Presentación de PowerPoint
Anuncio
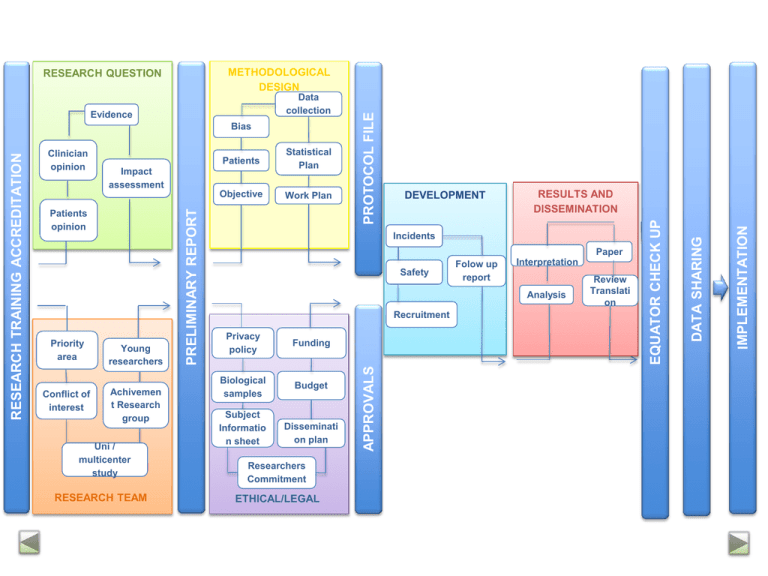
Conflict of interest Objective Work Plan Young researchers Achivemen t Research group Uni / multicenter study RESEARCH TEAM DEVELOPMENT RESULTS AND DISSEMINATION Incidents Paper Safety Folow up report Interpretation Analysis Recruitment Privacy policy Funding Biological samples Budget Subject Informatio n sheet Disseminati on plan Researchers Commitment ETHICAL/LEGAL Review Translati on IMPLEMENTATION Statistical Plan Impact assessment Patients opinion Priority area Patients APPROVALS Clinician opinion PRELIMINARY REPORT RESEARCH TRAINING ACCREDITATION Bias DATA SHARING Evidence EQUATOR CHECK UP Data collection PROTOCOL FILE METHODOLOGICAL DESIGN RESEARCH QUESTION RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION GOOD RESEARCH PRACTICE TRAINING ACCREDITATION Rationale: Good Clinical Practice recommendations indicate that all the health professionals must be trained for every aspect of their work, including research. Clinical research may involve risk to participants. Research Unit actions: Principal Investigators of a new project must have “IP Txartela” (PI card). It will be given if they have a large experience or they attend the following courses: “Ethical and legal issues in biomedical research” and “Good Practice for data management workshop” Information: Clinical Research Training Program, secretary at Araba Research Unit : unidadinvestigacion.araba@osakidetza.net 1 General Medical Council (2013) Good medical practice. www.gmc-uk.org/gmp RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION PRELIMINARY REPORT Rationale: the relevance and suitability of the research question and the background of the research team must be the key point of the adequacy of the study. This may be helpful as a first step to elaborate the research report in order to get financial support or future publication. Research Unit actions: a) To create a template for the preliminary report; b) Check and register reports. RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION PROTOCOL REGISTRY Rationale: it is believed that registry of clinical trials may reduce the risk of selective publication and increase transparency in research, thus both funding agencies and journal editors and managers are demanding registration of research1. Recently, new empirical evidence has been published that suggests inverse association between study registration and positive findings2. Although the greatest part of available evidence refers to clinical trials, there is no reason to think this could not be applied to other type of studies. Research Unit actions: a) To elaborate a template for presenting any type of study; b) To write a complete research report following Araba Research Unit template or, if applicable, using available guidelines: SPIRIT –clinical trials, PRISMA-P –systematic reviews; c) Registry of research protocol and/or report in Araba Research Unit, leaving proof of date and last version; d) Registry in clinicaltrials.com or other similar approved registries. 1 Krleza-Jerić K, Chan A-W, Dickersin K, Sim I, Grimshaw J, Gluud C. Principles for international registration of protocol information and results from human trials of health related interventions: Ottawa statement (part 1). BMJ. 2005;330 (7497):956-958. 2 Emdin C, Odutayo A, Hsiao A, Shakir M, Hopewell S, Rahimi K, Altman D. Association of Cardiovascular Trial Registration With Positive Study Findings: Epidemiological Study of Randomized Trials (ESORT). JAMA Int Med. 2014. Published online December 29, 2014 RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION LAW REQUIREMENTS Rationale: biomedical research with human beings is regulated by a complex legal and ethical framework in order to protect research participants. Clinical Research Ethical Committees guarantee that research is conducted applying ethical regulations. In Alava region, these committees are Araba University Hospital Clinical Research Ethical Committee and Basque Country Clinical Research Ethical Committee. Research Unit actions: a) To verify all required permissions are applied for according to the study scope and type of research design; b) if necessary, to advice with process paperwork. RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION EQUATOR STATEMENT AND APPROVAL OF RESEARCHERS Rationale: Although it has been discussed in the ‘preparation of the study’ stage, the tasks undertaken by each team member have to be updated and therefore, so have the roles regarding the authorship. This implies rechecking the ICMJE authorship criteria. For authorship to be considered, it is necessary to: 1) substantially contribute to the conception and design, data collection, or analysis and interpretation; 2) contribute to the manuscript or to critically revise it with a relevant intellectual component; 3) give final approval of the version to be published. Prior to submission each author should check for correctness of its name, initials, affiliation… Research Unit actions: a) Declaration of the PI of having filtered and adequated the manuscript to the criteria of the corresponding EQUATOR statement; b) Archive the manuscript provided with version number and signed approval of all the researchers. RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION DATA SHARING Rationale: The sharing of data from clinical studies will accelerate new discoveries avoiding, for example, study duplication and will optimize the profit devived from the effort of researchers. There are also some risks and challenges such as maintaining the privacy of the participants, protecting the rights of the promoters or guaranteeing the validity of subsequent studies1. According to the report of the IOM, these principles should be followed: 1) to maximize the benefits of the clinical studies while minimizing the risks of information sharing; 2) to respect the rights of the participants; 3) to increase the confidence of public opinion in public research; 4) to share the data in a reasonable and transparent manner. Research Unit actions: a) Raw data from study’s main article will be hosted in the research unit (RU) and will be available to researchers upon request for a maximum period of six months after publication; b) All the data collected during the study will be hosted in the RU and will be available to researchers upon request after a maximum period of 18 months after completion of the study; c) The RU will host all the documents generated throughout the course of the study which may contain information necessary for optimal understanding of the research and for an unbiased interpretarion of findings. This encompasses documents not routinelly available such as reports submitted to committees or regulatory agencies to justify the study, or the reviews made by the journals to which manuscripts about the study have been sent. 1 Institute of Medicine (IOM). 2015. Sharing clinical trial data: Maximizing benefits, minimizing risk. Washington, DC: The National Academies Press et al.: Preparing raw clinical data for publication: guidance for journal editors, authors, and peer reviewers. Trials 2010 11:9. 2 Hrynaszkiewicz RESEARCH TEAM RESEARCH QUESTION METHODOLOGY ETHICS/ LEGAL FIELD WORK RESULTS & DISSEMINATION IMPLEMENTATION OF FINDINGS Rationale: The strategy to implement the findings into the practice should consider, at the very least, the following aspects: the key audiences for the research, the implementation outcome variables, the implementation strategies that will support the delivery of health services.1 Research Unit actions: a) Rating joint research-innovation-BIOEF-OSTEBA alternatives for optimal implementation of the research results; b) Implementation of the procedures for legal protection of research results and, where appropriate, of the products corresponding transfer. 1 Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ 2013;347:f6753 doi: 10.1136/bmj.f6753 (Published 20 November 2013)