60 29 31 … 66 45 … CRETEIL 114 71 72 20 33 7 41 … 9 DALLAS 31 65 48 … 69 34 … … … HYDERABAD 20 64 64 … 68 … 82 … … LEUVEN-1 54 73 72 … 61 12 48 … 6 … MIRANO 104 71 71 22 26 4 62 … MOSCOW-SHEVCHENKO 46 … … 13 54 … … … 5 OSAKA-POLICE 41 71 83 … 46 … … … … PITTSBURGH 580 71 58 … 48 38 … … … SAO PAULO-BENEFICIENCA 47 66 80 … 75 … … … … SAO PAULO-DO CORACAO 94 64 51 … … … … … … SHEFFIELD 204 68 68 18 85 … 47 … … SIENNA-COTIGNOLA 442 73 79 … 57 13 … … 11 VALHALLA 78 … … … 25 46 … … … VICENZA 156 68 77 … 100 23 … … 20 WARSAW 77 65 65 27 76 … 63 … 12 1483 70 72 34 57 6 65 26 … … ALKK BADALONA 10 66 80 40 100 … … 20 BERGAMO-CARDIO 100 70 76 37 34 3 79 47 11 BOSTON-BETH 24 65 39 17 100 39 35 … 17 BOSTON-ST ELIZABETH 110 70 71 38 58 20 61 37 24 … CHIBA 30 68 90 40 83 … … … CREST 749 70 64 29 31 … … … … ICAROS 415 68 71 31 32 54 45 24 … KENTUCKY-2 43 67 … 16 0 … 81 … … KRAKOW 137 63 73 30 62 2 69 57 20 KYOTO 43 71 83 … 40 … … … … LEBANON 74 72 82 39 38 18 … … 26 LEIPZIG 42 70 81 43 31 … 74 … 5 NANCY-2 268 71 80 20 64 12 61 24 6 NEWARK 194 71 56 … 34 60 … … … REIMERS 753 70 74 21 26 5 63 … 7 RIO DE JANEIRO 79 65 62 … 80 … … … … SAPPHIRE 159 73 67 25 30 23 86 … 24 SHEFFIELD 333 68 70 16 100 0 43 … … STANFORD SAN JOSE 21 73 80 10 62 19 28 … 0 SZEGED 260 65 57 … 48 4 … … 13 VIENNA 471 72 81 31 37 … 42 48 9 WAKAYAMA 215 … … … … … … … … (Continued) e714 Table I. Publication Year 2005 Stroke December 2009 Continued First Author Analysis Country Accrual Period Design N Patients Zahn94 ALKK SG Germany 07/1996 – 07/2003 Cohort 729 Li95 BEIJING AR & SG China 10/1997–10/2004 Cohort 439 Bonaldi96 BERGAMO-VARESE AR Italy 07/2000–12/2003 Cohort 53 Hammer97 BRUSSELS AR & SG Belgium 11/2002–01/2005 Cohort 53 Eskandari98 CHICAGO AR USA 04/2001–02/2005 Cohort 168 Chen99 CHONGQING AR China 01/2003–06/2004 Cohort 28 174 100 Yen CLEVELAND SG USA 01/2000–09/2002 Cohort Lin101 DUSSELDORF-1 AR Germany 10/2002–10/2004 Cohort 55 Nagata102 FUKUOKA AR Japan 12/1999–08/2004 Cohort 84 Lin103 HOUSTON SG USA 01/2002–03/2005 Cohort 182 68 104 Cohen Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 JERUSALEM AR Israël 10/2001–04/2005 Cohort Dudek105 KRAKOW-DUDEK AR Poland NA Cohort 21 Bergeron106 MARSEILLE AR France 09/1991–09/2003 Cohort 193 Emanuelli107 MILAN-VASCULAR SURGERY AR Italy 1995 Cohort 113 MOSCOW-CARDIOVASC AR & SG Russia NA Cohort 20 OMAHA AR USA 12/2002–01/2005 Cohort 17 108 Bokeriia Pipinos109 Cosottini110 PISA-2 AR Italy 12/2003–10/2004 Cohort 52 Kadkhodayan111 SAINT-LOUIS-NEURO AR & SG USA 03/1996–12/2003 Cohort 131 SECURITY112 SECURITY AR USA NA Cohort 305 Roh113 SEOUL-SAMSUNG-1 AR Korea 07/1998–02/2001 Cohort 22 Ackerstaff114 ST ANTONIUS AR Netherlands 12/1997–04/2004 Cohort 550 Vos115 ST ANTONIUS SG Netherlands 12/1997–12/2003 Cohort 509 327 116 Groschel 2006 Study TUBINGEN AR Germany 04/1999–12/2004 Cohort Kastrup117 TUBINGEN-JENA SG Germany 06/1999–08/2004 Cohort 299 Boltuch118 VIENNA SG Austria 01/1997–06/1905 Cohort 651 Gray119 ARCHeR AR & SG USA 05/2000–09/2003 Cohort 581 120 Kasirajan White121 ATLANTA AR & SG USA 09/2000–09/2007 Cohort 127 BEACH AR & SG USA 02/2002–12/2003 Cohort 747 CABERNET122 CABERNET AR USA 02/2002–03/2004 Cohort 454 Katzen123 CASES-PMS SG USA 08/2003–10/2005 Cohort 1493 Criado124 CASES-PMS SG USA 08/2003–10/2005 Cohort 1493 Skelly125 CHICAGO-PHILADELPHIA AR USA 01/2002–10/2004 Cohort 97 Lanzer126 COSWIG AR & SG Germany 02/1999–05/2004 Cohort 143 Safian127 CREATE AR & SG USA NA Cohort 419 709 128 DENDERMONDE-BONHEIDEN AR & SG Belgium 01/2001–08/2005 Cohort Hauth129 DORTMUND AR Germany 01/2000–12/2000 Cohort 91 McDonnell130 DUBLIN AR & SG Australia NA Cohort 98 Mas131 EVA-3S AR & SG France 11/2000–09/2005 RCT 261 46 Hart 132 FARMINGTON AR USA 12/2003–12/2005 Cohort Rabe133 Park FRANKFURT-CVC AR Germany 03/2001–10/2003 Cohort 56 Rubartelli134 GENOA AR Italy NA Cohort 31 Halabi135 HAIFA AR & SG Israël 02/1998–08/2005 Cohort 169 136 HONG-KONG AR China 01/1997–01/2004 Cohort 70 Lin137 HOUSTON AR USA 02/2002–08/2006 Cohort 354 Kawaguchi138 KASHIHARA AR Japan 01/2002–03/2005 Cohort 38 Maleux139 LEUVEN-2 AR Belgium 01/2003–04/2005 Cohort 52 Wang (Continued) Touzé et al Table I. Risks After Carotid Angioplasty and Stenting e715 Continued Diabetes (%) Symptomatic Carotid Stenosis (%) 71 33 56 12 61 … 93 … Study N Attempted Procedures Mean Age, y Male (%) ALKK 729 70 BEIJING 478 68 Prior CEA/CAS (%) Prior CAD (%) Prior PAD (%) Contralateral Occlusion (%) 67 27 … … … 9 BERGAMO-VARESE 53 68 66 … 72 8 … … … BRUSSELS 53 72 75 40 34 … … … 8 CHICAGO 175 70 74 … 32 18 … … 4 CHONGQING 28 … 68 … 100 … … … … … CLEVELAND 193 … … … 36 … … … DUSSELDORF-1 55 … … … 56 5 … … … FUKUOKA 97 72 88 … 63 … 28 … … HOUSTON 200 71 95 50 25 … 72 … … Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 JERUSALEM 73 72 62 45 100 18 … … 12 KRAKOW-DUDEK 22 63 67 14 76 … 76 … … MARSEILLE 221 72 78 18 39 21 68 … … MILAN-VASCULAR SURGERY 113 70 81 27 49 12 35 … 14 MOSCOW-CARDIOVASC 22 60 80 10 35 5 80 … … OMAHA 17 65 88 41 47 29 … … … PISA-2 52 73 81 35 44 … 37 … … SAINT-LOUIS-NEURO 142 64 63 27 48 47 36 24 … SECURITY 305 75 64 31 21 21 … … 9 SEOUL-SAMSUNG-1 22 62 100 … 82 … … … 36 ST ANTONIUS 550 70 70 … 34 10 … … … ST ANTONIUS 509 71 71 … 33 10 … … … TUBINGEN 327 69 74 26 56 … 25 17 13 TUBINGEN-JENA 299 69 73 25 57 … 25 17 14 VIENNA 651 72 68 36 30 6 42 46 10 ARCHeR 581 70 67 38 24 35 66 … 17 ATLANTA 127 71 … … 28 34 … … … BEACH 747 71 64 33 25 38 22 … 18 CABERNET 454 73 65 33 24 21 63 … 19 CASES-PMS 1493 73 63 35 22 24 … … 12 CASES-PMS 1493 73 63 35 22 24 … … 12 CHICAGO-PHILADELPHIA 101 70 55 32 40 29 61 … … COSWIG 143 69 70 49 26 6 44 20 20 CREATE 419 74 61 34 17 24 14 … 10 DENDERMONDE-BONHEIDEN 709 72 59 23 43 3 … … … DORTMUND 94 67 75 46 70 11 67 58 … DUBLIN 110 69 69 … 74 … … … … EVA-3S 260 74 72 22 100 0 … 14 5 … FARMINGTON 46 69 50 41 22 … 76 … FRANKFURT-CVC 58 68 70 21 41 … 54 … 2 GENOA 31 71 77 19 36 … 77 … 3 HAIFA 185 71 63 40 … 50 76 31 … HONG-KONG 70 … 83 43 63 8 40 … 9 HOUSTON 380 71 96 57 24 … 76 … … KASHIHARA 38 70 82 … 100 … … … 21 LEUVEN-2 53 73 77 … 29 … … … … (Continued) e716 Table I. Publication Year Stroke December 2009 Continued Study Kypta140 LINZ SG Austria 12/1997–NA Cohort 718 Hofmann141 LINZ AR & SG Austria 12/1997–05/2005 Cohort 606 Alexandrescu142 MARCHE-EN-FAMENNE AR Belgium 02/2002–NA Cohort 26 Hill143 MAVERIC AR International 11/2001–12/2002 Cohort 51 Dalainas144 MILAN-SAN DONATO AR Italy 02/2001–10/2004 RCT 100 Mussack145 MUNCHEN-MUSSAK AR Germany NA Cohort 14 MUNCHEN-POPPERT AR Germany NA Cohort 41 NY, PRESBYTERIAN AR USA 01/1997–01/2005 Cohort 148 Iihara148 OSAKA AR & SG Japan 09/1998–08/2004 Cohort 92 Verzini149 PERUGIA AR & SG Italy 05/2001–04/2006 Cohort 570 Tinoco150 RIO DE JANEIRO AR Brazil 01/2004–01/2006 Cohort 40 Zindler151 ROTTERDAM AR Netherlands 01/1999–01/2004 Cohort 98 Marine152 SAINT-LOUIS-VASCULAR AR USA 09/2003–04/2005 Cohort 93 Carvalho153 SAO PAULO-SAO JOACHIM AR & SG Brazil 03/2000–06/2004 Cohort 113 146 Poppert Chaer147 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 154 Country Accrual Period Design SEOUL-YONGON DONG AR Korea 02/2004–12/2005 Cohort 72 Gonzalez-Marcos155 SEVILLE SG Spain NA Cohort 607 Setacci156 SIENNA-COTIGNOLA AR & SG Italy 12/2000–09/2005 Cohort 1053 Setacci157 SIENNA-RAVENNA SG Italy 12/2000–03/2006 Cohort 1729 Ringleb158 SPACE AR & SG Europe 03/2001–02/2006 RCT 567 Wu159 TAIWAN-KAOSIUNG AR China 03/2005–07/2005 Cohort 13 Kassaian160 TEHERAN AR Iran 12/2003–10/2004 Cohort 30 Ling161 TESCAS-C AR China NA RCT 166 Kwon 162 Gupta 2007 Analysis N Patients First Author TRIVANDRUM AR & SG India 01/1995–06/2006 Cohort 47 Asakura163 TSU CITY AR & SG Japan 2002–2004 Cohort 57 Reiter164 VIENNA AR & SG Austria 01/1997–06/2005 Cohort 698 Mehta165 ALKK SG Germany 07/1996–03/2006 Cohort 3070 Zahn166 ALKK AR & SG Germany 07/1996–12/2005 Cohort 2878 Matas167 BARCELONA-HEBRON AR Spain 01/2005–06/2006 Cohort 62 Faggioli168 BOLOGNA AR & SG Italy 01/2005–03/2007 Cohort 298 Bosiers169 BOSIERS-MULTI SG Italy-Belgium NA Cohort 3179 170 Ascher BROOKLYN AR USA NA Cohort 34 Parodi171 BUENOS-AERES AR Argentina-USA 09/1999–12/2003 Cohort 200 Gray172 CAPTURE AR USA 10/2004–03/2006 Cohort 3500 Gray173 CAPTURE SG USA 10/2004–03/2006 Cohort 3500 174 CAPTURE SG USA 10/2004–03/2006 Cohort 3500 Katzen175 CASES-PMS SG USA 08/2003–10/2005 Cohort 1493 Gurm176 CLEVELAND SG USA 02/1998–08/2005 Cohort 833 Rajagopal177 CLEVELAND SG USA 02/1998–08/2005 Cohort 915 Fairman 178 Huppert DARMSTADT AR Germany NA Cohort 120 Iyer179 DENDERMONDE-SIENACOTTIGNOLA SG Belgium-Italy 01/1997–01/2006 Cohort 3260 Pinter180 DUSSELDORF-2 AR Germany 03/2006–12/2006 Cohort 20 EXACT181 EXACT AR USA NA Cohort 1500 CAPTURE-2181 CAPTURE-2 AR USA NA Cohort 597 Wijtenburg182 HAINE-ST PAUL AR Belgium 2003–2005 Cohort Grunwald183 HOMBURG AR & SG Germany NA Cohort 90 Younis184 HOUSTON ST LUKE AR & SG USA 07/1995–09/2004 Cohort 363 (Continued) Table I. Touzé et al Risks After Carotid Angioplasty and Stenting Study N Attempted Procedures Mean Age, y Male (%) Diabetes (%) LINZ 742 … … LINZ 628 72 65 e717 Continued Symptomatic Carotid Stenosis (%) Prior CEA/CAS (%) Prior CAD (%) Prior PAD (%) Contralateral Occlusion (%) … 35 … … … … 29 35 8 45 17 10 31 MARCHE-EN-FAMENNE 29 74 65 42 88 … … … MAVERIC 52 69 84 24 … 29 … … 28 MILAN-SAN DONATO 100 65 60 17 23 … 50 35 … MUNCHEN-MUSSAK 14 70 79 … 64 … … … … … Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 MUNCHEN-POPPERT 41 71 66 … 44 … … … NY, PRESBYTERIAN 148 75 61 26 … 18 64 … 10 OSAKA 92 71 90 40 36 … 38 45 … PERUGIA 627 72 70 31 22 15 … 19 8 RIO DE JANEIRO 40 67 55 20 30 … 25 … 0 ROTTERDAM 98 68 89 … 100 … … … … SAINT-LOUIS-VASCULAR 93 70 63 30 0 28 58 38 … SAO PAULO-SAO JOACHIM 130 74 74 44 45 15 70 … 12 SEOUL-YONGON DONG 78 70 81 … 47 … 25 … 10 SEVILLE 607 … 78 … 100 … … … … SIENNA-COTIGNOLA 1222 72 86 … 65 … … … 9 SIENNA-RAVENNA 1981 … … 29 … … … … … SPACE 567 68 72 26 100 0 21 … 7 TAIWAN-KAOSIUNG 13 72 92 39 100 … … … … TEHERAN 30 66 60 47 26 … 100 … … TESCAS-C 166 63 … … … … … … … TRIVANDRUM 49 61 74 … 96 … 45 … 6 TSU CITY 60 69 92 … 50 … … … … VIENNA 698 73 69 36 30 … … … 10 ALKK 3070 71 73 32 49 … 67 25 … ALKK 2878 71 73 32 49 9 69 26 … BARCELONA-HEBRON 62 77 84 34 39 3 61 … 11 BOLOGNA 298 75 63 … … … … … 6 BOSIERS-MULTI 3179 72 67 26 41 6 … … … BROOKLYN 35 73 71 40 34 37 51 … … BUENOS-AERES 200 70 73 19 52 9 27 … 6 CAPTURE 3545 73 61 35 14 … 67 36 8 CAPTURE 3545 73 61 35 14 … 67 36 8 CAPTURE 3545 73 61 35 14 … 67 36 8 CASES-PMS 1493 73 63 35 22 24 … … 12 CLEVELAND 833 71 65 37 38 23 79 36 14 CLEVELAND 915 … … … … … … … … DARMSTADT 120 … … … 67 … … … … DENDERMONDE-SIENA-COTTIGNOLA 3260 … 67 26 41 7 … … … … DUSSELDORF-2 20 72 70 30 35 … 45 … EXACT 1500 … … … 10 … … … … CAPTURE-2 597 … … … 12 … … … … HAINE-ST PAUL 19 64 67 22 28 22 28 … … HOMBURG 90 66 66 … 17 … … … … HOUSTON ST LUKE 399 71 68 14 33 9 89 87 … (Continued) e718 Table I. Publication Year Stroke December 2009 Continued First Author Analysis Country Accrual Period Design N Patients Lojik185 HRADEK AR Czech Republic 09/2001– 08/2006 Cohort 204 Aydiner186 ISTAMBUL AR & SG Turkey 03/2002–12/2004 Cohort 26 KAOHSIUNG AR Taiwan NA Cohort 77 LINZ AR & SG Austria 08/1999–06/2006 Cohort 77 Timler189 LODZ AR Poland 01/2004–08/2006 Cohort 27 Brightwell190 LONDON-SURGERY AR UK 04/2005–06/2006 Cohort 24 Montorsi191 MILAN AR Italy NA Cohort 306 Spes192 MUNCHEN-STADTISCHES AR Germany 11/1999–05/2006 Cohort 371 Henry193 NANCY-3 AR France NA Cohort 34 Lam194 NY, PRESBYTERIAN SG USA 02/2003–08/2005 Cohort 133 Tsai195 ORANGE AR USA NA Cohort 105 Kawarada196 OSAKA-TOKUSHUKAI-2 AR Japan 12/1996–05/2006 Cohort 51 Veselka197 PRAGUE AR Czech Republic 09/2005–01/2007 Cohort 83 Folmar198 RALEIGH AR USA 01/2005–08/2006 Cohort 42 Fanelli199 ROMA-FANELLI AR Italy 02/2000–NA Cohort 230 Gandini200 ROMA-GANDINI AR Italy 04/1999–NA Cohort 612 Gossetti201 ROMA-SAPIENZA AR Italy 01/2005–01/2006 Cohort 50 Kadkhodayan202 SAINT-LOUIS-NEURO SG USA 03/1996–03/2005 Cohort 153 Wu 187 Topakian188 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 2008 Study Rapp203 SAN FRANCISCO-RAPP AR USA 02/2005–08/2006 Cohort 48 Sanchez204 SAO PAULO-DO CORACAO SG Brazil 01/2002–01/2005 Cohort 230 Suk205 SEOUL-SAMSUNG-2 AR & SG Korea 05/2002–10/2005 Cohort 70 Maynar206 TENERIFE AR Spain 06/2002–10/2004 Cohort 87 Saratzis207 THESSALONIKI AR Greece 05/2003–04/2005 Cohort 232 Criado208 TOLEDO AR Spain 03/2003–07/2005 Cohort 97 Hoffman209 BACASS AR Switzerland 11/1998–02/2002 RCT 10 Silvestro210 BRESCIA AR & SG Italy 2000–2005 Cohort 138 CAPTURE-2181,211 CAPTURE 2 AR & SG USA NA Cohort 2070 Uflacker212 CHARLESTON AR USA 01/2005–09/2007 Cohort 100 Cremonesi213 CREMONESI (MULTI) AR Italy-Germany 10/2006–03/2007 Cohort 124 Priban214 CSEKE BUDEJOVICE AR Czech Republic 01/2003–10/2006 Cohort 90 86 215 Ozturk IZMIR AR Turkey 06/2003–09/2007 Cohort Uddin216 KARACHI AR Pakistan 09/2002–12/2005 Cohort 17 Buszman217 KATOWICE AR & SG Poland 06/1997–03/2005 Cohort 223 Yuo218 LEBANON AR USA 10/2000–08/2006 Cohort 179 219 LIMOGES AR France 10/2003–01/2006 Cohort 50 Bianchi220 LOMA LINDA AR USA 10/2003–02/2006 Cohort 50 Jackson221 PHILADELPHIA AR & SG USA 07/2003–12/2005 Cohort 170 Barbato222 PITTSBURGH SG USA 12/2003–01/2006 Cohort 35 223 Ghorab Sayeed PITTSBURGH SG USA 06/1996–06/2005 Cohort 421 Theiss224 PROCAS SG Europe 07/1999–06/2005 Cohort 5341 Sanchez225 SAO PAULO-DO CORACAO AR Brazil 01/2002–01/2005 Cohort 230 Martinez-Fernandez226 SEVILLE AR Spain 1992 Cohort 359 227 SHIRAZ AR Iran 04/2005–03/2003 Cohort 42 Stingele228 SPACE SG Europe 03/2001–02/2006 RCT 567 Groschel229 TUBINGEN-GOTTINGEN SG Germany 01/2000–03/2007 Cohort 320 Kastrup230 TUBINGEN-GOTTINGEN SG Germany 04/1999–12/2006 Cohort 243 UDINE AR & SG Italy NA Cohort Kojuri 231 Piccoli 415 (Continued) Table I. Touzé et al Risks After Carotid Angioplasty and Stenting Study N Attempted Procedures Mean Age, y Male (%) Diabetes (%) Symptomatic Carotid Stenosis (%) HRADEK 212 65 67 … ISTAMBUL 28 70 72 42 KAOHSIUNG 83 … … LINZ 77 67 68 e719 Continued Prior CEA/CAS (%) Prior CAD (%) Prior PAD (%) Contralateral Occlusion (%) 47 12 … … 63 50 … 73 11 8 … … … … … … 34 100 0 … … … LODZ 27 … … … 96 … … … … LONDON-SURGERY 24 63 83 29 42 … 79 … … MILAN 306 70 … … 27 … … … … MUNCHEN-STADTISCHES 371 71 71 36 31 … 71 26 … NANCY-3 35 71 68 41 29 … … … … NY, PRESBYTERIAN 135 65 65 … 49 22 … … 20 … Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 ORANGE 105 … … … … … … … OSAKA-TOKUSHUKAI-2 53 70 78 56 54 … 66 32 10 PRAGUE 100 68 54 47 … … 69 … … RALEIGH 42 71 62 45 21 2 76 31 2 ROMA-FANELLI 230 … … … … 21 … … … ROMA-GANDINI 665 … … … 52 … … … … ROMA-SAPIENZA 50 … … … … … … … … SAINT-LOUIS-NEURO 153 66 63 27 50 47 36 20 … SAN FRANCISCO-RAPP 54 71 100 … 48 … … … … SAO PAULO-DO CORACAO 268 68 … … 74 7 … … … SEOUL-SAMSUNG-2 71 67 84 … 66 … … … … TENERIFE 100 71 82 25 58 … 59 … … THESSALONIKI 232 76 65 33 71 4 33 28 … TOLEDO 103 72 80 21 36 … 34 … … BACASS 10 69 80 30 100 … 20 … 10 BRESCIA 154 72 63 33 46 … 55 … 6 CAPTURE 2 2070 73 61 … … … … … … CHARLESTON 100 70 80 … 72 36 … … … CREMONESI (MULTI) 124 72 71 34 24 … … … … CSEKE BUDEJOVICE 90 71 69 31 70 12 44 … … IZMIR 87 68 78 30 81 … … … … KARACHI 18 … 82 … 88 … … … … KATOWICE 256 65 68 26 38 … 93 25 … LEBANON 196 72 79 38 39 21 … … … LIMOGES 50 70 76 26 62 … … … … LOMA LINDA 50 70 96 44 28 12 46 20 … PHILADELPHIA 215 71 53 … 41 29 … … 17 PITTSBURGH 36 76 64 28 17 … 61 … … PITTSBURGH 429 72 62 … 35 32 … … 14 PROCAS 5341 70 71 … 55 8 … … … SAO PAULO-DO CORACAO 268 68 … … 74 7 … … … SEVILLE 359 63 78 40 87 … … 29 … SHIRAZ 42 67 71 24 … … 100 … … SPACE 567 68 72 26 100 0 21 … 7 TUBINGEN-GOTTINGEN 320 69 70 28 100 … 28 … … TUBINGEN-GOTTINGEN 243 68 75 28 55 … 22 … 17 UDINE 415 … … … 60 … … … … (Continued) e720 Table I. Publication Year Stroke December 2009 Continued Design N Patients 01/2002– 07/2007 Cohort 179 07/1999–03/2003 Cohort 56 Cohort 99 First Author Study Analysis Country De Gregorio232 ZARAGOZA AR & SG Spain Kimiagar233 ZERIFIN AR Israël ZURICH AR Switzerland 07/2003–11/2006 234 Roffi Accrual Period Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 AR, Absolute Risk; SG, Subgroups; NA, Not Available; RCT, Randomised Clinical Trial. 1. Munari LM, Belloni G, Perretti A, Ghia HF, Moschini L, Porta M. Carotid percutaneous angioplasty. Neurol Res 1992;14:156 – 8. 2. Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology 1996;201:627–36. 3. Eckert B, Zanella FE, Thie A, Steinmetz J, Zeumer H. Angioplasty of the internal carotid artery: Results, complications and follow-up in 61 cases. Cerebrovascular Diseases 1996;6:97–105. 4. Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42– 62. 5. Criado FJ, Wellons E, Clark NS. Evolving indications for and early results of carotid artery stenting. Am J Surg 1997;174:111– 4. 6. Crawley F, Clifton A, Buckenham T, Loosemore T, Taylor RS, Brown MM. Comparison of hemodynamic cerebral ischemia and microembolic signals detected during carotid endarterectomy and carotid angioplasty. Stroke 1997;28:2460 – 4. 7. Vozzi CR, Rodriguez AO, Paolantonio D, Smith JA, Wholey MH. Extracranial carotid angioplasty and stenting. Initial results and short-term follow-up. Tex Heart Inst J 1997;24:167–72. 8. Mendelsohn FO, Weissman NJ, Lederman RJ, et al. Acute hemodynamic changes during carotid artery stenting. Am J Cardiol 1998;82:1077– 81. 9. Naylor AR, Bolia A, Abbott RJ, et al. Randomized study of carotid angioplasty and stenting vs carotid endarterectomy: a stopped trial. J Vasc Surg 1998;28:326 –34. 10. Mathur A, Roubin GS, Iyer SS, et al. Predictors of stroke complicating carotid artery stenting. Circulation 1998;97:1239 – 45. 11. Teitelbaum GP, Lefkowitz MA, Giannotta SL. Carotid angioplasty and stenting in high-risk patients. Surgical Neurology 1998;50:300 –11. 12. Henry M, Amor M, Masson I, et al. Angioplasty and stenting of the extracranial carotid arteries. J Endovasc Surg 1998;5:293–304. 13. Gross CM, Kramer J, Uhlich F, et al. Treatment of carotid artery stenosis by elective stent placement instead of carotid endarterectomy in patients with severe coronary artery disease. Thromb Haemost 1999;82 Suppl 1:176 – 80. 14. Sievert H, Pfeil W, Bosenberg I, Spies H, Theis R, Beykirch KF. 关Primary stent implantation in the internal carotid artery兴. Dtsch Med Wochenschr 1999;124:1262– 6. 15. Arakawa Y, Ishii A, Ueno Y, et al. 关Self-expanding stent (Wallstent) supported angioplasty for carotid artery stenosis兴. No Shinkei Geka 1999;27:817–23. 16. Matushita K, Akai F, Yokoi Y, Aoki A, Taneda M. Initial and long-term results and peri-procedural complication in 35 extracranial stentings. Intervent Neuroradiol 1999;5:55– 60. 17. Reifart N. 关Carotid percutaneous transluminal angioplasty in cardiological patients with increased surgical risk兴. Z Kardiol 2000;89 Suppl 8:27–31. 18. Parodi JC, La MR, Ferreira LM, et al. Initial evaluation of carotid angioplasty and stenting with three different cerebral protection devices. J Vasc Surg 2000;32:1127–36. 19. Qureshi AI, Luft AR, Janardhan V, et al. Identification of patients at risk for periprocedural neurological deficits associated with carotid angioplasty and stenting. Stroke 2000;31:376 – 82. 20. Jacksch R, Schiele TM, Knobloch W, Niehues R, Hauser ER, Massalha K. 关Carotid stenting with the new slotted tube stent—prospective multicenter study. Essen experiences兴. Z Kardiol 2000;89 Suppl 8:40 – 6. 21. Griewing B, Brassel F, von SU, Al Ahmar MT, Kessler C. Carotid artery stenting in patients at surgical high risk: clinical and ultrasound findings. Cerebrovasc Dis 2000;10:44 – 8. 22. Gupta A, Bhatia A, Ahuja A, Shalev Y, Bajwa T. Carotid stenting in patients older than 65 years with inoperable carotid artery disease: a single-center experience. Catheter Cardiovasc Interv 2000;50:1– 8. 23. Kaul U, Singh B, Bajaj R, et al. Elective stenting of extracranial carotid arteries. J Assoc Physicians India 2000;48:196 –200. 24. Link J, Manke C, Rosin L, et al. Carotid endarteriectomy and carotic stenting. Pilot study of a prospective, randomized and controlled comparison. Radiologe 2000;40:813–20. 25. Malek AM, Higashida RT, Phatouros CC, et al. Stent angioplasty for cervical carotid artery stenosis in high-risk symptomatic NASCET-ineligible patients. Stroke 2000;31:3029 –33. 26. Yoon YS, Shim WH, Kim SM, Park KJ, Cho SY. Carotid artery stenting in patients with symptomatic coronary artery disease. Yonsei Med J 2000;41:89 –97. 27. Shawl F, Kadro W, Domanski MJ, et al. Safety and efficacy of elective carotid artery stenting in high-risk patients. J Am Coll Cardiol 2000;35:1721– 8. 28. Dangas G, Laird JR Jr, Satler LF, et al. Postprocedural hypotension after carotid artery stent placement: predictors and short- and long-term clinical outcomes. Radiology 2000;215:677– 83. 29. Endovascular vs surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357:1729 –37. 30. Dietz A, Berkefeld J, Theron JG, et al. Endovascular treatment of symptomatic carotid stenosis using stent placement: long-term follow-up of patients with a balanced surgical risk/benefit ratio. Stroke 2001;32:1855–9. 31. Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting vs carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol 2001;38:1589 –95. 32. Roubin GS, New G, Iyer SS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation 2001;103:532–7. 33. Pappada G, Marina R, Fiori L, et al. Stenting of atherosclerotic stenoses of the extracranial carotid artery. Acta Neurochir (Wien) 2001;143:1005–11. 34. Baudier JF, Licht PB, Roder O, Andersen PE. Endovascular treatment of severe symptomatic stenosis of the internal carotid artery: early and late outcome. Eur J Vasc Endovasc Surg 2001;22:205–10. 35. Kirsch EC, Khangure MS, Van Schie GP, Lawrence-Brown MM, Stewart-Wynne EG, McAuliffe W. Carotid arterial stent placement: Results and follow-up in 53 patients. Radiology 2001;220:737– 44. (Continued) Table I. Touzé et al Risks After Carotid Angioplasty and Stenting Study N Attempted Procedures Mean Age, y Male (%) Diabetes (%) ZARAGOZA 203 66 86 ZERIFIN 60 69 80 ZURICH 100 68 82 e721 Continued Symptomatic Carotid Stenosis (%) Prior CEA/CAS (%) Prior CAD (%) Prior PAD (%) Contralateral Occlusion (%) … 94 … … … … 50 100 20 77 39 16 24 25 8 … … 11 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 36. Orlandi G, Fanucchi S, Fioretti C, et al. Characteristics of cerebral microembolism during carotid stenting and angioplasty alone. Arch Neurol 2001;58:1410 –3. 37. Ahmadi R, Willfort A, Lang W, et al. Carotid artery stenting: effect of learning curve and intermediate-term morphological outcome. J Endovasc Ther 2001;8:539 – 46. 38. Alberts MJ. Results of the multicenter prospective randomized trail of carotid artery stenting vs carotid endarterectomy. Stroke 2001;32:325. 39. Criado FJ, Lingelbach JM, Ledesma DF, Lucas PR. Carotid artery stenting in a vascular surgery practice. J Vasc Surg 2002;35:430 – 4. 40. Guimaraens L, Sola MT, Matali A, et al. Carotid angioplasty with cerebral protection and stenting: report of 164 patients (194 carotid percutaneous transluminal angioplasties). Cerebrovasc Dis 2002;13:114 –9. 41. Wang D, Sheng A, Gong T, et al. 关Initial application of brain protection device in dilatation and stenting of carotid and vertebral artery stenosis兴. Zhonghua Wai Ke Za Zhi 2002;40:893–5. 42. Bonaldi G. Angioplasty and stenting of the cervical carotid bifurcation: report of a 4-year series. Neuroradiology 2002;44:164 –74. 43. Qureshi AI, Suri MF, Ali Z, et al. Carotid angioplasty and stent placement: a prospective analysis of perioperative complications and impact of intravenously administered abciximab. Neurosurgery 2002;50:466 –73. 44. Castriota F, Cremonesi A, Manetti R, et al. Impact of cerebral protection devices on early outcome of carotid stenting. J Endovasc Ther 2002;9:786 –92. 45. Koch C, Kucinski T, Eckert B, Wittkugel O, Rother J, Zeumer H. 关Endovascular therapy of high-degree stenoses of the neck vessels-stent-supported percutaneous angioplasty of the carotid artery without cerebral protection兴. Rofo 2002;174:1506 –10. 46. Milosevic Z, Zvan B, Zaletel M, Surlan M. Carotid angioplasty with cerebral protection. Acta Clin Croat 2002;41:15–21. 47. Kaposzta Z, Clifton A, Molloy J, Martin JF, Markus HS. S-nitrosoglutathione reduces asymptomatic embolization after carotid angioplasty. Circulation 2002;106:3057– 62. 48. Stankovic G, Liistro F, Moshiri S, et al. Carotid artery stenting in the first 100 consecutive patients: results and follow up. Heart 2002;88:381– 6. 49. Grego F, Frigatti P, Amista P et al. Prospective comparative study of two cerebral protection devices in carotid angioplasty and stenting. J Cardiovasc Surg (Torino) 2002;43:391–7. 50. Gray WA, White HJ Jr, Barrett DM, Chandran G, Turner R, Reisman M. Carotid stenting and endarterectomy: a clinical and cost comparison of revascularization strategies. Stroke 2002;33:1063–70. 51. Madyoon H, Braunstein E, Callcott F, et al. Unprotected carotid artery stenting compared to carotid endarterectomy in a community setting. J Endovasc Ther 2002;9:803–9. 52. Kao HL, Lin LY, Lu CJ, Jeng JS, Yip PK, Lee YT. Long-term results of elective stenting for severe carotid artery stenosis in Taiwan. Cardiology 2002;97:89 –93. 53. Ahmadi R, Schillinger M, Lang W, Mlekusch W, Sabeti S, Minar E. Carotid artery stenting in older patients: is age a risk factor for poor outcome? J Endovasc Ther 2002;9:559 – 65. 54. Moller-Hartmann W, Mull M, Krings T, Klotzsch C, Gunther RW, Thron A. Carotid Artery Stenting with and without Cerebral Protection: Report of 43 Procedures. Rivista di Neuroradiologia 2003;16:1327–29. 55. McKinlay S, White RA, Diethrich EB, et al. Carotid Revascularization Using Endarterectomy or Stenting Systems (CARESS): Phase I Clinical Trial. J Endovasc Ther 2003;10:1021–30. 56. Becquemin JP, Ben El KH, Desgranges P, Kobeiter H. Carotid stenting vs carotid surgery: a prospective cohort study. J Endovasc Ther 2003;10:687–94. 57. Gable DR, Bergamini T, Garrett WV, et al. Intermediate follow-up of carotid artery stent placement. Am J Surg 2003;185:183–7. 58. Rath PC, Lakshmi G, Agarwala M, et al. Carotid artery stenting with filter protection. Indian Heart J 2003;55:241– 4. 59. Maleux G, Bernaerts P, Thijs V, et al. Extracranial carotid artery stenting in surgically high-risk patients using the Carotid Wallstent endoprosthesis: midterm clinical and ultrasound follow-up results. Cardiovasc Intervent Radiol 2003;26:340 – 6. 60. Cernetti C, Reimers B, Picciolo A, et al. Carotid artery stenting with cerebral protection in 100 consecutive patients: immediate and two-year follow-up results. Ital Heart J 2003;4:695–700. 61. Shevchenko I, Krotovskii GS, Shcherbiuk AN, et al. 关Comparative assessment of the results of open carotid endarterectomy and carotid aftery stenting兴. Khirurgiia (Mosk) 2003;12– 8. 62. Koshimae N, Morimoto T, Nagata K. Stenting of extracranial carotid artery stenosis. Intervent Neuroradiol 2003;9 (suppl 1):137– 41. 63. Wholey MH, Wholey MH, Eles G, et al. Evaluation of glycoprotein IIb/IIIa inhibitors in carotid angioplasty and stenting. J Endovasc Ther 2003;10:33– 41. 64. Piske RL, Ferreira MS, Campos CMS, et al. The cerebral protection technique in angioplasty plus stenting of carotid: An effective procedure against cerebral embolism. Arquivos de Neuro-Psiquiatria 2003;61:296 –302. 65. Costa JR Jr, Cano MN, Oliveira DC, et al. Percutaneous implantation of endoprostheses in the carotid arteries. Arq Bras Cardiol 2003;80:77– 6. 66. Tan KT, Cleveland TJ, Berczi V, McKevitt FM, Venables GS, Gaines PA. Timing and frequency of complications after carotid artery stenting: what is the optimal period of observation? J Vasc Surg 2003;38:236 – 43. 68. Pucillo AL, Mateo RB, Aronow WS. Effect of carotid angioplasty-stenting on short-term mortality and stroke. Heart Dis 2003;5:378 –9. 69. Causin F, Castellan L, Iannucci G, et al. Perioperative and immediate outcome of endovascular treatment of extracranial carotid artery steno-occlusive disease. Rivista di Neuroradiologia 2003;16:93–9. 70. Dabrowski M, Bielecki D, Golebiewski P, Kwiecinski H. Percutaneous internal carotid artery angioplasty with stenting: early and long-term results. Kardiol Pol 2003;58:469 – 80. 71. Zahn R, Mark B, Niedermaier N, et al. Embolic protection devices for carotid artery stenting: better results than stenting without protection? Eur Heart J 2004;25:1550 – 8. 72. Garcia-Sanchez S, Millan-Torne M, Capellades-Font J, Muchart J, Callejas P, Vila-Moriente N. 关Ischemic brain lesions following carotid revascularisation procedures: a comparative study using diffusion-weighted magnetic resonance imaging兴. Rev Neurol 2004;38:1013–7. (Continued) e722 Stroke December 2009 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 73. Sganzerla P, Bocciarelli M, Savasta C, et al. The treatment of carotid artery bifurcation stenoses with systematic stenting: experience of first 100 consecutive cardiological procedures. J Invasive Cardiol 2004;16:592–5. 74. Linfante I, Hirsch JA, Selim M, Schlaug G, Caplan LR, Reddy AS. Safety of latest-generation self-expanding stents in patients with NASCET-ineligible severe symptomatic extracranial internal carotid artery stenosis. Arch Neurol 2004;61:39 – 43. 75. Clark DJ, Lessio S, O’Donoghue M, Schainfeld R, Rosenfield K. Safety and utility of intravascular ultrasound-guided carotid artery stenting. Catheter Cardiovasc Interv 2004;63:355– 62. 76. Kobayashi E, Uchino Y, Ono J, Yamaura A. Treatment outcome of carotid stenting and CEA in the same period. Intervent Neuroradiol 2004;10:93– 6. 77. Hobson Ii RW, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-Day stroke and death rates in the CREST lead-in phase. J Vasc Surg 2004;40:1106 –11. 78. Biasi GM, Froio A, Diethrich EB, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004;110:756 – 62. 79. Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting vs carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 2004;54:318 –24. 80. Pieniazek P, Kablak-Ziembicka A, Przewlocki T, et al. Carotid artery stenting with proximal or distal brain protection: Early outcome. Kardiologia Polska 2004;61:48 –56. 81. Sadato A, Satow T, Ishii A, Ohta T, Hashimoto N. Use of a large angioplasty balloon for predilation is a risk factor for embolic complications in protected carotid stenting. Neurol Med Chir (Tokyo) 2004;44:337– 42. 82. Powell RJ, Schermerhorn M, Nolan B, et al. Early results of carotid stent placement for treatment of extracranial carotid bifurcation occlusive disease. J Vasc Surg 2004;39:1193–9. 83. Schmidt A, Diederich KW, Scheinert S, et al. Effect of two different neuroprotection systems on microembolization during carotid artery stenting. J Am Coll Cardiol 2004;44:1966 –9. 84. Henry M, Polydorou A, Henry I, Polydorou A, Hugel M. Carotid angioplasty under cerebral protection with the PercuSurge GuardWire System. Catheter Cardiovasc Interv 2004;61:293–305. 85. Choi HM, Hobson RW, Goldstein J, et al. Technical challenges in a program of carotid artery stenting. J Vasc Surg 2004;40:746 –51. 86. Reimers B, Schluter M, Castriota F, et al. Routine use of cerebral protection during carotid artery stenting: Results of a multicenter registry of 753 patients. American Journal of Medicine 2004;116:217–22. 87. Kihara EN, Andrioli MS, Zukerman E, et al. Endovascular treatment of carotid artery stenosis: retrospective study of 79 patients treated with stenting and angioplasty with and without cerebral protection devices. Arq Neuropsiquiatr 2004;62:1012–5. 88. Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting vs endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–501. 89. McKevitt FM, Macdonald S, Venables GS, Cleveland TJ, Gaines PA. Complications following carotid angioplasty and carotid stenting in patients with symptomatic carotid artery disease. Cerebrovascular Diseases 2004;17:332– 8. 90. Chang DW, Schubart PJ, Veith FJ, Zarins CK. A new approach to carotid angioplasty and stenting with transcervical occlusion and protective shunting: Why it may be a better carotid artery intervention. J Vasc Surg 2004;39:994 –1002. 91. Sztriha LK, Voros E, Sas K, et al. Favorable early outcome of carotid artery stenting without protection devices. Stroke 2004;35:2862– 6. 92. Sabeti S, Schillinger M, Mlekusch W, et al. Contralateral high-grade carotid artery stenosis or occlusion is not associated with increased risk for poor neurologic outcome after elective carotid stent placement. Radiology 2004;230:70 – 6. 93. Terada T, Tsuura M, Matsumoto H, et al. Technique and clinical results of carotid stenting under distal protection. Intervent Neuroradiol 2004;10:31–3. 94. Zahn R, Ischinger T, Mark B, et al. Embolic protection devices for carotid artery stenting: is there a difference between filter and distal occlusive devices? J Am Coll Cardiol 2005;45:1769 –74. 95. Li SM, Li D, Ling F, Miao ZR, Wang ML. Carotid artery stenting: Experience of a single institute in China. Intervent Neuroradiol 2005;11:205–212. 96. Bonaldi G, Aiazzi L, Baruzzi F, et al. Angioplasty and stenting of the cervical carotid bifurcation under filter protection: a prospective study in a series of 53 patients. J Neuroradiol 2005;32:109 –17. 97. Hammer FD, Lacroix V, Duprez T, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg 2005;42:847–53. 98. Eskandari MK, Longo GM, Matsumura JS, et al. Carotid stenting done exclusively by vascular surgeons: first 175 cases. Ann Surg 2005;242:431– 6. 99. Chen KN, Chi LX, Shi SG, Fan WH. Endovascular stenting of carotid stenosis under cerebral protection device: Short-term follow-up of the incidence of cerebral ischemia in 28 patients. Chinese Journal of Clinical Rehabilitation 2005;9:14 –5. 100. Yen MH, Lee DS, Kapadia S, et al. Symptomatic patients have similar outcomes compared with asymptomatic patients after carotid artery stenting with emboli protection. Am J Cardiol 2005;95:297–300. 101. Lin JC, Kolvenbach RR, Pinter L. Protected carotid artery stenting and angioplasty via transfemoral vs transcervical approaches. Vasc Endovascular Surg 2005;39:499 –503. 102. Nagata S, Kazekawa K, Aikawa H, et al. Hemodynamic stability under general anesthesia in carotid artery stenting. Radiat Med 2005;23:427–31. 103. Lin PH, Bush RL, Peden EK, et al. Carotid artery stenting with neuroprotection: assessing the learning curve and treatment outcome. Am J Surg 2005;190:850 –7. 104. Cohen JE, Umansky F, Rajz G, Ben-Hur T. Protected stent-assisted carotid angioplasty in symptomatic high-risk NASCET-ineligible patients. Neurol Res 2005;27 Suppl 1:S59 –S63. 105. Dudek D, Bartus S, Rakowski T, Zmudka K, Dubiel JS. MO.MA - A new cerebral stroke protection system during carotid artery stenting. Kardiologia Polska 2005;62:565–70. 106. Bergeron P, Roux M, Khanoyan P, Douillez V, Bras J, Gay J. Long-term results of carotid stenting are competitive with surgery. J Vasc Surg 2005;41:213–21. 107. Emanuelli G, Marina R, Bizzi U, Perona F, Pecora N. Endovascular therapy vs surgery in carotid stenosis: Personal experience. Italian Journal of Vascular and Endovascular Surgery 2005;12:47–54. 108. Bokeriia LA, Alekian BG, Buziashvili I, et al. 关Short- and long-term effects of internal carotid arteries stenting in high-risk patients兴. Zh Nevrol Psikhiatr Im S S Korsakova 2005;105:12– 8. 109. Pipinos II, Johanning JM, Pham CN, Soundararajan K, Lynch TG. Transcervical approach with protective flow reversal for carotid angioplasty and stenting. J Endovasc Ther 2005;12:446 –53. 110. Cosottini M, Michelassi MC, Puglioli M, et al. Silent cerebral ischemia detected with diffusion-weighted imaging in patients treated with protected and unprotected carotid artery stenting. Stroke 2005;36:2389 –93. (Continued) Touzé et al Risks After Carotid Angioplasty and Stenting e723 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 111. Kadkhodayan Y, Derdeyn CP, Cross DT, III, Moran CJ. Procedure complications of carotid angioplasty and stent placement without cerebral protection devices. Neurosurg Focus 2005;18:e1– e7. 112. SECURITY: Multicenter Evaluation of Carotid Stenting with a Distal Protection Filter. 2005. Available at: www.fda.gov/cdrh/pdf4/P040038b.pdf. 113. Roh HG, Byun HS, Ryoo JW, et al. Prospective analysis of cerebral infarction after carotid endarterectomy and carotid artery stent placement by using diffusion-weighted imaging. AJNR Am J Neuroradiol 2005;26:376 – 84. 114. Ackerstaff RG, Suttorp MJ, Van Den Berg JC, et al. Prediction of early cerebral outcome by transcranial Doppler monitoring in carotid bifurcation angioplasty and stenting. J Vasc Surg 2005;41:618 –24. 115. Vos JA, Van Den Berg JC, Ernst SM, et al. Carotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology 2005;234:493–9. 116. Groschel K, Ernemann U, Riecker A, Schmidt F, Terborg C, Kastrup A. Incidence and risk factors for medical complications after carotid artery stenting. J Vasc Surg 2005;42:1101– 6. 117. Kastrup A, Groschel K, Schulz JB, Nagele T, Ernemann U. Clinical predictors of transient ischemic attack, stroke, or death within 30 days of carotid angioplasty and stenting. Stroke 2005;36:787–91. 118. Boltuch J, Sabeti S, Amighi J, et al. Procedure-related complications and early neurological adverse events of unprotected and protected carotid stenting: temporal trends in a consecutive patient series. J Endovasc Ther 2005;12:538 – 47. 119. Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg 2006;44:258 – 68. 120. Kasirajan K, Milner R, Dodson TF, Smith RB, Salam A, Chaikof E. Neuroprotection during carotid angioplasty and stenting: Comparison of no protection, occlusion, or filters. International Journal of Angiology 2006;15:20 –24. 121. White CJ, Iyer SS, Hopkins LN, Katzen BT, Russell ME. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day results. Catheter Cardiovasc Interv 2006;67:503–12. 122. Carotid Artery Revascularization Using the Boston Scientific FilterWire EX/EZ and the EndoTex NexStent. 2006. www.fda.gov/cdrh/pdf5/p050025b.pdf. 123. Katzen B, Yadav J, Criado FJ, Strickman N, Mishkel G, Massop DW, Foster M, Schreiber T. The CASES-PMS study of carotid stenting with distal emboli protection: effect of age⬎⫽80 on 30-day outcomes. Am J Cardiol 2006;98关Supplement 1兴:11. 124. Criado E, Katzen B, Yadav J, Strickman N, Mishkel G, Schreiber T, Foster M, Massop DW. The CASES-PMS study: 30-day outcomes in diabetic vs non-diabetic patients undergoing carotid artery stenting with distal emboli protection. Am J Cardiol 2006;98关Supplement 1兴:243 mol/L. 125. Skelly CL, Gallagher K, Fairman RM, et al. Risk factors for restenosis after carotid artery angioplasty and stenting. J Vasc Surg 2006;44:1010 –5. 126. Lanzer P, Weser R, Prettin C. Carotid-artery stenting in a high-risk patient population–single centre, single operator results. Clin Res Cardiol 2006;95:4 –12. 127. Safian RD, Bresnahan JF, Jaff MR, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol 2006;47:2384 –9. 128. Hart JP, Peeters P, Verbist J, Deloose K, Bosiers M. Do device characteristics impact outcome in carotid artery stenting? J Vasc Surg 2006;44:725–30. 129. Hauth EA, Drescher R, Jansen C, et al. Complications and follow-up after unprotected carotid artery stenting. Cardiovasc Intervent Radiol 2006;29:511– 8. 130. McDonnell CO, Fearn SJ, Baker SR, Goodman MA, Price D, Lawrence-Brown MM. Value of diffusion-weighted MRI during carotid angioplasty and stenting. Eur J Vasc Endovasc Surg 2006;32:46 –50. 131. Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy vs stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006;355:1660 –71. 132. Park B, Aiello F, Dahn M, Menzoian JO, Mavanur A. Follow-up results of carotid angioplasty with stenting as assessed by duplex ultrasound surveillance. Am J Surg 2006;192:583– 8. 133. Rabe K, Sugita J, Godel H, Sievert H. Flow-reversal device for cerebral protection during carotid artery stenting - Acute and long-term results. Journal of Interventional Cardiology 2006;19:55– 62. 134. Rubartelli P, Brusa G, Arrigo A, et al. Transcranial Doppler monitoring during stenting of the carotid bifurcation: evaluation of two different distal protection devices in preventing embolization. J Endovasc Ther 2006;13:436 – 42. 135. Halabi M, Gruberg L, Pitchersky S, et al. Carotid artery stenting in surgical high-risk patients. Catheter Cardiovasc Interv 2006;67:513– 8. 136. Wang YQ, Saiwah DH, Wang Y, Zheng JT, Jiang HF, Chen BH. Safety and efficacy of carotid artery stenting. J Vasc Interv Radiol 2006;26:6 –10. 137. Lin PH, Zhou W, Guerrero MA, et al. Carotid artery stenting with distal protection using the carotid wallstent and filterwire neuroprotection: single-center experience of 380 cases with midterm outcomes. Vascular 2006;14:237– 44. 138. Kawaguchi S, Sakaki T, Iwahashi H, et al. Effect of carotid artery stenting on ocular circulation and chronic ocular ischemic syndrome. Cerebrovasc Dis 2006;22:402– 8. 139. Maleux G, Demaerel P, Verbeken E, et al. Cerebral ischemia after filter-protected carotid artery stenting is common and cannot be predicted by the presence of substantial amount of debris captured by the filter device. AJNR Am J Neuroradiol 2006;27:1830 –3. 140. Kypta A, Hofman R, Kerschner K, Steinwender C, Leisch F. Gender differences in patients undergoing carotid artery stenting–Acute results, complications and long term follow-up. Am J Cardiol 98关Supplement 1兴, 244 mol/L. 2006. 141. Hofmann R, Niessner A, Kypta A, et al. Risk score for peri-interventional complications of carotid artery stenting. Stroke 2006;37:2557– 61. 142. Alexandrescu V, Ngongang C, Proumen J, et al. Filter-protected carotid stenting via a minimal cervical access with transitory aspirated reversed flow during initial passage of the target lesion. J Endovasc Ther 2006;13:196 –204. 143. Hill MD, Morrish W, Soulez G, et al. Multicenter evaluation of a self-expanding carotid stent system with distal protection in the treatment of carotid stenosis. AJNR Am J Neuroradiol 2006;27:759 – 65. 144. Dalainas I, Nano G, Bianchi P, Stegher S, Malacrida G, Tealdi DG. Dual antiplatelet regime vs acetyl-acetic acid for carotid artery stenting. Cardiovasc Intervent Radiol 2006;29:519 –21. 145. Mussack T, Hauser C, Klauss V, et al. Serum S-100B protein levels during and after successful carotid artery stenting or carotid endarterectomy. J Endovasc Ther 2006;13:39 – 46. 146. Poppert H, Wolf O, Theiss W, et al. MRI lesions after invasive therapy of carotid artery stenosis: a risk-modeling analysis. Neurol Res 2006;28:563–7. 147. Chaer RA, Derubertis BG, Trocciola SM, et al. Safety and efficacy of carotid angioplasty and stenting in high-risk patients. Am Surg 2006;72:694 – 8. 148. Iihara K, Murao K, Sakai N, Yamada N, Nagata I, Miyamoto S. Outcome of carotid endarterectomy and stent insertion based on grading of carotid endarterectomy risk: a 7-year prospective study. J Neurosurg 2006;105:546 –54. 149. Verzini F, Cao P, De Rango P, et al. Appropriateness of learning curve for carotid artery stenting: An analysis of periprocedural complications. J Vasc Surg 2006;44:1211–2. 150. Tinoco ECDA, Da Silva LF, Luquini BB, Campanha R, Nascimento M, Horta L. Prospective and comparative study between endarterectomy and stent angioplasty with cerebral protection in carotid atherosclerotic lesions: 30-Day results. Jornal Vascular Brasileiro 2006;5:257– 62. (Continued) e724 Stroke December 2009 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 151. Zindler JD, Hendriks JM, Koudstaal PJ, Pattynama PM, van Sambeek MR, van Dijk LC. 关Complications within 30 days following placement of a carotid stent with cerebral protection in patients with considerable symptomatic carotid stenosis; Erasmus MC, Rotterdam, 1999 –2004兴. Ned Tijdschr Geneeskd 2006;150:730 – 4. 152. Marine LA, Rubin BG, Reddy R, Sanchez LA, Parodi JC, Sicard GA. Treatment of asymptomatic carotid artery disease: similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J Vasc Surg 2006;43:953– 8. 153. Carvalho Lujan RA, Lucas LA, Gracio ADF, Lobato ADC. Endovascular treatment of carotid obstructive disease in high risk patients: Immediate results. Jornal Vascular Brasileiro 2006;5:23–29. 154. Kwon BJ, Han MH, Kang HS, Jung C. Protection filter-related events in extracranial carotid artery stenting: a single-center experience. J Endovasc Ther 2006;13:711–22. 155. Gonzalez-Marcos JR, Gonzalez A, Martinez E, Boza F, Mayol A, Gil-Peralta A. Carotid artery stenting in women with internal carotid artery disease. Int J Stroke. 2006;1(Suppl 1):65. 156. Setacci C, De DG, Chisci E, et al. Is carotid artery stenting in octogenarians really dangerous? J Endovasc Ther 2006;13:302–9. 157. Setacci C, De Donato G, Setacci F, Chisci E, Cappelli A, Pieraccini M, Castriota F, Cremonesi A. Is diabetes mellitus a risk factor for carotid angioplasty and stenting? Am J Cardiol 2006;98关Supplement 1兴:12 mol/L. 158. Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty vs carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial Lancet 2006;368:1239 – 47. 159. Wu CJ, Cheng CI, Hung WC, et al. Feasibility and safety of transbrachial approach for patients with severe carotid artery stenosis undergoing stenting. Catheter Cardiovasc Interv 2006;67:967–71. 160. Kassaian SE, Kazemi-Saleh D, Alidoosti M, et al. A novel scoring system for identifying high-risk patients undergoing carotid stenting. Arch Iran Med 2006;9:129 –37. 161. Ling F, Jiao LQ. Preliminary report of trial of endarterectomy vs stenting for the treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chinese Journal of Cerebrovascular Diseases 2006;3:4 – 8. 162. Gupta AK, Purkayastha S, Kapilamoorthy TR, et al. Carotid artery stenting: results and long-term follow-up. Neurol India 2006;54:68 –72. 163. Asakura F, Kawaguchi K, Sakaida H, et al. Diffusion-weighted magnetic resonance imaging in carotid angioplasty and stenting with balloon embolic protection devices. Neuroradiology 2006;48:100 –12. 164. Reiter M, Bucek RA, Effenberger I, et al. Plaque echolucency is not associated with the risk of stroke in carotid stenting. Stroke 2006;37:2378 – 80. 165. Mehta RH, Zahn R, Hochadel M, et al. Comparison of in-hospital outcomes of patients with vs without previous carotid endarterectomy undergoing carotid stenting (from the German ALKK CAS Registry). Am J Cardiol 2007;99:1288 –93. 166. Zahn R, Ischinger T, Hochadel M, et al. Carotid artery stenting in octogenarians: Results from the ALKK Carotid Artery Stent (CAS) Registry. European Heart Journal 2007;28:370 –75. 167. Matas M, Alvarez B, Ribo M, Molina C, Maeso J, varez-Sabin J. Transcervical carotid stenting with flow reversal protection: experience in high-risk patients. J Vasc Surg 2007;46:49 –54. 168. Faggioli G, Ferri M, Gargiulo M, et al. Measurement and impact of proximal and distal tortuosity in carotid stenting procedures. J Vasc Surg 2007;46:1119 –24. 169. Bosiers M, De DG, Deloose K, et al. Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg 2007;33:135– 41. 170. Ascher E, Hingorani AP, Marks N. Duplex-assisted internal carotid artery balloon angioplasty and stent placement. Perspect Vasc Surg Endovasc Ther 2007;19:41–7. 171. Parodi JC, Schonholz C, Parodi FE, Sicard G, Ferreira LM. Initial 200 cases of carotid artery stenting using a reversal-of-flow cerebral protection device. J Cardiovasc Surg (Torino) 2007;48:117–24. 172. Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv 2007;69:341– 8. 173. Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv 2007;70:1025–33. 174. Fairman R, Gray WA, Scicli AP, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg 2007;246:551– 6. 175. Katzen BT, Criado FJ, Ramee SR, et al. Carotid artery stenting with emboli protection surveillance study: thirty-day results of the CASES-PMS study. Catheter Cardiovasc Interv 2007;70:316 –23. 176. Gurm HS, Rajagopal V, Sachar R, et al. Impact of diabetes mellitus on outcome of patients undergoing carotid artery stenting: Insights from a single center registry. Catheterization and Cardiovascular Interventions 2007;69:541– 45. 177. Rajagopal V, Kapadia SR, Gurm HS, Bhatt DL, Madhwal S, Abou-Chebl A, Karha J, Mazighi M, Chacko M, Jefferson MK, Simpfendorfer C, Bajzer C, Yadav JS. Octogenarian status is an independent risk factor for adverse outcome after carotid stenting. J Am Coll Cardiol. 2007;49(Suppl 2):B33. 178. Huppert PE, Kotterer O, Claus D, Wiest A. Carotid stent angioplasty with filter protection: microscopical findings and clinical results. CIRSE 2007 1308.4. 179. Iyer V, De DG, Deloose K, et al. The type of embolic protection does not influence the outcome in carotid artery stenting. J Vasc Surg 2007;46:251– 6. 180. Pinter L, Cagiannos C, Ruzsa Z, Bakoyiannis C, Kolvenbach R. Report on initial experience with transradial access for carotid artery stenting. J Vasc Surg 2007;45:1136 – 41. 181. EXACT/CAPTURE-2: Postmarketing carotid stent registry data. 2007. 31-1-2009. 182. Wijtenburg E, Schillaci A, Thomas T, Lismonde M, Remy P, Van RP. Preliminary results of carotid artery stenting in a non-academic hospital. Acta Chir Belg 2007;107:143–5. 183. Grunwald IQ, Papanagiotou P, Struffert T, et al. Reversal of flow during carotid artery stenting: use of the Parodi antiembolism system. Neuroradiology 2007;49:237– 41. 184. Younis GA, Gupta K, Mortazavi A, et al. Predictors of carotid stent restenosis. Catheter Cardiovasc Interv 2007;69:673– 82. 185. Lojik M, Krajiekova D, Kubikova M, et al. 关Endovascular treatment of carotid artery stenosis with cerebral protection: 5-year experience兴. Rozhl Chir 2007;86:513–20. 186. Aydiner O, Boztosun B, Sirvanci M, et al. 关Early and late outcomes of carotid artery stenting兴. Anadolu Kardiyol Derg 2007;7:152–7. 187. Wu CJ, Yip HK, Youssef AA. Trans radial/trans brachial carotid stenting: safety and feasibility trial. Am J Cardiol 2007;100关Suppl 1兴:86L. 188. Topakian R, Strasak AM, Sonnberger M, et al. Timing of stenting of symptomatic carotid stenosis is predictive of 30-day outcome. Eur J Neurol 2007;14:672– 8. (Continued) Touzé et al Risks After Carotid Angioplasty and Stenting e725 Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 189. Timler D, Stelagowski M, Kasielska A, Bogusiak K, Tazbir J. Analysis of surgical treatment of patients with carotid artery stenosis endarterectomy vs stenting. Acta Chir Belg 2007;107:146 –50. 190. Brightwell RE, Sherwood RA, Athanasiou T, Hamady M, Cheshire NJ. The neurological morbidity of carotid revascularisation: using markers of cellular brain injury to compare CEA and CAS. Eur J Vasc Endovasc Surg 2007;34:552– 60. 191. Montorsi P, Galli S, Ghulam Ali S, Ravagnami P, Fabbiochi F, Lualdi A, Trabattoni D, De Martini S, Teruzzi G, Grancini L, Calligaris G, Bartorelli AL. Is carotid artery stenting a durable technique? Acute and up to 4 years long-term clinical results from a single-center consecutive series of patients. Am J Cardiol 2007;100关Suppl 1兴:88L. 192. Spes CH, Schwende A, Beier F, et al. Short- and long-term outcome after carotid artery stenting with neuroprotection: single-center experience within a prospective registry. Clin Res Cardiol 2007;96:812–21. 193. Henry M, Polydorou A, Henry I, et al. New distal embolic protection device the FiberNet 3 dimensional filter: first carotid human study. Catheter Cardiovasc Interv 2007;69:1026 –35. 194. Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg 2007;45:875– 80. 195. Tsai FY. Stroke prevention during carotid stenting: anticoagulation vs distal protection. J Vasc Interv Radiol 2007;18关Supplement兴:S51. 196. Kawarada O, Yokoi Y, Takemoto K, Morioka N. Double-wire technique in balloon-protected carotid artery stenting. J Interv Cardiol 2007;20:55– 62. 197. Veselka J, Cerna D, Zimolova P, et al. Thirty-day outcomes of direct carotid artery stenting with cerebral protection in high-risk patients. Circ J 2007;71:1468 –72. 198. Folmar J, Sachar R, Mann T. Transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc Interv 2007;69:355– 61. 199. Fanelli F, Corona M, Nardis P, Pucci A, Boatta E, Allegritti M, Passariello R. Carotid artery stenting with cerebral protection device: seven-year single center experience. CIRSE 2007 , 1308.3. 2007. 200. Gandini R, Fabiano S, Pampana E, Stefanini M, Spinelli A, Reale CA, Di Primio M, Simonetti G. Endovascular treatment of internal carotid artery stenosis: immediate and six-year follow-up. CIRSE 2007, 2007.3. 201. Gossetti B, Gattuso R, Irace L, et al. Embolism to the brain during carotid stenting and surgery. Acta Chir Belg 2007;107:151– 4. 202. Kadkhodayan Y, Cross DT, III, Derdeyn CP, Moran CJ. Carotid angioplasty and stenting in the elderly. Neuroradiology 2007;49:933– 8. 203. Rapp JH, Wakil L, Sawhney R, et al. Subclinical embolization after carotid artery stenting: new lesions on diffusion-weighted magnetic resonance imaging occur postprocedure. J Vasc Surg 2007;45:867–72. 204. Sanchez A, Kambara A, Sousa AG, Moreira S, Costa JR Jr, Chaves A, Rodrigues C, Sousa-Nunes PM, Medeiros W, Vieira E, Cano MN, Feres F, Abizaid A, Sousa JE. Long term comparison of carotid stenting for symptomatic vs asymptomatic patients. Am J Cardiol 2007;100关Supplement 1兴:187L. 205. Suk JK, Hong GR, Jeon P, et al. Cerebral ischemia detected with diffusion-weighted MR imaging after protected carotid artery stenting: Comparison of distal balloon and filter device. Kor J Radiol 2007;8:276 – 85. 206. Maynar M, Baldi S, Rostagno R, et al. Carotid stenting without use of balloon angioplasty and distal protection devices: Preliminary experience in 100 cases. Am J Neuroradiol 2007;28:1378 – 83. 207. Saratzis N, Saratzis A, Melas N, et al. Carotid artery stent placement with embolic protection: single-center experience. J Vasc Interv Radiol 2007;18:337– 42. 208. Criado E, Fontcuberta J, Orgaz A, Flores A, Doblas M. Transcervical carotid stenting with carotid artery flow reversal: 3-year follow-up of 103 stents. J Vasc Surg 2007;46:864 –9. 209. Hoffman A, Engelter T, Taschner C, et al. Carotid artery stenting vs carotid endarterectomy-a prospective randomised controlled single-centre trial with long-term follow-up (BACASS). Schweizer Archiv für Neurologie und Psychiatrie 2008;159:84 –9. 210. Silvestro A, Civelli P, Laffranchini G, Troianiello B, Graziani L. Influence of anatomic factors on the feasibility and safety of carotid stenting in a series of 154 consecutive procedures. J Cardiovasc Med (Hagerstown) 2008;9:137– 41. 211. Arjomand H, Gray WA, Hymanson A, Petrovich L, LaBlanc P, Alani F, Surabhi S, Goldberg S, McCormick D. Outcome of carotid artery stenting in female vs male patients: a gender analysis of the CAPTURE-2 registry. J Am Coll.Cardiol 2008;52关Supplement 2兴:B78. 212. Uflacker R, Schonholz C. Carotid artery stenting, the first 100 patients with zero complication rate: how can we improve our learning curve. J Vasc Interv Radiol 2008;19关Supplement兴, S97. 213. Cremonesi A, Rubino P, Grattoni C, Scheinert D, Castriota F, Biamino G. Multicenter experience with a new ⬙hybrid⬙ carotid stent. J Endovasc Ther 2008;15:186 –92. 214. Priban V, Fiedler J, Barankova L, Bombic M, Sterba L. Carotid endarterectomy and carotid angioplasty stenting as complementary treatment methods: Three years experience. Ceska Slov Neurol Neurochir 2008;71:75– 80. 215. Ozturk V, Men S, Ugurel B, et al. Management of carotid artery stenosis: Experiences in Dokuz Eylul University Hospital. J Neurol Sci 2008;25:25–31. 216. Uddin MA, Haq T, Chishty IA, Sajjad Z, Salam B. Endovascular stenting for carotid artery stenosis: results of local experience at Aga Khan University Hospital, Karachi. J Pak Med Assoc 2008;58:11–5. 217. Buszman P, Debinski M, Gruszka A, et al. Early and late outcomes of percutaneous transluminal angioplasty of cephalad arteries. Kardiol Pol 2008;66:233– 42. 218. Yuo TH, Goodney PP, Powell RJ, Cronenwett JL. ⬙Medical high risk⬙ designation is not associated with survival after carotid artery stenting. J Vasc Surg 2008;47:356 – 62. 219. Ghorab K, Macian F, Adoukounou T, Magy L, Chapot R, Vallat JM. Carotid angioplasty stenting revisited: clinical and radiological (MRI) outcome. Cerebrovasc Dis 2008;25:21–5. 220. Bianchi C, Ou HW, Bishop V, et al. Carotid Artery Stenting in High-Risk Patients: Midterm Mortality Analysis. Ann Vasc Surg 2008;22:185–9. 221. Jackson BM, English SJ, Fairman RM, Karmacharya J, Carpenter JP, Woo EY. Carotid artery stenting: identification of risk factors for poor outcomes. J Vasc Surg 2008;48:74 –9. 222. Barbato JE, Dillavou E, Horowitz MB, et al. A randomized trial of carotid artery stenting with and without cerebral protection. J Vasc Surg 2008;47:760 –5. 223. Sayeed S, Stanziale SF, Wholey MH, Makaroun MS. Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J Vasc Surg 2008;47:81–7. 224. Theiss W, Hermanek P, Mathias K, et al. Predictors of death and stroke after carotid angioplasty and stenting: a subgroup analysis of the Pro-CAS data. Stroke 2008;39:2325–30. 225. Sanchez A, Kambara A, Martins S, Costa JR Jr, Rodrigues C, Medeiros W, Vieira E, Sousa-Nunes PM, Feres F, Abizaid A, Cano MN, Sousa AG, Sousa JE. Long term (2 years) follow-up of consecutive patients treated with percutaneous carotid intervention. Am J Cardiol 2008;100关Supplement 1兴, 186L. 226. Martinez-Fernandez E, Boza GF, Gonzalez-Marcos JR, Gil-Peralta A, Gonzalez GA, Mayol DA. Risk factors and neurological consequences of syncopes induced by internal carotid artery angioplasty. Stroke 2008;39:1336 –9. (Continued) e726 Stroke December 2009 227. Kojuri J, Ostovan MA, Zamiri N, Zolghadr AA, Bani Hashemi MA, Borhani HA. Procedural outcome and midterm result of carotid stenting in high-risk patients. Asian Cardiovasc Thorac Ann 2008;16:93– 6. 228. Stingele R, Berger J, Alfke K, et al. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol 2008;7:216 –22. 229. Groschel K, Knauth M, Ernemann U, Pilgram SM, Schnaudigel S, Kastrup A. Early treatment after a symptomatic event is not associated with an increased risk of stroke in patients undergoing carotid stenting. Eur J Neurol 2008;15:2–5. 230. Kastrup A, Groschel K, Nagele T, et al. Effects of age and symptom status on silent ischemic lesions after carotid stenting with and without the use of distal filter devices. AJNR Am J Neuroradiol 2008;29:608 –12. 231. Piccoli G, Agarwal N, Bais B, Vit A, Gasparini D. Protected carotid artery angioplasty and stenting: acute and long term outcomes in a large population. CIRSE 2007, 1308.2. 232. De Gregorio MA, Laborda A, Medrano J, Tejero C, Perez A, Mostacero E, Barrufet M, Gomez-Arrue J. Carotid artery stent placement with and without embolic protection. Retrospective analysis of 203 cases. Int J Stroke 2008;1关Supplement 1兴:S32. 233. Kimiagar I, Klein C, Rabey JM, et al. Carotid artery stenting in high risk patients with carotid artery stenosis not eligible for endarterectomy: clinical outcome after 5 years. Isr Med Assoc J 2008;10:121– 4. 234. Roffi M, Greutmann M, Eberli FR, et al. Starting a carotid artery stenting program is safe. Catheter Cardiovasc Interv 2008;71:469 –73. Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Systematic Review of the Perioperative Risks of Stroke or Death After Carotid Angioplasty and Stenting Emmanuel Touzé, Ludovic Trinquart, Gilles Chatellier and Jean-Louis Mas Downloaded from http://stroke.ahajournals.org/ by guest on November 19, 2016 Stroke. published online November 5, 2009; Stroke is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2009 American Heart Association, Inc. All rights reserved. Print ISSN: 0039-2499. Online ISSN: 1524-4628 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://stroke.ahajournals.org/content/early/2009/11/05/STROKEAHA.109.562041.citation Data Supplement (unedited) at: http://stroke.ahajournals.org/content/suppl/2016/04/10/STROKEAHA.109.562041.DC1.html Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Stroke can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Stroke is online at: http://stroke.ahajournals.org//subscriptions/ Comentarios, opiniones y revisiones Revisión sistemática de los riesgos perioperatorios de ictus y muerte tras la angioplastia carotídea con implantación de stent Emmanuel Touzé, PhD; Ludovic Trinquart, MSc; Gilles Chatellier, PhD; Jean-Louis Mas, MD Antecedentes y objetivo. No se ha demostrado que la angioplastia carotídea con implantación de stent (ACIS) sea igual de segura que la endarterectomía carotídea (EDAC) por lo que respecta a los riesgos de complicaciones periintervención, aunque después del periodo postoperatorio, los riesgos observados son comparables, lo cual sugiere que la ACIS puede ser una opción aceptable en pacientes seleccionados. Sin embargo, no se han establecido claramente los factores de riesgo para el ictus y la muerte perioperatorios. El objetivo de este estudio fue estimar los riesgos absolutos de ictus o muerte a 30 días tras la ACIS e investigar los orígenes de la heterogeneidad. Métodos. Llevamos a cabo una búsqueda de artículos publicados entre enero de 1990 y junio de 2008, mediante el empleo de las bases de datos de MEDLINE, EMBASE y COCHRANE, una búsqueda manual, libros de resúmenes de congresos y sitios web oficiales. Dos revisores seleccionaron de manera independiente y por duplicado los artículos relativos a los riesgos de la ACIS, con independencia del tipo de tratamiento, el diseño de estudio, el contexto o el idioma de publicación. Los 2 revisores se encargaron de realizar una extracción de los datos y de evaluar la calidad de los estudios. Resultados. Se incluyeron un total de 206 estudios independientes (con 54.713 pacientes). El riesgo global de ictus o muerte a 30 días fue del 4,7% (IC del 95%, 4,1 a 5,2), con una heterogeneidad sustancial entre los diversos estudios. Los pacientes sintomáticos presentaban una probabilidad de sufrir complicaciones aproximadamente doble de la existente en los pacientes con estenosis asintomáticas. El riesgo de ictus o muerte a 30 días fue del 7,6% (3,6 a 9,1) en los pacientes sintomáticos y del 3,3% (2,6 a 4,1) en los pacientes asintomáticos. Los riesgos aumentaban con la edad, la hipertensión y los antecedentes de enfermedad coronaria; no estaban relacionados con el sexo ni con la presencia de una oclusión carotídea contralateral; y eran menores en los pacientes con una reestenosis carotídea tras la EDAC y en los tratados utilizando un dispositivo de protección cerebral. Además, los riesgos han disminuido a lo largo del tiempo. Conclusiones. Los riesgos de ACIS presentan una variación sustancial en los diversos estudios. En general son mayores que los de la EDAC en pacientes sintomáticos. Es probable que algunos factores sean útiles para seleccionar a los candidatos adecuados para la ACIS. (Traducido del inglés: Systematic Review of the Perioperative Risks of Stroke or Death After Carotid Angioplasty and Stenting. Stroke. 2009;40:e683-e693.) Palabras clave: stroke n carotid disease n stenting n angioplasty n atherosclerosis n systematic review L a estenosis de la arteria carótida interna extracraneal explica del 15% al 20% de los ictus isquémicos, según cuál sea la población estudiada1. La eficacia de la endarterectomía carotídea (EDAC) para la prevención del ictus en pacientes con estenosis carotídea está bien establecida, sobre todo en los pacientes con estenosis sintomáticas1-3. La angioplastia carotídea con implantación de stent (ACIS), una posible alternativa terapéutica a la EDAC, se ha evaluado en unos pocos ensayos aleatorizados y en múltiples estudios no aleatorizados, con la participación de muchos especialistas, como neurólogos, radiólogos, cardiólogos, cirujanos vasculares y neurocirujanos, la mayor parte de los cuales han aplicado ya esta técnica en su práctica clínica4. Sin embargo, en recientes ensayos aleatorizados y metaanálisis no se ha podido demostrar que la ACIS sea igual de segura que la EDAC en lo relativo a los riesgos de complicaciones periintervención4–12 y las guías terapéuticas actuales recomiendan que no se utilice la ACIS en los pacientes que son candidatos adecuados para el tratamiento quirúrgico2,3,13. No obstante, los ensayos clínicos han indicado también que, después del periodo perioperatorio, el riesgo de ictus homolateral es muy bajo y comparable en los pacientes tratados con ACIS y con EDAC10,14,15, Recibido el 8 de julio de 2009; revisión final recibida el 25 de agosto de 2009; aceptado el 4 de setiembre de 2009. Université Paris Descartes, INSERM U894 (E.T., J.-L.M.), Hôpital Sainte-Anne, Service de Neurologie, e INSERM CIE4 (L.T., G.C.), Assistance Publique-Hôpitaux de Paris, Unité de recherche clinique, Hôpital Européen Georges Pompidou, París, Francia. Los dos primeros autores contribuyeron por igual a la obtención y análisis de los datos y a la redacción del artículo. Correspondencia: Emmanuel Touzé, MD, PhD, Université Paris Descartes, INSERM U894, Department of Neurology, Hôpital Sainte-Anne, 1 rue Cabanis, 75014 Paris, Francia. E-mail e.touze@ch-sainte-anne.fr © 2009 American Heart Association, Inc. Stroke está disponible en http://www.strokeaha.org DOI: 10.1161/STROKEAHA.109.562041 22 Touzé y cols. Revisión sistemática de los riesgos perioperatorios de ictus y muerte... 23 Tabla 1. Estrategia de búsqueda en MEDLINE y EMBASE Estrategia de búsqueda en PubMed (“Carotid stenosis” Mesh AND (“stents” Mesh OR “angioplasty” Mesh OR “angioplasty, balloon” Mesh ) AND (“treatment outcome” Mesh OR “postoperative complications” Mesh OR “myocardial infarction” Mesh OR “stroke” Mesh OR “brain ischemia” Mesh OR “death” Mesh OR “death, sudden, cardiac” Mesh OR “mortality” Mesh )) OR (“carotid stenosis” AND (“carotid angioplasty” OR “stent”*) AND (“stroke” OR “myocardial infarction” OR “death” OR “mortality”) AND (“1990” PDat :”2008/06” PDat ) AND (“humans” Mesh ) ) Estrategia de búsqueda en EMBASE (“Carotid artery obstruction”/exp AND (“stent”/exp OR “angioplasty”/exp OR “percutaneous transluminal angioplasty”/exp) AND (“treatment outcome”/exp OR “postoperative complication”/exp OR “heart infarction”/exp OR “cerebrovascular accident”/exp OR “stroke”/exp OR “brain ischemia”/exp OR “death”/exp OR “sudden death”/exp) NOT “review” /lim AND “humans” /lim AND 1990 –2008 /py) lo cual sugiere que la ACIS puede ser una opción aceptable en pacientes seleccionados que presentan un riesgo bajo de complicaciones periintervención. Sin embargo, en la actualidad no existe ningún método que permita seleccionar a los pacientes que son candidatos adecuados para una ACIS. Además, aunque la ACIS se utiliza ampliamente en algunos centros, no se sabe si el riesgo absoluto de la ACIS observado en los ensayos clínicos aleatorizados puede generalizarse a la práctica clínica cotidiana. Los riesgos de complicaciones tras la EDAC y su relación con diferentes subgrupos se han estimado en varios estudios y metaanálisis16–20, pero no existen datos similares par la ACIS. Algunos estudios han puesto de manifiesto que es probable que el riesgo de complicaciones tras la ACIS esté relacionado con algunas características de los pacientes y con aspectos técnicos. Sin embargo, el número de complicaciones observadas en estudios específicos fue generalmente bajo, y ello impide extraer una conclusión fiable. Por otra parte, muchos de los estudios se han centrado en pacientes con un riesgo quirúrgico percibido alto, lo que lleva a plantear la hipótesis de que estos pacientes deben ser candidatos adecuados para una ACIS4,21. Sin embargo, es posible que las comorbilidades asociadas a un riesgo perioperatorio superior con la EDAC aumenten también el riesgo periintervención en la ACIS. Así pues, hemos realizado una revisión sistemática de los estudios en los que se ha descrito el riesgo de ictus, muerte e infarto de miocardio (IM) tras la ACIS, con objeto de estimar los riesgos absolutos e investigar la posible relación entre los riesgos observados y el diseño del estudio, la población incluida, los factores clínicos y los aspectos técnicos. Métodos Antes de realizar la revisión, elaboramos un protocolo que incluía los fundamentos y objetivos, junto con una descripción de los métodos de investigación propuestos y los planes para la obtención y el análisis de los datos. El manuscrito se preparó según lo establecido en las guías MOOSE22. Criterios de selección y estrategia de búsqueda Se consideraron elegibles para la revisión los estudios que cumplían las siguientes características: (1) incluían a pacien- tes con estenosis sintomáticas y/o asintomáticas situadas en la región de la bifurcación carotídea; (2) los pacientes eran tratados con angioplastia, fuera cual fuera el tipo de tratamiento concreto utilizado (angioplastia con balón con o sin implantación de stent), la vía de acceso arterial y el uso de protección cerebral; y (3) era posible extraer información relativa al número de episodios (ictus, IM o muerte). Se excluyeron los estudios para los que se había reclutado tan solo a un grupo de población específico (reestenosis tras una EDAC, estenosis post-radioterapia, displasia fibromuscular, disección carotídea, y pacientes tratados en un contexto de urgencia). Realizamos una búsqueda de los artículos publicados entre enero de 1990 y junio de 2008 sobre los riesgos de ictus, muerte o IM tras una ACIS, con independencia del diseño de estudio, el contexto o el idioma de publicación. Se llevaron a cabo búsquedas electrónicas con el empleo de MEDLINE y EMBASE , utilizando tanto los términos de clasificación de temas médicos (medical subject heading terms) como las palabras del texto (Tabla 1), y en la base de datos de la COCHRANE Library (CENTRAL y DARE). Realizamos búsquedas manuales en las listas de bibliografía de todos los artículos incluidos, de todos los artículos de revisión relevantes, de nuestros archivos personales y de las páginas de índice de las 3 revistas en las que se había identificado un mayor número de artículos elegibles en las búsquedas electrónicas (Journal of Vascular Surgery, Journal of Endovascular Therapy y Catheter Cardiovascular Interventions). Con objeto de identificar los estudios recientes todavía no publicados en forma de artículos completos, realizamos también una búsqueda en los libros de resúmenes de los congresos recientes (Joint World Congress on Stroke 2006, American Heart Association International Stroke Conferences 2007 y 2008, European Stroke Conferences 2007 y 2008, congresos de la Cardiovascular and Interventional Radiological Society of Europe 2006 y 2007, congresos de Transcatheter Cardiovascular Thera­peutics 2006 y 2007, sesiones del American College of Cardiol­ogy Scientific 2007 y 2008, y congresos de la Society of Interven­tional Radiology 2007 y 2008), así como de los sitios web del registro de ensayos clínicos (www.clinicaltrials.gov), la Food and Drug Administration de EEUU (www.fda.gov) y la Agencia Europea del Medicamento (www. emea.europa.eu). Selección de los estudios y obtención de los datos Dos revisores se encargaron de evaluar, de manera independiente y por duplicado, la elegibilidad de las referencias bibliográficas identificadas mediante la estrategia de búsqueda, mediante el examen de los títulos y luego de los resúmenes de los artículos. En cada paso, las discrepancias aparecidas se resolvieron mediante el debate. La selección final se realizó tras haber revisado los artículos completos correspondientes a todos los artículos que o bien cumplían los criterios de selección o bien no estaba del todo clara la selección según lo indicado en el resumen. En los casos de publicaciones múltiples relativas a la misma población, se eligió la que presentaba un número más elevado de pacientes para el análisis del riesgo absoluto. Se obtuvieron datos adicionales de subgrupos del ensayo SPACE solicitándolos a los autores8. Los 2 24 Stroke Marzo 2010 Medline y Embase 53 referencias de otras fuentes Cochrane Library, FDA, EMEA, libros de resúmenes, archivos personales, listas de bibliografía 1.796 títulos examinados 605 resúmenes examinados 457 referencias elegibles para una revisión completa Figura 1. Diagrama de flujo de la selección de los estudios. 510 referencias analizadas con el texto completo Población no independiente (149) Resultados no disponibles (69) Muy seleccionados (56) 206 poblaciones independientes 234 referencias incluidas revisores se encargaron de extraer los datos mediante un formulario estandarizado (que puede solicitarse a los autores). La calidad de cada estudio fue evaluada con un esquema ya existente23 que adaptamos a nuestro contexto y que incluía la siguiente lista de criterios: (1) diseño (ensayo aleatorizado frente a estudios de cohortes/registros), (2) contexto (estudio de un solo centro frente a estudio multicéntrico), (3) forma de inclusión de los pacientes (prospectiva frente a retrospectiva, y consecutiva frente a no consecutiva), (4) descripción de la población (adecuada frente a insuficiente) y (5) evaluación del resultado (evaluación sistemática por un neurólogo tras la intervención, sí frente a no). La descripción de la población se consideró adecuada cuando se describían suficientemente el marco de referencia del muestreo, el reclutamiento, los criterios de inclusión y exclusión y las características basales de la muestra en estudio. La evaluación sistemática por parte de un neurólogo hacía referencia a que todos los pacientes fueran examinados por un neurólogo a los 30 días (resultados a los 30 días) o antes del alta (episodios periintervención o intrahospitalarios), tanto si el paciente presentaba un episodio de la variable de valoración como si no. Síntesis y análisis de los datos Las variables de valoración principales fueron los riesgos a 30 días de ictus; ictus o muerte; o ictus, IM o muerte. Las variables de valoración secundarias fueron los riesgos intrahospitalarios y periintervención (en las primeras 24 horas). Cuando no estaba claro el momento exacto de la evaluación de las complicaciones, sin un seguimiento sistemático a los 30 días, consideramos que se trataba de episodios de complicaciones periintervención. Las estimaciones combinadas del riesgo se calcularon por separado para las diferentes variables de valoración. Cada proporción individual obtenida se transformó primero en una cantidad con la transformación estabilizadora de varianzas de Freeman-Tukey24. Se calculó una media ponderada de las proporciones transformadas, utilizando un modelo de efectos aleatorios de DerSimonianLaird25. La proporción combinada se calculó mediante la retrotransformación de esta media ponderada26. Con objeto de explorar los posibles orígenes de la heterogeneidad, realizamos en primer lugar comparaciones de subgrupos según los siguientes factores: forma de presentación clínica (sintomática frente a asintomática; ictus frente a ataque isquémico transitorio; episodio cerebral frente a episodio ocular), edad (>75 a 80 frente a <75 a 80 años), sexo, diabetes mellitus, enfermedad coronaria (EC), enfermedad arterial periférica (EAC), oclusión carotídea contralateral, reestenosis tras la EDAC frente a lesión aparecida de novo, estructura de la placa (ulcerada frente a lisa, presencia de calcificación), momento de realización de la ACIS (<14 días frente a >14 días tras el episodio de isquemia cerebral), lado de la lesión tratada y uso de un dispositivo de protección cerebral. Estas comparaciones se llevaron a cabo dentro de cada estudio. Calculamos los riesgos relativos (RR) combinados para el conjunto de todos los estudios utilizando un metaanálisis de efectos fijos, según el método de MantelHaenszel, o bien con el empleo de un metaanálisis de efectos aleatorios de DerSimonian-Laird, según fuera apropiado. A continuación realizamos comparaciones indirectas de los riesgos absolutos combinados, según la forma de presentación clínica y las características de calidad del estudio que se han definido antes y evaluamos los posibles cambios del riesgo a lo largo del tiempo, mediante un análisis de metarregresión. Utilizamos un modelo normal logístico que especificaba la distribución binomial de la variable dependiente (riesgo de ictus o muerte a 30 días) y un efecto aleatorio para tener en cuenta la varianza compartida dentro del estudio27. Se calculó el año de mitad de cohorte, definido como el punto medio del periodo de inclusión, para cada estudio si se disponía de información sobre el periodo de inclusión, y se consideró una covariable. Evaluamos los sesgos de publicación mediante un análisis visual simple de los gráficos de embudo en el metaanálisis de los riesgos absolutos, puesto que no hay pruebas estadísticas validadas para la detección de la asimetría, y utilizamos gráficos de embudo y la prueba de Egger en las comparaciones de subgrupos28. En todos los análisis, se evaluó la inconsistencia de los resultados en los diversos estudios utilizando el parámetro estadístico Q de Cochran y Touzé y cols. Revisión sistemática de los riesgos perioperatorios de ictus y muerte... 25 Tabla 2. Calidad, características de la población y aspectos técnicos de los estudios incluidos Número Número de de estudios (%), pacientes (Número de intervenciones) Total 206* Calidad del estudio Tabla 3. Estimaciones combinadas de los riesgos absolutos de ictus, muerte o IM, según el momento de valoración de los resultados tras la ACIS Ictus Ictus, muerte Ictus, IM, muerte Episodios a 30 días Contexto Multicéntrico 36 (17) 34 898 (35 502) 113 63 170 (83) 19 815 (20 684) Número de estudios incluidos 118 Un solo centro 10 (5) 1613 (1613) Número de pacientes 27 186 25 237 17 291 Número de intervenciones 28 149 26 145 17 858 Diseño ECA (EDAC frente a ACIS) ECA (otros)† 3 ( 1) 144 (144) 193 (94) 52 956 (54 430) Prospectiva 83 (40) 24 878 (25260) Retrospectiva 42 (20) 8580 (8881) No se indica claramente 81 (40) 21 255 (22045) Riesgo combinado (IC del 95%) P(het) 119 (58) 29 485 (30250) I² (IC del 95%) 87 (42) 25 228 (25936) Episodios intrahospitalarios Se describe el marco de referencia del muestreo Se describen los criterios de inclusión 154 (75) 140 (68) 38 056 (39 354) 36 487 (37 429) Se describen las características basales 132 (64) Evaluación realizada por un neurólogo Registro Inclusión de pacientes Consecutiva No se indica claramente Descripción de la población 3,9% (3,4 a 4,4) 0,0001 67% (60 a 73) 4,7% (4,1 a 5,2) 0,0001 69% (62 a 74) 5,3% (4,6 a 6,0) 0,0001 64% (52 a 72) Número de estudios incluidos 53 48 19 34832 (3552) Número de pacientes 11 694 7912 1723 79 (39) 26 286 (26 835) 12 073 8243 1806 Se indica la definición de la variable de valoración de ictus 87 (43) 37 499 (38 292) Número de intervenciones Presencia de al menos 1 neurólogo en la relación de autores 66 (32) 9075 (9410) Evaluación de las variables de valoración Número de estudios con datos disponibles Mediana (RIC) Características de la población Número de pacientes 206 90 (41 to 204) Número de intervenciones 20 6 94 (43 to 215) Porcentaje de varones 179 71 (65 to 80) Media de edad, años 180 70 (67 to 71) Porcentaje de pacientes sintomáticos 180 50 (33 to 78) Porcentaje de pacientes diabéticos 128 31 (24 to 38) Porcentaje de pacientes con reestenosis carotídea 95 14 (7 to 24) Porcentaje de pacientes con estenosis carotídea post-irradiación 47 5 (2 to 9) Porcentaje de pacientes con EC 104 60 (40 to 71) Porcentaje de pacientes con EAC 44 28 (20 to 37) Porcentaje de pacientes con oclusión carotídea contralateral 79 10 (6 to 14) Riesgo combinado (IC del 95%) P(het) 3,9% (3,2 a 4,6) 0,0001 0,0001 0,11 I² (IC del 95%) 56% (41 a 68) 54% (36 a 67) 30% (0 a 60) Ningún paciente tratado con protección cerebral, n estudios (%) 201 54 (27) Todos los pacientes tratados con protección cerebral, n estudios (%) 201 71 (35) Porcentaje de intervenciones satisfactorias, mediana (RIC) 109 98 (97–100) RIC indica rango intercuartiles. *Incluye también resúmenes. †ECA en los que se comparan diferentes estrategias en pacientes tratados con ACIS. 4,6% (3,5 a 5,9) 53 40 13 Número de pacientes 9003 3893 979 Número de intervenciones 9413 4199 1006 3,7% (2,6 a 5,0) 4,0% (2,6 a 5,7) Episodios periintervención* Número de estudios incluidos Riesgo combinado (IC del 95%) P(het) Aspectos técnicos 4,1% (3,3 a 5,0) I² (IC del 95%) 3,5% (2,7 a 4,4) 0,0001 71% (61 a 78) 0,0001 66% (53 a 76) 0,23 21% (0 a 59) P (het) indica el valor de P asociado a la prueba de χ² para la heterogeneidad; I², porcentaje de la variabilidad en las estimaciones del efecto que se debe a la heterogeneidad y no al error de muestreo (aleatorio). Se calcularon estimaciones combinadas del riesgo por separado para las distintas variables de valoración. Cada proporción individual se transformó primero en una cantidad con la transformación estabilizadora de la varianza de Freeman-Tukey24. Se calculó una media ponderada de las proporciones transformadas mediante un modelo de efectos aleatorios de DerSimonian-Laird24. Se calculó la proporción combinada mediante la retrotransformación de esta media ponderada. *Incluye también los episodios en los que no estaba claro el momento de la determinación. el parámetro estadístico I2 con el IC del 95% asociado; este último correspondía al porcentaje de variabilidad debida a la heterogeneidad entre los estudios y no al error de muestreo 26 Stroke Marzo 2010 Figura 2. Valores combinados de los RR de ictus y de ictus o muerte en diferentes subgrupos. p(het) indica el valor de probabilidad asociado a la prueba estadística de χ2 de Cochran para la heterogeneidad; I2, porcentaje de la variabilidad en las estimaciones del efecto que se debe a la heterogeneidad y no al error de muestreo (aleatorio); NA, no evaluable; AIT, ataque isquémico transitorio; y p(sig), valor de p para la significación. Utilizamos un modelo de efectos fijos para calcular las estimaciones combinadas, excepto cuando sucedía que p(het) < 0,10 o I2 > 30%, en cuyo caso se utilizó un modelo de efectos aleatorios. Véanse las Figuras II y III del suplemento para los valores de cada metaanálisis individual. Se combinaron los resultados evaluados a los 30 días, en el momento del alta, durante la intervención o cuando no se conocía el momento de valoración. La comparación corresponde a sí frente a no, salvo que se indique lo contrario. A, RR combinado (IC del 95%) para ictus o muerte (véase también la Figura II del suplemento). B, RR combinado (IC del 95%) para ictus (véase también la Figura III disponible solamente online). (aleatorio)29,30. Según el manual Cochrane, la heterogeneidad se clasificó como moderada (I2 ≥30%), substancial (I2 ≥50%) o considerable (I2 ≥75%)31. Consideramos significativo un valor de probabilidad bilateral <0,05. El análisis estadístico se realizó con los programas SAS versión 9.1 y MIX (http:// mix-for-metaanálisis.info). Resultados De los 1.796 artículos identificados en nuestra búsqueda electrónica en MEDLINE y EMBASE, se examinaron 605 resúmenes y se obtuvieron 457 artículos para la evaluación del texto completo (Figura 1). Identificamos otros 53 artículos o resúmenes a partir de otras fuentes. De las 510 re- Touzé y cols. Revisión sistemática de los riesgos perioperatorios de ictus y muerte... 27 Figura 3. Riesgo absoluto de ictus o muerte a 30 días (%) tras la ACIS en 91 estudios (18.538 pacientes) según el año de mitad de cohorte, junto con una metarregresión de efectos aleatorios de resumen. El área de cada círculo es inversamente proporcional a la varianza del riesgo absoluto. ferencias bibliográficas analizadas de manera detallada, 206 correspondían a estudios independientes que fueron considerados elegibles (133 solamente en cuanto al riesgo absoluto, 62 en cuanto al riesgo absoluto y los subgrupos, y 11 en cuanto a los subgrupos solamente). Dada la existencia de múltiples publicaciones de algunos registros, con diferentes análisis de subgrupos, los 206 estudios independientes dieron origen a 234 presentaciones de datos (212 artículos completos, 19 resúmenes, 2 documentos de la Food and Drug Administration de EEUU y 1 documento presentado en un sitio web) relevantes para nuestro análisis. En la Tabla 2 se presentan las características resumidas de los estudios incluidos, y la lista de referencias bibliográficas y características de los trabajos individuales pueden consultarse en la Tabla I del suplemento disponible online en http://ictus.ahajournals. org. En el conjunto de 206 estudios independientes (54.713 pacientes), había 10 ensayos clínicos aleatorizados (ECA) en los que se comparaba la ACIS con la EDAC (1.613 pa­ cientes)5,7,8,21,32–37, 3 ECA en los que se comparaban estrategias diferentes en pacientes tratados con ACIS (144 pacientes)38–40 y 193 registros (52.956 pacientes). Había 32 estudios (2.922 pacientes) en los que un 95% o más de los pacientes tenían una estenosis sintomática, 2 estudios (136 pacientes) en los que el 95% o más de los pacientes tenían una estenosis carotídea asintomática, y 172 estudios (51.655 pacientes) que incluían a pacientes tanto sintomáticos como asintomáticos. Aproximadamente la mitad (51%) de los estudios iniciaron el reclutamiento de pacientes antes de 2000. Como se muestra en la Tabla 2, un 83% de los estudios se habían realizado en un solo centro y en el 40% se indicaba que eran prospectivos. La descripción de la población era adecuada en el 46%, y la evaluación de los resultados fue realizada por un neurólogo independiente en el 40% de los estudios publicados en forma de artículos completos. Se identificó una población plenamente descrita y una evaluación neurológica de los resultados en 49 (26%) de los estudios publicados en forma de artículo completo. De los 172 estudios que incluyeron a pacientes sintomáticos y asintomáticos, 36 (21%) presentaron los riesgos de ACIS estratificados según la forma de presentación clínica. De los 173 estudios que indicaban claramente el tipo de tratamiento realizado, 161 (93%) eran estudios en los que más del 90% de los pacientes fueron tratados con implantación de stents. Las características principales de los ECA y los registros fueron muy similares en lo relativo a la edad (mediana, 69 frente a 70 años), la proporción de varones (mediana, 71% frente a 71%), la proporción de pacientes con oclusión carotídea contralateral (mediana, 10% frente a 10%) y la proporción de pacientes con EC (mediana, 57% frente a 61%). La proporción de pacientes sintomáticos fue mayor en los ECA que en los registros (mediana, 81% frente a 49%). En cambio, en los registros era más probable que en los ECA haber incluido a pacientes con reestenosis tras una EDAC (mediana, 15% frente a 8%), la presencia de pacientes con diabetes (mediana, 32% frente a 24%) y el haber tratado a pacientes utilizando dispositivos de protección cerebral (mediana, 83% frente a 42%). 28 Stroke Marzo 2010 Figura 4. Riesgo combinado de ictus o muerte a 30 días tras la ACIS, estratificado según la indicación clínica y con una estratificación adicional respecto al diseño de estudio y a si los resultados fueron evaluados o no por un neurólogo. La línea a trazos corresponde al riesgo a 30 días combinado para los diversos ECA y en todos los datos de registros. Se realizó una evaluación neurológica independiente en todos los ECA. Algunos ECA no pudieron ser incluidos en este análisis porque no se dispuso de una evaluación de los resultados de ictus o muerte a los 30 días. En la Tabla 3 se indican las estimaciones combinadas de los riesgos absolutos según el momento en el que se realizaba la evaluación del resultado. El riesgo a 30 días de ictus fue del 3,9% (IC del 95%, 3,4 a 4,4; 118 estudios; 27.186 pacientes); el de ictus o muerte fue del 4,7% (IC del 95%, 4,1 a 5,2; 113 estudios; 25.237 pacientes); y el de ictus, muerte o IM fue del 5,3% (IC del 95%, 4,6 a 6,0; 63 estudios; 17.291 pacientes). Los correspondientes riesgos intrahospitalarios y periintervención fueron ligeramente inferiores. Sin embargo, había una heterogeneidad sustancial entre los distintos estudios. La exclusión de los resúmenes o de los estudios postcomercialización que podrían haber incluido a pacientes considerados también en los estudios individuales publicados, no modificó las estimaciones (datos no mostrados). Por lo que respecta a la calidad de los estudios, los análisis de metarregresión indicaron que el riesgo de ictus o muerte a 30 días no estaba relacionado con el contexto de realización del estudio (multicéntrico 4,8% frente a unicéntrico, 4,6%, p = 0,77), ni con el reclutamiento de pacientes consecutivos (sí 4,6% frente a no 4,8%, p = 0,89). Sin embargo, el riesgo era más alto cuando había una descripción adecuada de la población (sí 5,2% frente a no 4,0%, p =0,04), en el caso de una inclusión prospectiva (sí 5,2% frente a no 4,2%, p = 0,07), y cuando la evaluación era realizada por un neurólogo (sí 5,4% frente a no 4,1%, p = 0,02). No observamos indicio alguno de sesgo de publicación en el análisis visual de los gráficos de embudo del tamaño muestral de los estudios en relación con el riesgo absoluto de ictus o muerte, puesto que había igual número de estudios de menor tamaño con riesgos de complicaciones altos y bajos (Figura I del suplemento, accesible online en http://ictus.ahajournals.org). Los resultados fueron similares para el ictus y para el conjunto de ictus, IM o muerte (datos no mostrados). En la Figura 2 se presenta un resumen de los RR combinados de ictus y de ictus o muerte con la ACIS para los diferentes análisis de subgrupos preespecificados, por separado en función de los síntomas clínicos, las características de los Touzé y cols. Revisión sistemática de los riesgos perioperatorios de ictus y muerte... 29 pacientes (incluidos los factores de riesgo vascular y los antecedentes patológicos previos), las características de la estenosis y los factores técnicos. (Pueden consultarse los gráficos de Forest de los correspondientes análisis en las Figuras II y III del suplemento). Las estenosis sintomáticas (RR = 1,86; IC del 95%, 1,61 a 2,14), los episodios cerebrales frente a los oculares (RR = 2,28; IC del 95%, 1,08 a 4,77), la edad >75 a 80 años (RR = 1,93; IC del 95%, 1,66 a 2,24), la EC (RR = 1,41; IC del 95%, 0,97 a 2,06), los antecedentes de bypass arterial coronario (RR = 2,21; IC del 95%, 1,03 a 4,72), y la EAC (RR = 2,04; IC del 95%, 0,92 a 4,52) se asociaron a un riesgo superior de ictus o muerte tras la ACIS. Hubo también una tendencia a un riesgo superior de complicaciones en los pacientes que presentaban placas calcificadas. En cambio, el riesgo de ictus o muerte tras la ACIS fue inferior en los pacientes tratados por una enfermedad carotídea causada por una reestenosis tras una EDAC, en comparación con los tratados por una estenosis carotídea aterosclerótica (RR = 0,45; IC del 95%, 0,28 a 0,71). El empleo de sistemas de protección cerebral se asoció a un menor riesgo de ictus o muerte (RR = 0,57; IC del 95%, 0,43 a 0,76). El riesgo de ictus o muerte no estaba relacionado con el sexo, la oclusión carotídea contralateral, la diabetes mellitus, la ulceración de la placa, el momento de realización de la ACIS o el lado de la lesión tratada. Se observaron resultados similares en cuanto al resultado de ictus, excepto porque la hipertensión se asociaba de manera significativa a un riesgo superior de complicaciones (RR = 1,86; IC del 95%, 1,30 a 2,68). A diferencia de la heterogeneidad sustancial observada en las estimaciones combinadas de los riesgos absolutos, la heterogeneidad en estas estimaciones combinadas de los RR fue nula o tan solo moderada. No observamos indicio alguno de sesgos de publicación en estos análisis en los gráficos de embudo ni en la prueba de Egger (datos no mostrados). El riesgo combinado de ictus a 30 días fue del 6,3% (IC del 95%, 4,8 a 8,0) en los estudios con un año de mitad de cohorte anterior a 1998, del 5,0% (IC del 95%, 4,1 a 5,9) en los estudios con un año de mitad de cohorte situado entre 1998 y 2002, y del 3,9% (IC del 95%, 3,0 a 4,9) en los estudios con un año de mitad de cohorte posterior a 2002. Un análisis de metarregresión tomando el año de mitad de cohorte como covariable puso de manifiesto una reducción significativa del riesgo de ictus o muerte a los 30 días a lo largo del tiempo, que correspondía a una reducción del RR de ≈6% anual (91 estudios, p <0,0001; Figura 3). Se obtuvo un resultado similar al utilizar el año de publicación en vez del año de mitad de cohorte (p <0,0001) o al considerar el ictus solamente en vez del ictus o la muerte (datos no presentados). En la Figura 4 se muestran los riesgos absolutos combinados a 30 días para el ictus o la muerte, estratificados según la indicación clínica y con una estratificación adicional según el diseño del estudio y según que los resultados fueran evaluados o no por un neurólogo. En los pacientes con estenosis sintomáticas, el riesgo absoluto global de ictus o muerte a los 30 días fue del 7,6% (IC del 95%, 6,3 a 9,1; 42 estudios; 4.910 pacientes). Ese riesgo era superior en la ECA (10,8%; IC del 95%, 6,8 a 15,5) en comparación con los registros que incluían solamente a pacientes sintomáticos (7,3%; IC del 95%, 5,3 a 9,6; p = 0,16) y en comparación con los subgru- pos de pacientes sintomáticos incluidos en otros registros (7,0%; IC del 95%, 5,2 a 9,0; p = 0,04). El riesgo absoluto de ictus o muerte a 30 días no fue significativamente mayor en los estudios en los que hubo una evaluación neurológica independiente en comparación con los estudios en los que no estaba claro que el método de evaluación de los resultados estuviera a cargo de un neurólogo independiente. En los pacientes con estenosis asintomáticas, sólo hubo un ECA en el que no se hubiera evaluado claramente el riesgo de ictus o muerte a 30 días34, y 1 de los estudios de registro incluyó únicamente a pacientes asintomáticos41. El riesgo absoluto global de ictus o muerte fue del 3,3% (IC del 95%, 2,6 a 4,1; 23 estudios; 8.504 pacientes). Al igual que para la estenosis sintomática, los riesgos no fueron significativamente mayores en los estudios en los que se utilizó una evaluación neurológica independiente. Todos estos resultados fueron similares al utilizar la presencia de al menos 1 neurólogo en la relación de autores como indicador de calidad, en vez de la evaluación neurológica independiente (datos no presentados). Discusión En primer lugar, hemos evidenciado que el riesgo global de ictus o muerte a 30 días después de una ACIS es de ≈5%, pero presenta variaciones sustanciales en los distintos estudios. Estas variaciones pueden ser consecuencia de diferencias en la combinación de tipos de casos, el diseño, la calidad del estudio o la pericia de los médicos que realizan las intervenciones. En segundo lugar, los riesgos de la ACIS dependen de la indicación clínica, de tal manera que los pacientes sintomáticos tienen una probabilidad de presentar complicaciones aproximadamente doble de la de los pacientes con estenosis asintomáticas; y dependen también de características de los pacientes que son asimismo factores de riesgo quirúrgico elevado, como la edad, la hipertensión y los antecedentes de EC (incluido el bypass arterial coronario). En cambio, otros factores de riesgo quirúrgico elevado establecidos o bien no parecieron influir en el riesgo de complicaciones de la ACIS (sexo femenino y oclusión carotídea contralateral) o bien se asociaron incluso a un riesgo inferior (reestenosis carotídea tras una EDAC). En consecuencia, nuestros resultados sugieren claramente que hay factores clínicos simples que es probable que faciliten la selección de los candidatos adecuados para la ACIS en futuros ensayos clínicos de comparación de esta técnica con la EDAC, y finalmente en la práctica clínica. Por último, nuestros resultados sugieren que los riesgos del tratamiento han disminuido a lo largo del tiempo, y que el uso de un dispositivo de protección cerebral se asocia a un riesgo de complicaciones inferior. Identificamos un total de 206 estudios que presentaban datos sobre los riesgos de la ACIS, en 54.713 pacientes. Debe señalarse que los pacientes de ECA suponían solamente un 3% de la población total, lo cual subraya el grado en el que esta técnica se ha venido aplicando en la práctica clínica a pesar del bajo nivel de la evidencia existente. Dada la heterogeneidad sustancial existente entre los estudios, nuestras estimaciones combinadas de los riesgos operatorios absolutos no pueden interpretarse de manera directa. Sin embargo, el IC del 95% obtenido en un metaanálisis de efectos aleatorios describe bien la incertidumbre relativa al riesgo medio. 30 Stroke Marzo 2010 Por ejemplo, para el conjunto de todos los estudios, el riesgo de ictus o muerte a 30 días fue, con el intervalo de confianza del 95%, al menos igual al 4,1% y de hasta un 5,2%. Es interesante señalar que se ha observado una heterogeneidad similar en revisiones sistemáticas previas de los riesgos de la EDAC16–18. La heterogeneidad existente entre los estudios tienen varios orígenes que pudimos identificar. Está claramente establecido que el efecto beneficioso de la EDAC depende en gran medida de la indicación clínica, de tal manera que se observa un efecto beneficioso superior en los pacientes sintomáticos en comparación con los asintomáticos, así como que el riesgo de complicaciones perioperatorias es superior en los pacientes sintomáticos17,18,42,43. En una revisión sistemática anterior se observó que solamente ≈25% de los estudios realizados en la EDAC habían estratificado sus resultados en función de que los pacientes fueran asintomáticos o sintomáticos16. De forma análoga, nosotros observamos que solamente un 21% de los estudios de la ACIS presentaban los riesgos estratificados según la indicación clínica. Hemos evidenciado que, al igual que en la EDAC, los pacientes sintomáticos tienen una probabilidad aproximadamente doble de la de los pacientes asintomáticos de sufrir complicaciones después de la ACIS. Este resultado se basa en análisis de subgrupos, es decir, en la comparación de los pacientes sintomáticos y asintomáticos dentro de los mismos estudios, y no observamos una heterogeneidad entre los distintos estudios a este respecto. Para los pacientes sintomáticos, observamos que el riesgo de ictus o muerte a 30 días de la ACIS era del 7,6%, con un límite inferior del IC del 95% del 6,3%, valor éste que es superior al riesgo a 30 días asociado a la EDAC según lo observado en una revisión sistemática anterior (5,1%; IC del 95% 4,6 a 5,6)16. Este nivel de riesgo es también superior al umbral de riesgo establecido por los comités ad hoc de las guías del American Heart Association Stroke Council, que indican que el riesgo combinando de ictus y muerte como consecuencia de la EDAC no debe ser superior al 5% para los pacientes con ataques isquémicos transitorios y al 7% en los pacientes con ictus2,3. Aun siendo cuestionable, esta comparación de los riesgos absolutos combinados concuerda plenamente con el metaanálisis de ECA de comparación de la ACIS con la EDAC en pacientes sintomáticos y pone de manifiesto que la ACIS se asocia a un aumento del 40% en el riesgo de ictus o muerte a 30 días12. Hemos observado también que el riesgo fue mayor en los ECA en comparación con los registros. Es probable que la definición de la estenosis sintomática fuera diferente en los distintos estudios, aunque esta información no puede extraerse con facilidad de las publicaciones (por ejemplo, algunos registros consideraban los ictus correspondientes a cualquier territorio o en cualquier periodo de tiempo). Además, al igual que en el caso de la EDAC18, nuestros resultados sugieren que la calidad de la evaluación neurológica explica en parte las diferencias observadas. Aunque la mayoría de los pacientes incluidos en los registros presentaban estenosis asintomáticas, obtuvimos datos específicos muy limitados sobre los riesgos de la ACIS en esos pacientes. El riesgo global de ictus o muerte a 30 días con la ACIS fue del 3,3%, con un límite inferior del IC del 95% de 2,6%. Ese nivel de riesgo está próximo al que se da con la EDAC (2,8%; IC del 95%, 2,4 a 3,2)16 y al umbral de riesgo del 3% establecido en las directrices para la estenosis asintomática2,3. Muchos registros se han centrado en los pacientes que tienen un riesgo quirúrgico elevado según un conjunto de criterios, que varían en número y tipo, y han planteado la hipótesis de que esos pacientes debieran ser candidatos adecuados para la ACIS4. Los factores que se citan con frecuencia como asociados a un riesgo quirúrgico superior son factores anatómicos como las lesiones no accesibles quirúrgicamente, la EDAC o la irradiación cervical previas, la edad avanzada, la oclusión carotídea contralateral y las comorbilidades médicas. Sin embargo, no hay una evidencia que indique que en los pacientes de riesgo quirúrgico elevado se obtenga un efecto beneficioso con alguna otra estrategia de revascularización en comparación con el tratamiento médico solo44. Además, es posible que las comorbilidades asociadas a un mayor riesgo perioperatorio en la EDAC aumenten también el riesgo periintervención de la ACIS. Los estudios en los que se ha investigado si los factores que identifican a los pacientes de riesgo quirúrgico elevado tienen alguna influencia en los riesgos de la ACIS han tenido generalmente una potencia estadística baja para poder extraer conclusiones fiables. Aunque los análisis previos de ECA y de registros han indicado de manera uniforme que la edad tiene tan solo una influencia pequeña en el riesgo de complicaciones tras la EDAC45, los pacientes ancianos se consideran un grupo de riesgo quirúrgico elevado y posibles candidatos adecuados para la ACIS. De hecho, nosotros observamos que la edad se asociaba a un aumento de ≈2 veces en el riesgo de complicaciones tras la ACIS, lo cual sugiere que la edad tienen más influencia en los riesgos de la ACIS que en los riesgos de la EDAC. Es interesante señalar que varios estudios han indicado que los pacientes de mayor edad tienen una mayor probabilidad de presentar vasos tortuosos con una calcificación intensa que probablemente aumentan el riesgo de embolización durante la manipulación de la guía y los cambios de catéter en algunas de las fases de la ACIS46,47. Otra observación importante de nuestro análisis es que, a diferencia de la EDAC, en la que las mujeres tienen un riesgo de complicaciones superior al de los varones, los riesgos de la ACIS no muestran una relación con el sexo. Sin embargo, aunque en ambos casos están próximos a 1 y no son significativos, los RR combinados de las mujeres respecto a los varones en cuanto al ictus y en cuanto al ictus o la muerte, se encuentran a cada lado del valor 1. De hecho, los resultados obtenidos para el ictus eran consecuencia en gran parte de los del registro CAPTURE, en el que las mujeres presentaron un riesgo de ictus ligeramente superior en el análisis univariado, pero no en los análisis multivariados 48. La ausencia de efecto del sexo sobre el riesgo de ACIS se ha demostrado también en 2 estudios recientes, publicados fuera del periodo de inclusión definido para nuestra revisión sistemática49,50. La inclusión de esos estudios no hubiera modificado nuestras estimaciones [RR combinado para el ictus = 1,02; IC del 95%, 0,87 a 1,27; p(het) = 0,87; RR combinado para el ictus o la muerte = 0,90; IC del 95%, 0,74 a 1,10; p(het) =0,84]. Touzé y cols. Revisión sistemática de los riesgos perioperatorios de ictus y muerte... 31 Nuestra observación de que los riesgos de la ACIS no dependen de la oclusión carotídea contralateral y de que son inferiores en los pacientes con una reestenosis tras la EDAC es también importante, puesto que permite identificar a una posible población diana en la que la ACIS podría compararse con la EDAC. Por último, aunque se obtuvieron a partir de datos más limitados, y coincidiendo con lo indicado por datos previos sobre la EDAC, nuestros resultados sugieren que los riesgos de la ACIS son mayores en los pacientes que sufrieron un episodio cerebral en comparación con los que presentaron un episodio ocular, y que los antecedentes previos de EC pueden no ser útiles para la selección de los candidatos adecuados para la ACIS. Hay algunas razones que podrían explicar que los factores de riesgo para las complicaciones puedan diferir en la EDAC y la ACIS. El mayor riesgo de complicaciones tras la EDAC en las mujeres suele atribuirse al hecho de que la arteria carótida interna es más pequeña en ellas que en los varones, lo cual predispone a los errores técnicos o a la trombosis postoperatoria inmediata, si bien esta hipótesis ha sido puesta en duda51,52. La cirugía de la reestenosis carotídea se asocia a un riesgo elevado de complicaciones, debido probablemente a las importantes modificaciones fibrosas postoperatorias que se producen en el tejido cervical y al hecho de que la reestenosis se deba con frecuencia a una hiperplasia de la mioíntima más que a la aterosclerosis53. Aunque en los pacientes sintomáticos con reestenosis, el efecto beneficioso a largo plazo de la EDAC continúa justificando el riesgo quirúrgico inmediato y hace que sea inferior al riesgo del tratamiento médico solo, la única evidencia derivada de estudios aleatorizados sugiere que los pacientes asintomáticos con reestenosis evolucionan ligeramente mejor con el tratamiento médico1. Dado que la ACIS no requiere una incisión cervical ni arterial, es probable que los factores anatómicos relacionados con el sexo o con la EDAC previa sean superados por la ACIS. La oclusión carotídea contralateral puede comprometer los mecanismos de compensación y, por consiguiente, la perfusión cerebral durante el pinzamiento de la arteria carotídea que es necesario para practicar la EDAC. La menor duración de la oclusión carotídea durante la ACIS, en comparación con la EDAC, podría explicar la ausencia de aumento del riesgo operatorio durante la ACIS. En cambio, otros factores como la edad, la hipertensión y los antecedentes de EC o EAC, tienen una intensa asociación con la gravedad y la extensión de la aterosclerosis y es probable que estén relacionados con el riesgo de complicaciones tromboembólicas durante el avance arterial por la aorta y la arteria carótida. Con el empleo de un análisis de metarregresión, observamos que los riesgos de la ACIS han disminuido a lo largo del tiempo, entre 1993 y 2006. Esto puede ser consecuencia de mejoras en la técnica de la ACIS, los dispositivos utilizados o la formación de los especialistas, así como de una mejor selección de los pacientes candidatos para la ACIS a lo largo del tiempo. El desarrollo de dispositivos de protección frente a la embolia durante la intervención de ACIS puede haber sido un avance importante. Las revisiones sistemáticas anteriores de series de casos sin asignación aleatoria indicaron que el uso de los dispositivos de protección cerebral parece reducir las complicaciones tromboembólicas durante la ACIS54 y también la incidencia de nuevas lesiones isquémicas, mayoritariamente asintomáticas en la resonancia magnética con ponderación de difusión obtenida en las primeras 48 horas siguientes a la ACIS55,56. Nuestros resultados, obtenidos a partir de un mayor número de estudios, concuerdan con estos datos previos. Sin embargo, hubo una heterogeneidad significativa entre los distintos estudios en este análisis. De hecho, la aparente ventaja de los dispositivos de protección cerebral podría ser ilusoria. Ciertamente, el uso de estos dispositivos de protección ha aumentado con el paso del tiempo, y el aparente efecto protector puede haberse visto afectado por los efectos de confusión derivados de los avances en las técnicas de implantación de stents y en la selección de los pacientes a lo largo del tiempo. Podría reflejar también la selección de los pacientes. Además, continúa sin haber datos de estudios aleatorizados en los que se compare la ACIS con o sin protección cerebral, y puesto que los dispositivos de protección deben superar la estenosis arterial, es posible que los propios dispositivos pudieran causar complicaciones. Nuestro estudio tiene varias posibles limitaciones. En primer lugar, la existencia de factores de confusión constituye una amenaza importante en un metaanálisis de estudios observacionales, puesto que los análisis de subgrupos se basan en comparaciones univariadas. Solamente un metaanálisis de los datos individuales permitiría abordar esta cuestión. Sin embargo, nuestros análisis de subgrupos fueron muy uniformes en los diversos estudios y disponen de explicaciones fisiopatológicas plausibles. Además, con el empleo de un enfoque similar para la EDAC, todos los factores de riesgo para las complicaciones observadas en las revisiones sistemáticas de los estudios de registros se reprodujeron en un análisis combinado de los datos individuales de ECA16– 20,43. Así pues, es improbable que nuestros resultados sean falsamente positivos. En segundo lugar, aunque incluimos los estudios publicados en cualquier idioma y utilizamos múltiples fuentes de datos, los sesgos de publicación podrían haber distorsionado nuestros resultados, puesto que los registros con un riesgo de complicaciones bajo podrán tener una mayor probabilidad de haber sido publicados. Sin embargo, con el empleo de gráficos de dispersión de puntos sencillos, puesto que no hay ninguna prueba estadística validada para valorar el sesgo de publicación en un metaanálisis de riesgos absolutos, no observamos indicio alguno de sesgos de publicación. Además, los sesgos de publicación son improbables en los análisis de subgrupos, dado que es improbable que las posibilidades de publicación estén relacionadas con los resultados de los análisis de subgrupo, y no observamos indicio alguno de sesgos de este tipo en los gráficos de embudo. Por otra parte, los RR no suelen depender del riesgo absoluto. En tercer lugar, la posible inclusión de datos duplicados podrían haber distorsionado nuestros resultados57. Sin embargo, examinamos detalladamente la relación de autores y el contexto de cada artículo con objeto de excluir en la mayor medida posible las poblaciones duplicadas. Además, realizamos análisis de sensibilidad excluyendo algunos registros amplios que podrían haber incluido a pacientes cuyos datos se hubieran publicado también en estudios unicéntricos más pequeños, y los resultados obtenidos fueron similares. En cuarto lugar, la heterogeneidad 32 Stroke Marzo 2010 existente en la calidad de los datos constituye otro problema en los metaanálisis de estudios observacionales. Aunque la calidad de la evaluación de las variables de valoración fue diferente en los distintos estudios, nuestros resultados no se vieron influidos por este parámetro. En quinto lugar, algunos análisis de subgrupos (por ejemplo, tipo de episodio cerebrovascular, aspecto superficial de la placa o antecedentes de bypass arterial coronario) se basaron en un número relativamente bajo de estudios y requerirían datos de confirmación adicionales. Por último, hay otros posibles factores de riesgo para las complicaciones que no pudieron ser evaluados. Por ejemplo, el papel de la experiencia del operador y la curva de aprendizaje no pudieron evaluarse en nuestra revisión sistemática, puesto que no había una definición estandarizada de esos factores en los distintos estudios. Es probable que los factores anatómicos arteriales influyan también en la viabilidad y los riesgos de la ACIS58. En resumen, los riesgos de la ACIS son globalmente superiores a los de la EDAC en los pacientes sintomáticos. Nuestros resultados respaldan la recomendación de las actuales guías en cuanto a que la ACIS no debe utilizarse en los pacientes que son candidatos adecuados para el tratamiento quirúrgico. Sin embargo, también sugieren que existen factores que es probable que faciliten la selección de los candidatos adecuados para la ACIS en futuros ensayos y finalmente en la práctica clínica. Agradecimientos Quisiéramos agradecer especialmente a Peter A. Ringleb y a los investigadores del SPACE que nos proporcionaran datos de subgrupos no publicados de este ensayo. Damos las gracias a Marta Pasquini, Enrico Floβmann, Kaori Floβmann, Hu Chau, Maria Koziak, Daniel Freddy, Didier Leys, Ghislain Nokam, Barish Turak y Suzanne Vobecky por su ayuda en la extracción de los datos de artículos publicados en lenguas diferentes del francés y el inglés. Agradecemos también a Bernard Beyssen y Olivier Naggara sus recomendaciones sobre aspectos técnicos y a Isabelle Laurent su apoyo técnico. Ninguna. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Declaraciones de intereses Bibliografía References 1. Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, Stern B, Wilterdink J. Carotid endarterectomy–an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:794 – 801. 2. Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006; 37:1583–1633. 3. Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-Sponsored by the Council on Cardiovascular Radiology and 16. 17. 18. 19. 20. 21. 22. Intervention: The American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577– 617. Touzé E, Calvet D, Chatellier G, Mas JL. Carotid stenting. Curr Opin Neurol. 2008;21:56 – 63. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729 –1737. Coward LJ, Featherstone RL, Brown MM. Safety and efficacy of endovascular treatment of carotid artery stenosis compared with carotid endarterectomy: a Cochrane systematic review of the randomized evidence. Stroke. 2005;36:905–911. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660 –1671. Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239 –1247. Ringleb PA, Chatellier G, Hacke W, Favre JP, Bartoli JM, Eckstein HH, Mas JL. Safety of endovascular treatment of carotid artery stenosis compared with surgical treatment: a meta-analysis. J Vasc Surg. 2008; 47:350 –355. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Ansel G, Strickman NE, Wang H, Cohen SA, Massaro JM, Cutlip DE. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579. Jeng JS, Liu HM, Tu YK. Carotid angioplasty with or without stenting versus carotid endarterectomy for carotid artery stenosis: a meta-analysis. J Neurol Sci. 2008;270:40 – 47. Ederle J, Featherstone RL, Brown MM. Randomized controlled trials comparing endarterectomy and endovascular treatment for carotid artery stenosis: a Cochrane systematic review. Stroke. 2009;40:1373–1380. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touze E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Henon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885– 892. Bond R, Rerkasem K, Rothwell PM. Systematic review of the risks of carotid endarterectomy in relation to the clinical indication for and timing of surgery. Stroke. 2003;34:2290 –2301. Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke. 1996;27:266 –269. Rothwell PM, Slattery J, Warlow CP. A systematic review of the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis. Stroke. 1996;27:260 –265. Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ. 1997;315:1571–1577. Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351: 1493–1501. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. Observational 283:2008 –2012 Hayden JA, Co studies in syste Freeman M, T square root. An DerSimonian R Trials. 1986;7: Trinquart L, To lessons from a atherosclerosis. Gao S. Combin Comput. 2004; Egger M, Dav detected by a s Higgins JP, Th Stat Med. 2002 Higgins JP, Th tency in meta-a Higgins JPT, G of Intervention Collaboration, Alberts MJ. Re carotid artery s Abstract. Brooks WH, M angioplasty and in a community Brooks WH, M angioplasty and asymptomatic hospital. Neuro Hoffman A, En EW, Lyrer P. prospective ra follow-up (BA 84 – 89. Ling F, Jiao L stenting for th (TESCAS-C). Naylor AR, Bo London NJ, B stenting versus 1998;28:326 –3 Dalainas I, Nan antiplatelet reg Cardiovasc Int Kaposzta Z, S-nitrosoglutath angioplasty. Ci Jordan WD Jr, cost compariso my for the tre 16 –22. Marine LA, Ru Treatment of as after carotid s standard-risk p Redgrave JN, R Curr Opin Neu Rothwell PM, MR, Warlow domised contr stenosis. Lance Ederle J, Feath vascular treatm patients not su Vertebral Arte brovasc Dis. 2 Bond R, Rerka associations be endarterectomy Bazan HA, Pra aortic arch cal affirms the value of tenting. Curr Opin h carotid stenosis in Angioplasty Study 1729 –1737. d efficacy of endod with carotid endndomized evidence. oulin T, Becquemin Watelet J, Pruvo JP, F, Touze E, Giroud X. Endarterectomy e carotid stenosis. HH, Fraedrich G, Kunze A, Marx P, Zeumer H, Hacke otected angioplasty ents: a randomised i JM, Eckstein HH, otid artery stenosis J Vasc Surg. 2008; J, Bajwa TK, Ansel o JM, Cutlip DE. ectomy in high-risk or without stenting sis: a meta-analysis. ed controlled trials nt for carotid artery ;40:1373–1380. transient ischaemic edrich G, Hacke W, n O. Results of the erectomy (SPACE) multinational, pro3–902. eau H, Viguier A, , Touze E, Julia P, zelin G, Houdart E, ni R, Chatellier G. ymptomatic Severe from a randomised, iew of the risks of ation for and timing comparison of the my for symptomatic . view of the risks of tic carotid stenosis. angiographic preectomy: systematic P, Barnett HJ. Endion to clinical sub–924. n BT, Mishkel GJ, pma JJ, Snead DB, ery stenting versus Med. 2004;351: son GD, Rennie D, nalysis of observag. Meta-analysis Of S-nitrosoglutathione reduces asymptomatic embolization after carotid Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe angioplasty. Circulation. 2002;106:3057–3062. Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, 40. Jordan WD Jr, Roye GD, Fisher WS III, Redden D, McDowell HA. A multicentre trial. Lancet Neurol. 2008;7:885– 892. cost comparison of balloon angioplasty and stenting versus endarterecto16. Bond R, Rerkasem K, Rothwell PM. Systematic review of the risks of my for the treatment of carotid artery stenosis. J Vasc Surg. 1998;27: carotid endarterectomy in relation to the clinical indication for and timing 16 –22. of surgery. Stroke. 2003;34:2290 –2301. 41. Marine LA, Rubin BG, Reddy R, Sanchez LA, Parodi JC, Sicard GA. 17. Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the Touzé y cols. Revisión sistemáticaTreatment de los riesgos perioperatorios de ictus y muerte... 33 of asymptomatic carotid artery disease: similar early outcomes risks of stroke and death due to carotid endarterectomy for symptomatic after carotid stenting for high-risk patients and endarterectomy for and asymptomatic stenosis. Stroke. 1996;27:266 –269. standard-risk patients. J Vasc Surg. 2006;43:953–958. 18. Rothwell PM, Slattery J, Warlow CP. A systematic review of the risks of Observational in Epidemiology group. JAMA. 2000; 42. Redgrave JN, Rothwell PM. Asymptomatic carotid stenosis: what to do. stroke and deathStudies due to endarterectomy for(MOOSE) symptomatic carotid stenosis. 283:2008 –2012. –265. Curr Opin Neurol. 2007;20:58 – 64. Stroke. 1996;27:260 23.Rothwell Hayden JA, P, Bombardier C. CP. Evaluation the quality of prognosis 43. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg 19. PM,Cote Slattery J, Warlow Clinicalofand angiographic prestudiesofin stroke systematic Ann carotid Intern Med. 2006;144:427– 437. MR, Warlow CP, Barnett HJ. Analysis of pooled data from the randictors and reviews. death from endarterectomy: systematic 24.review. Freeman M, 1997;315:1571–1577. Tukey J. Transformations related to the angular and the domised controlled trials of endarterectomy for symptomatic carotid BMJ. square root. Math M, Stat. 1950;21:607– 611. CP, Barnett HJ. Endstenosis. Lancet. 2003;361:107–116. 20. Rothwell PM,Ann Eliasziw Gutnikov SA, Warlow 25.arterectomy DerSimonian Laird N. Meta-analysis in clinical trials. ControlsubClin 44. Ederle J, Featherstone RL, Brown MM. Long-term outcome of endoforR, symptomatic carotid stenosis in relation to clinical Trials.and 1986;7:177–188. vascular treatment versus medical care for carotid artery stenosis in groups timing of surgery. Lancet. 2004;363:915–924. 26.Yadav Trinquart L, TouzéMH, E. Pitfalls meta-analysis of observational studies: patients not suitable for surgery and randomised in the Carotid and 21. JS, Wholey Kuntz in RE, Fayad P, Katzen BT, Mishkel GJ, lessonsTK, from a systematic review of theJaff risks of stenting forSnead intracranial Vertebral Artery Transluminal Angioplasty study (CAVATAS). CereBajwa Whitlow P, Strickman NE, MR, Popma JJ, DB, atherosclerosis. 2009;40:e586 – e587. brovasc Dis. 2009;28:1–7. Cutlip DE, Firth Stroke. BG, Ouriel K. Protected carotid-artery stenting versus 27.endarterectomy Gao S. Combining using theN logistic J Stat 45. Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the in binomial high-riskdata patients. Engl Jnormal Med. model. 2004;351: Comput. 2004;74:293. associations between age and sex and the operative risks of carotid 1493–1501. 28.Stroup Egger DF, M, Berlin Davey JA, SG,Morton Schneider Minder C. Bias in endarterectomy. Cerebrovasc Dis. 2005;20:69 –77. 22. SC, M, Olkin I, Williamson GD,meta-analysis Rennie D, detected a simple, graphical test. BMJ. – 634. 46. Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A. Increased Moher D, by Becker BJ, Sipe TA, Thacker SB.1997;315:629 Meta-analysis of observa29.tional Higgins JP, Thompson SG. Quantifying a meta-analysis. aortic arch calcification in patients older than 75 years: implications studies in epidemiology: a proposal heterogeneity for reporting. in Meta-analysis Of for carotid artery stenting in elderly patients. J Vasc Surg. 2007;46: Stat Med. 2002;21:1539 –1558. 841– 845. 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsis47. Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The tency in meta-analyses. BMJ. 2003;327:557–560. impact of increasing age on anatomic factors affecting carotid angioplasty 31. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Updated September 2008. The Cochrane and stenting. J Vasc Surg. 2007;45:875– 880. Collaboration, 2008. Available from www.cochrane-handbook.org. 48. Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, Hopkins 32. Alberts MJ. Results of the multicenter prospective randomized trial of LN, Atkinson R, Raabe R, Barnwell S, Green R. The CAPTURE registry: carotid artery stenting vs. carotid endarterectomy. Stroke. 2001;32:325. results of carotid stenting with embolic protection in the post approval Abstract. setting. Cathet Cardiovasc Interv. 2007;69:341–348. 33. Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid 49. Howard VJ, Voeks JH, Lutsep HL, Mackey A, Milot G, Sam AD, Tom angioplasty and stenting versus carotid endarterectomy: randomized trial M, Hughes SE, Sheffet AJ, Longbottom M, Avery JB, Hobson RW, Brott in a community hospital. J Am Coll Cardiol. 2001;38:1589 –1595. TG. Does sex matter? thirty-day stroke and death rates after carotid artery 34. Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid stenting in women versus men: results from the Carotid Revascularization angioplasty and stenting versus carotid endarterectomy for treatment of Endarterectomy Versus Stenting Trial (CREST) Lead-in Phase. Stroke. asymptomatic carotid stenosis: a randomized trial in a community 2009;40:1140 –1147. hospital. Neurosurgery. 2004;54:318 –324. 50. Goldstein LJ, Khan HU, Sambol EB, Kent KC, Faries PL, Vouyouka AG. 35. Hoffman A, Engelter T, Taschner C, Mendellowitsch A, Merlo A, Radue Carotid artery stenting is safe and associated with comparable outcomes EW, Lyrer P. Carotid artery stenting versus carotid endarterectomy–a in men and women. J Vasc Surg. 2009;49:315–323. prospective randomised controlled single-centre trial with long-term 51. Lee JW, Pomposelli F, Park KW. Association of sex with perioperative follow-up (BACASS). Schweizer Archiv Neurol Psychiatr. 2008;159: mortality and morbidity after carotid endarterectomy for asymptomatic 84 – 89. carotid stenosis. J Cardiothorac Vasc Anesth. 2003;17:10 –16. 36. for Ling F, Jiao LQ. stenting Preliminary report patients. of trial ofJ endarterectomy versus 52. Rockman CB, Castillo J, Adelman MA, Jacobowitz GR, Gagne PJ, carotid artery in elderly Vasc Surg. 2007;46: stenting Lamparello PJ, Landis R, Riles TS. Carotid endarterectomy in female 841– 845. for the treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chin J Cerebrovasc Dis. 2006;3:4 patients: are the concerns of the Asymptomatic Carotid Atherosclerosis 47. Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent– 8. KC, Faries PL. The 37. impact NaylorofAR, Bolia A, RJ, Pyefactors IF, Smith J, Lennard Lloyd AJ, Study valid? J Vasc Surg. 2001;33:236 –240. increasing ageAbbott on anatomic affecting carotidN, angioplasty London NJ, J Bell study 53. Lal BK. Recurrent carotid stenosis after CEA and CAS: diagnosis and and stenting. VascPR. Surg.Randomized 2007;45:875– 880. of carotid angioplasty and stenting endarterectomy: a stopped trial. JM,Vasc Surg. management. Semin Vasc Surg. 2007;20:259 –266. 48. Gray WA,versus Yadav carotid JS, Verta P, Scicli A, Fairman R, Wholey Hopkins 1998;28:326 54. Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. LN, Atkinson –334. R, Raabe R, Barnwell S, Green R. The CAPTURE registry: Early outcome of carotid angioplasty and stenting with and without 38. results Dalainas I, Nano stenting G, Bianchi P, embolic Stegher S, Malacrida DG. Dual of carotid with protection inG, theTealdi post approval cerebral protection devices: a systematic review of the literature. Stroke. antiplatelet regime versus Interv. acetyl-acetic acid for carotid artery stenting. setting. Cathet Cardiovasc 2007;69:341–348. 2003;34:813– 819. Cardiovasc Intervent 49. Howard VJ, Voeks JH,Radiol. Lutsep 2006;29:519 HL, Mackey–521. A, Milot G, Sam AD, Tom 55. Kastrup A, Nagele T, Groschel K, Schmidt F, Vogler E, Schulz J, 39.M,Kaposzta Z,Sheffet Clifton A, Molloy M, J, Avery Martin JF, Markus HS. Hughes SE, AJ, Longbottom JB, Hobson RW, Brott Ernemann U. Incidence of new brain lesions after carotid stenting with S-nitrosoglutathione reduces asymptomatic embolization after carotid TG. Does sex matter? thirty-day stroke and death rates after carotid artery and without cerebral protection. Stroke. 2006;37:2312–2316. angioplasty. Circulation. 2002;106:3057–3062. stenting in women versus men: results from the Carotid Revascularization 56. Schnaudigel S, Groschel K, Pilgram SM, Kastrup A. New brain lesions 40. Endarterectomy Jordan WD Jr, Versus Roye GD, FisherTrial WS (CREST) III, Redden D, McDowell HA. A Stenting Lead-in Phase. Stroke. after carotid stenting versus carotid endarterectomy: a systematic review cost comparison of balloon angioplasty and stenting versus endarterecto2009;40:1140 –1147. of the literature. Stroke. 2008;39:1911–1919. my for the treatment of carotid artery stenosis. J Vasc Surg. 1998;27: 50. Goldstein LJ, Khan HU, Sambol EB, Kent KC, Faries PL, Vouyouka AG. 57. Senn SJ. Overstating the evidence– double counting in meta-analysis and 16 –22. Carotid artery stenting is safe and associated with comparable outcomes related problems. BMC Med Res Methodol. 2009;9:10. 41. Marine LA, Rubin BG, Reddy R, Sanchez LA, Parodi JC, Sicard GA. in men and women. J Vasc Surg. 2009;49:315–323. 58. Silvestro A, Civelli P, Laffranchini G, Troianiello B, Graziani L. Treatment of asymptomatic carotid artery disease: similar early outcomes 51. Lee JW, Pomposelli F, Park KW. Association of sex with perioperative Influence of anatomical factors on the feasibility and safety of carotid after carotid stenting for high-risk patients and endarterectomy for mortality and morbidity after carotid endarterectomy for asymptomatic stenting in a series of 154 consecutive procedures. J Cardiovasc Med standard-risk patients. J Vasc Surg. 2006;43:953–958. (Hagerstown, Md). 2008;9:137–141. carotid stenosis. J Cardiothorac Vasc Anesth. 2003;17:10 –16. 42. Redgrave JN, Rothwell PM. Asymptomatic carotid stenosis: what to do. Curr Opin Neurol. 2007;20:58 – 64. 43. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. 44. Ederle J, Featherstone RL, Brown MM. Long-term outcome of endovascular treatment versus medical care for carotid artery stenosis in patients not suitable for surgery and randomised in the Carotid and Vertebral Artery Transluminal Angioplasty study (CAVATAS). Cerebrovasc Dis. 2009;28:1–7. 45. Bond R, Rerkasem K, Cuffe R, Rothwell PM. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20:69 –77. 46. Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A. Increased aortic arch calcification in patients older than 75 years: implications 52. Rockman CB, Lamparello PJ, patients: are the Study valid? J V 53. Lal BK. Recurr management. Se 54. Kastrup A, Gro Early outcome cerebral protect 2003;34:813– 81 55. Kastrup A, Na Ernemann U. In and without cer 56. Schnaudigel S, after carotid ste of the literature 57. Senn SJ. Overst related problem 58. Silvestro A, C Influence of an stenting in a se (Hagerstown, M