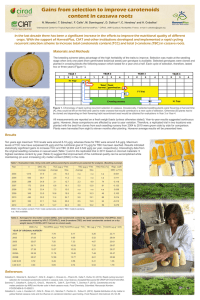
This article was published in an Elsevier journal. The attached
Anuncio

This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Available online at www.sciencedirect.com Aquatic Botany 88 (2008) 311–316 www.elsevier.com/locate/aquabot Allelopathic potential of two invasive alien Ludwigia spp. Sophie Dandelot a,*, Christine Robles b, Nicolas Pech c, Arlette Cazaubon a, Régine Verlaque b a Laboratoire d’Ecologie des Eaux Continentales Méditerranéennes (Case C 31), Institut Méditerranéen d’Ecologie et de Paléoécologie, UMR 6116, Faculté des Sciences et Techniques de Saint-Jérôme, Université Paul Cézanne, 13397 Marseille Cedex 20, France b Laboratoire de Biosystématique et d’Ecologie Méditerranéenne (Case 4), Institut Méditerranéen d’Ecologie et de Paléoécologie, UMR 6116, Université de Provence, Centre Saint Charles, Place Victor Hugo, 13331 Marseille Cedex 3, France c Laboratoire Evolution Génome Environnement (Case 36), Institut Méditerranéen d’Ecologie et de Paléoécologie, UMR 6116, Université de Provence, Centre Saint Charles, Place Victor Hugo, 13331 Marseille Cedex 3, France Received 29 January 2007; received in revised form 28 November 2007; accepted 3 December 2007 Available online 8 December 2007 Abstract The allelopathic potential of two invasive alien Ludwigia [Onagraceae: L. peploides (Kunth) Raven and L. grandiflora (Michaux) Greuter and Burdet], that have developed quasi-monotypic stands in many aquatic ecosystems in France, was investigated. Since allelopathy involves the release of compounds into the environment, the water of monospecific experimental cultures was directly tested against two target species: Lactuca sativa L., the standard cultivar for bioassays, and Nasturtium officinale R. Brown, a resistant and widespread native hydrophyte. The treatment was carried out at the three main phases of development of both Ludwigia in February, May and August. For each experiment, the germination, mortality and culture yield percentages, the seedling growth (radicle and hypocotyl elongation) and the health of 15-day-old-seedlings were measured. The water of each Ludwigia tank induced: (1) a decrease in germination for watercress in August (control: 68.6%, L. peploides: 48.6%, L. grandiflora: 61.1%); (2) an increase in mortality in May only for watercress (control: 3.4%, L. peploides: 13.5%, L. grandiflora: 12%) and in August for both target species (up to 22.3% vs. 3% for lettuce and 27% vs. 12.5% for watercress); (3) a disturbance of seedling elongation for lettuce in all seasons; and (4) a seedling chlorosis of both target species, particularly in May and August. This study showed that L. peploides and L. grandiflora possess an allelopathic activity that influences the water quality throughout the year. Combined with the various competitive attributes, allelopathy may contribute to the great success of these two invasive Ludwigia in Europe. In threatened wetland communities of the Mediterranean area, in particular, allelopathy might have an important impact by diminishing the seedling survival of the most vulnerable species. # 2008 Elsevier B.V. All rights reserved. Keywords: Ludwigia peploides; L. grandiflora; Allelopathy; Invasion; Lettuce; Watercress; Southern France; Wetland 1. Introduction In France, two alien amphibious Ludwigia (Onagraceae) have for the past 20 years caused major ecological and economic problems. These water primroses are morphologically very similar and are often confused: L. peploides (Kunth) Raven and L. grandiflora (Michaux) Greuter and Burdet. Introduced at Montpellier (Lez River: Hérault) in 1830, probably from America, these taxa have become the most aggressive weeds in all shallow-water habitats in France and * Corresponding author. E-mail addresses: sophie.dandelot@netcourrier.com (S. Dandelot), regine.verlaque@univ-provence.fr (R. Verlaque). 0304-3770/$ – see front matter # 2008 Elsevier B.V. All rights reserved. doi:10.1016/j.aquabot.2007.12.004 neighbouring countries. Their inexorable spread has been achieved because of their intensive growth and easy propagation by cuttings (Dandelot et al., 2005b). In the South of France, both Ludwigia exhibit a seasonal pattern of development: in early spring leaves appear at the water surface; by June up to 50 cm of stems emerge; they flower from July to October, producing a strong total biomass (2–3 kg dry weight m 2); in November all aerial stems fall and in winter persistent organs form with sediments a dense blackish mat (Dandelot, 2004). The major effects of these invasions are: reduction of the flow and hyper-sedimentation leading to silting up, in particular in ponds, the drastic decline of the local biodiversity (dense monotypic stands) and in summer water hypoxia and major alterations in bacterial communities (Dutartre et al., 1997; Dandelot et al., 2005a). These vigorous Author's personal copy 312 S. Dandelot et al. / Aquatic Botany 88 (2008) 311–316 ornamental plants can survive and flower under the worst conditions (pollution, salinity, drought, etc.). In addition, they are avoided by herbivores and pathogens (Dandelot, 2004), owing to the production of numerous noxious or repellent compounds (Ghani, 1998). All these features suggest that both Ludwigia might possess a hitherto unstudied allelopathic potential. Allelopathy is defined as any effect, direct or indirect, positive or negative, of one species on the growth of another through the release of chemical compounds into the environment (Rice, 1984). Allelopathic interactions can play a key role with regard to the success of invasive plants since they may alter physiological processes, and thus influence the structure of communities (Rice, 1992; Bais et al., 2002, 2003; Inderjit and Duke, 2003). Better known in terrestrial environment, allelopathy has also been found in prolific submerged macrophytes (Gross, 1999, 2003; Ervin and Wetzel, 2003) as Ceratophyllum and Myriophyllum spp. (Jasser, 1995; Nakai et al., 2000). Most bioassays, using plant extracts, have demonstrated growth inhibition in phytoplankton or lettuce seedlings, but only a few studies have described allelopathic effects of emergent macrophytes on hydrophytes living in the same ecosystem (Elakovich and Wooten, 1989, 1995; Gopal and Goel, 1993). The aim of this initial work is to study, over a yearly cycle of development, the allelopathic potential of L. peploides and L. grandiflora with regard to the germination, mortality and seedling elongation of two target species. The first, Lactuca sativa L., is the standard cultivar for bioassays due to its sensitivity to allelochemicals (Leather and Einhellig, 1987; Ervin and Wetzel, 2003). The second, Nasturtium officinale R. Brown, has been chosen because this native European hydrophyte germinates easily, and a few stems of this resistant species sometimes occur in Ludwigia stands in France. Since allelopathy involves the release of compounds into the environment (Rice, 1984), we directly sampled water from Ludwigia tanks for our tests. 2. Materials and methods In May 2003, about a hundred samples of Ludwigia were randomly taken in the naturalized Southern France populations of L. peploides (Durance River) and L. grandiflora (Siagne River). For each species, two tanks (90 cm 30 cm 50 cm: 0.27 m2) were placed in the open air and each tank filled with 30 L of tap drinking water. Thirty stems with roots of Ludwigia were washed, and then placed at regular intervals within each tank, in order to obtain the mean density observed in situ. During the year, tap water was daily added in order to maintain a constant volume (30 L/tank). Tap drinking water was used because: (1) it is free from allelochemicals and various contaminants, (2) it has an osmotic potential compatible with seed germinations, and (3) it has the nutrient content required for the development of both Ludwigia in tanks. Lettuce and watercress seeds were bought from local suppliers. Five treatments per target species were tested: (1) water from L. peploides tank 1, (2) water from L. peploides tank 2, (3) water from L. grandiflora tank 1, (4) water from L. grandiflora tank 2, and (5) tap water as control. Our experiments were carried out during the three main phases of both Ludwigia growths in Europe: February, May and August 2004. Bioassays were carried out in vitro under conditions of natural photoperiod and at room temperature (20– 25 8C). Each treatment was tested on seven replicates of 20 seeds, arranged at regular intervals and numbered in sterile Petri dishes (9 cm in diameter) with a Whatman filter-paper No. 4. Thus, for each season, 140 seeds for control and 280 seeds for each invader were tested. Daily during each month of experimentation, 1 mL of water taken within the Ludwigia tanks, and tap water for control, was added to each Petri dish. At each season, the germination, viability, culture yield percentages and seedling growth (radicle and hypocotyl elongation) have been measured. The number of germinations and abnormal seedlings were recorded daily. The percentage of very abnormal, i.e. non-viable, seedlings represents the early mortality percentage, calculated in relation to the number of germinations. The culture yield corresponds to the percentage of the number of seedlings with a ‘‘normal aspect’’ in relation to the total number of seeds tested. For each normal seedling, the measurement of the radicle and hypocotyl length was performed 15 days after its germination. Comparison of percentages was tested using x2-test where we approximated the distribution of statistical tests by permutations (1000 when necessary). Differences in mean lengths were tested using analyses of variance (one-way or twoway ANOVA) and multiple comparison tests by Tukey’s method. All statistical analyses were performed using S-PLUS 6 (2001). 3. Results 3.1. Germination For all treatments, germination percentages were always very high for lettuce (95–99%) and much lower for watercress (48–74%). For lettuce, results did not significantly differ during the experiment between seeds growing in water from the two Ludwigia tanks and the control (2.11 < x2 < 3.41; d.f. = 2; 0.18 < P < 0.35). The pattern was the same for watercress in February (x2 = 0.38; d.f. = 2; P = 0.85) and May (x2 = 5.45; d.f. = 2; P = 0.06: Fig. 1), but in August germination percentages of seeds growing in both Ludwigia waters were significantly reduced (x2 = 17.62; d.f. = 2; P = 0.0001), particularly with L. peploides (48.6% vs. control: 68.6% and L. grandiflora: 61.1%). 3.2. Mortality and yield Lethal anomalies encountered were characterized by: abortion of seedlings, poorly or mis-formed cotyledons, withered or necrotized seedlings, absence of radicle and arrested growth after germination. Mortality percentages differed widely according to the time, the treatment (tap or Ludwigia waters) and the target species. In February, percentages were low for all treatments (0–2% for lettuce, Author's personal copy S. Dandelot et al. / Aquatic Botany 88 (2008) 311–316 313 Fig. 1. Mean germination (Germ) and seedling mortality (Mort) percentages of watercress according to the three treatments in February, May and August. Values not different at a = 5% are denoted with the same letter. Bars indicate standard error. 7–4% for watercress) and without significant differences compared to control (x2 = 2.9; d.f. = 2; P = 0.22 for lettuce, and x2 = 1.24; d.f. = 2; P = 0.54 for watercress). In May, results showed wide divergences according to the target species. Mortality percentages were very low for all control seedlings (<3.5%). For lettuce, they increased up to 4.7% with L. peploides water and 6.6% with L. grandiflora water, but without significant difference (x2 = 3.75; d.f. = 2; P = 0.15). Watercress seedlings exhibited greater mortality in both Ludwigia waters: 13.5% and 12%, respectively. These percentages were significantly different compared to control (x2 = 6.74; d.f. = 2; P = 0.03) and similar for both Ludwigia (Fig. 1). In August, both Ludwigia waters strongly and significantly increased the mortality percentages of lettuce (x2 = 31.83; d.f. = 2; P < 10 6) and watercress seedlings (x2 = 8.79; d.f. = 2; P = 0.01). In summer, L. grandiflora water had a more severe lethal impact on both target species (lettuce: 22.3%, watercress: 27%) than L. peploides (respectively, 11 and 17.7%). Lastly, culture yield percentages did not significantly differ in February and May for the two target species (0.2 < x2 < 2.78; d.f. = 2; 0.25 < P < 0.9: Table 1). On the other hand, in August we observed a significant decrease for lettuce (x2 = 22.84; d.f. = 2; P = 10 5) and watercress (x2 = 15.31; d.f. = 2; P = 10 4). For lettuce the negative impact appeared to be greater with L. grandiflora than with L. peploides, whereas watercress seedlings showed the same sensitivity towards both invaders. 3.3. Chlorosis and elongation of seedlings The first obvious result of this experiment was the similarity of behaviour of the two target species with regard to chlorosis. Compared to healthy controls whose hypocotyls were always green, those of lettuce and watercress seedlings growing in both Ludwigia waters were yellowish, particularly in spring and summer, and even etiolated (especially lettuce seedlings). On the other hand, with regard to seedling elongation, the two target species showed contrasting behaviour. Whatever the season, the size of watercress seedlings did not show significant differences (one-way ANOVA, 0.06 < F < 2.85; 0.06 < P < 0.9 for radicles, and 0.29 < F < 2.57; 0.07 < P < 0.7 for hypocotyls). In contrast, the response of lettuce seedlings changed according to the season and the plant part. For radicle lengths (Fig. 2), the three treatments had a significantly different effect in February (one-way ANOVA, F 2,588 = 50.75; P < 10 10), because both Ludwigia waters had a clearly stimulating effect on root elongation. In May, this effect decreased and only the seedlings growing in L. peploides water were significantly different from the two others (one-way ANOVA, F 2,649 = 9.35; P < 10 4). Lastly in August, the impact of both Ludwigia waters became negative, especially Table 1 Mean culture yield percentages (number of ‘‘normal’’ seedlings/total number of seeds tested), and standard error, of the two target species 15 days after germination according to the three treatments in February, May and August February Control L. peploides L. grandiflora May August Lettuce Watercress Lettuce Watercress Lettuce Watercress 97.1 1.4 93.6 1.5 ns 95.4 1.3 ns 66.4 4 67.1 2.8 ns 65.4 2.8 ns 94.3 2 93.9 1.4 ns 90.7 1.7 ns 61.4 4.1 64.3 2.9 ns 60.4 2.9 ns 94.3 2 a 86.8 2 *** b 77.1 2.5 *** c 60 4.1 a 40 2.9 *** b 44.6 2.9 *** b Values not different at a = 5% are denoted with the same letter. P: ns non-significant; * significant at 0.05; ** significant at 0.01; *** significant at 0.001. Author's personal copy 314 S. Dandelot et al. / Aquatic Botany 88 (2008) 311–316 Fig. 2. Mean length (cm) of lettuce radicles on normal 15-day-old-seedlings according to the three treatments in February, May and August. Values not different at a = 5% are denoted with the same letter. Bars indicate standard error. with L. grandiflora (one-way ANOVA, F 2,588 = 11.17; P = 10 5). Conversely, for hypocotyl lengths, a slight positive effect was observed in February, but only the treatment with L. peploides water significantly differed from the two others (oneway ANOVA, F 2,661 = 3.87; P = 0.021). This effect increased in May, to reach a maximum in August, especially with L. peploides water. In May, the two Ludwigia impacts were similar (3.7 and 3.6 cm) and distinct from the control (3.3 cm) (oneway ANOVA, F 2,649 = 14.72; P < 10 6), but in August the three treatments significantly differed (respectively, 3.2, 3.8 and 3.6 cm) (one-way ANOVA, F 2,588 = 24.92; P < 10 10). 4. Discussion Generally seeds, protected by their teguments, seem less sensitive to allelochemicals than seedlings (Elakovich, 1999; Quayyum et al., 1999). Although the lettuce germination was never affected by the two Ludwigia waters, that of watercress showed a clear decrease in August. However, the most significant results of this study concern the seedling mortality of the two target species, which increased with the yearly development of the two invaders: (i) no effect in February during the winter Ludwigia dormancy; (ii) a slight (for lettuce) or clear effect (for watercress) in May when their leaf-rosettes grow; (iii) a strong effect in August (particularly with L. grandiflora) when Ludwigia are flowering. Although watercress is one of the most resistant hydrophytes, possessing allelopathic and anti-herbivory properties (Newman et al., 1996), its seedlings exhibit as early as the spring and during the summer higher mortality percentages than that of lettuce, which is well known for its sensitivity to allelochemicals (Leather and Einhellig, 1987). In contrast to watercress, the size of lettuce seedlings was strongly altered during tests and exhibited an unbalanced development pattern (radicle/hypocotyl ratio). In winter, both Ludwigia waters had a clearly stimulating effect on root elongation. In summer, in agreement with previous studies, growth inhibition was greater on roots than on hypocotyls. The negative and positive impact on seedling growth observed suggests that both alien Ludwigia could influence, throughout the year, the water quality. This concomitant inhibition and stimulation may result from: (1) direct alterations of roots by the water, (2) specific growth re-orientation related to allelochemical stress (Pasternak et al., 2005), and (3) perhaps the release of hormones by invaders (Rice, 1986; Neori et al., 2000; Inderjit and Duke, 2003). With hydrophyte extracts, the stimulation has been essentially found on lettuce and Lemna (Quayyum et al., 1999); its intensity varied widely according to allelochemical concentrations (maximal effect with low concentrations) and the development period of the donor (Elakovich and Wooten, 1989; Wooten and Elakovich, 1991). Except in winter, all seedlings of the two target species suffered chlorosis in Ludwigia waters. Photosynthesis disturbance is an important and widespread mode of action of many aquatic plants against competitors, via allelochemicals (Gross, 2003). Adding chlorosis to the disturbed development can compromise the long-term survival of most seedlings in invaded wetland communities. The occurrence of allelopathy and the feasibility of transferring bioassays to the natural environment is still a matter of debate (Inderjit and Callaway, 2003), particularly in aquatic ecosystems. Nearly all previous studies were carried out either with crude extracts of adult plants, or with some of its active compounds tested at different concentrations, on the germination and seedling growth of target species. In fact, the rare experiments using water extracts of lake sediments, associated with allelopathic macrophytes, showed no significant result, because exudate concentrations were insufficient (Quayyum et al., 1999; Seal et al., 2004). Moreover, the wellknown allelopathic compounds were not detected in any water samples, probably due to their rapid degradation (Glomski et al., 2002). In this context, our significant results, obtained directly with water from Ludwigia tanks, are new, relevant and close to certain natural situations. According to Gross (2003) ‘‘allelochemicals released by donor organisms into the water need to be sufficiently hydrophilic and reach their target organisms in effective concentrations despite considerable dilution’’. In the Mediterranean area, the long summer drought produces a strong drop in the water-level and an increase in compound concentrations, notably in closed systems. So compared to natural stands, our experimental conditions probably minimise the impact, because tanks are maintained at constant volume (30 L) and the duration was limited to 1 year. Compounds released by Ludwigia are probably produced by roots, foliar leachage and decomposition, but especially by several secretory organs (trichomes and glands) that excrete liquid lipophilic substances creating iridescence on the water surface (Dandelot, 2004). Lipophilic complexes, which tend to remain near the donor, Author's personal copy S. Dandelot et al. / Aquatic Botany 88 (2008) 311–316 have been implicated in plant defences and as a strong potential source of allelochemicals in wetlands (Gross, 1999, 2003; Fahn, 2000). Studies of putative allelochemicals will be complex because Ludwigia spp. synthesize: tannins, triterpenes, flavonoids, polyphenols, alkaloids, linoleic acids, fatty substances, saponins, etc. (Ghani, 1998) that can act in synergy. Most of them exert either phytotoxic, allelopathic (Singhvi and Sharma, 1984) or repellent effects (Neori et al., 2000; Ervin and Wetzel, 2003; Gross, 2003; Inderjit and Duke, 2003). But more significant is the strong antibacterial activity found in the closely related species L. adscendens (L.) Hara (Ahmed et al., 2005) and in both invaders (Le Petit, personal communication). This might explain the lack of decay or turbidity in our aquaria, even after several years. This property suggests that Ludwigia are not necessarily the single source of putative allelochemicals: complex interactions may also occur with the microflora (Algae, Bacteria). The relief and climate of the Mediterranean area result in major seasonal changes in the wetlands (devastating autumnal and spring floods, long summer drought and water resources management). This ecosystem is inhabited by endangered flora, with about 1/3 of short-living species (Verlaque et al., 2001), that require regular regeneration by seeds. Colonization by Ludwigia might contribute to the impoverishment of this flora, by reducing the seedling survival of the more vulnerable native taxa. Moreover, from mid-spring to mid-autumn, the strong biomass and height of Ludwigia (>1 m) limit interspecific competition for space, light and nutrients, and also prevent flowering of nearly all hydrophytes. In Ludwigia stands only a few etiolated shoots of early resistant or allelopathic macrophytes survive (e.g. Nasturtium, Myriophyllum, Ceratophyllum spp.), before the growth period of the invaders (Dandelot, 2004). In conclusion, this study shows that both alien Ludwigia possess real allelopathic potential that varies strongly in function of four main factors: (1) the season, (2) the target species, (3) the parameter studied (germination, mortality, elongation, chlorosis), and (4) the invaders. Further studies are required to complete this initial work, in particular with a view to analysing the nature of the putative allelochemicals and the complex hydrophyte–microflora interactions. The inexorable spread of these pests in the Mediterranean area is all the more worrying because aquatic ecosystems harbour a rich and highly vulnerable flora. References Ahmed, F., Selim, M.S.T., Shilpi, J.A., 2005. Antibacterial activity of Ludwigia adscendens. Fitoterapia 76, 473–475. Bais, H.P., Walker, T.S., Stermitz, F.R., Hufbauer, R.A., Vivanco, J.M., 2002. Enantiomeric-dependent phytotoxic and antimicrobial activity of ()-Catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol. 128, 1173–1179. Bais, H.P., Vepachedu, R., Gilroy, S., Callaway, R.M., Vivanco, J.M., 2003. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301, 1377–1380. 315 Dandelot, S., 2004. Les Ludwigia spp. invasives du Sud de la France: Historique, Biosystématique, Biologie et Ecologie. PhD Thesis. Université d’AixMarseille III, Marseille. Dandelot, S., Matheron, R., Le Petit, J., Verlaque, R., Cazaubon, A., 2005a. Variations temporelles des paramètres physicochimiques et microbiologiques de trois écosystèmes aquatiques (Sud-Est de la France) envahis par des Ludwigia. C. R. Biol. 328, 991–999. Dandelot, S., Verlaque, R., Dutartre, A., Cazaubon, A., 2005b. Ecological, dynamic and taxonomic problems due to Ludwigia (Onagraceae) in France. Hydrobiologia 551, 131–136. Dutartre, A., Haury, J., Planty-Tabacchi, A.M., 1997. Introduction des macrophytes aquatiques et riverains dans les hydrosystèmes français métropolitains: essai de bilan. B. Fr. Pêche Piscic 344/345, 407–420. Elakovich, S.D., 1999. Bioassays applied to allelopathic herbaceous vascular hydrophytes. In: Inderjit, Dakshini, K.M., Einhellig, F.A. (Eds.), Principles and Practices in Plant Ecology: Allelochemical Interactions. CRC Press, Boca Raton, pp. 45–56. Elakovich, S.D., Wooten, J.W., 1989. Allelopathic potential of sixteen aquatic and wetland plants. J. Aquat. Plant Manage. 27, 78–84. Elakovich, S.D., Wooten, J.W., 1995. Allelopathic, herbaceous, vascular hydrophytes. In: Inderjit, Dakshini, K.M., Einhellig, F.A. (Eds.), Allelopathy: Organisms, Processes, and Applications. Amer. Chem. Soc., Washington, pp. 58–73. Ervin, G.N., Wetzel, R.G., 2003. An ecological perspective of allelochemical interference in land–water interface communities. Plant Soil 256, 13–28. Fahn, A., 2000. Structure and function of secretory cells. Adv. Bot. Res. 31, 55– 66. Ghani, A., 1998. Medicinal Plants of Bangladesh: Chemical Constituents and Uses. Asiatic Society of Bangladesh, Dhaka, p. 220. Glomski, L.A.M., Wood, K.V., Nocholson, R.L., Lembi, C.A., 2002. The search for exudates from Eurasian Watermilfoil and Hydrilla. J. Aquat. Plant Manage. 40, 17–22. Gopal, B., Goel, U., 1993. Competition and allelopathy in aquatic plant communities. Bot. Rev. 59, 155–210. Gross, E.M., 1999. Allelopathy in benthic and littoral areas: case studies on allelochemicals from benthic cyanobacteria and submersed macrophytes. In: Inderjit, Dakshini, K.M., Foy, C.L. (Eds.), Principles and Practices in Plant Ecology: Allelochemical Interactions. CRC Press, pp. 179–199. Gross, E.M., 2003. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 22, 313–339. Inderjit, Callaway, R.M., 2003. Experimental designs for the study of allelopathy. Plant Soil 256, 1–11. Inderjit, Duke, S.O., 2003. Ecophysiological aspects of allelopathy. Planta 217, 529–539. Jasser, I., 1995. The influence of macrophytes on a phytoplankton community in experimental conditions. Hydrobiologia 306, 21–32. Leather, G.R., Einhellig, F.A., 1987. Bioassays of naturally occuring allelochemicals for phytotoxicity. J. Chem. Ecol. 14, 1821–1828. Nakai, S., Inoue, Y., Hosomi, M., Murakami, A., 2000. Myriophyllum spicatum released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 34, 3026–3032. Neori, A., Reddy, K.R., Čisková-Končalová, H., 2000. Bioactive chemicals and biological-biochemical activities and their functions in rhizosphere of wetland plants. Bot. Rev. 66, 350–378. Newman, R.M., Kerfoot, W.C., Hanscom, Z., 1996. Watercress allelochemical defends high-hydrogen foliage against consumption: effects on freshwater invertebrate herbivores. Ecology 77, 2312–2323. Pasternak, T., Rudas, V., Potters, G., Jansen, M.A.K., 2005. Morphogenetic effects of abiotic stress: reorientation of growth in Arabidopsis thaliana seedlings. Environ. Exp. Bot. 53, 299–314. Quayyum, H.A., Mallik, A.U., Lee, P.F., 1999. Allelopathic potential of aquatic plants associated with wild rice (Zizania palustris). I. Bioassay with plant and lake sediment samples. J. Chem. Ecol. 25, 209–218. Rice, E.L., 1984. Allelopathy. Academic Press, London. Author's personal copy 316 S. Dandelot et al. / Aquatic Botany 88 (2008) 311–316 Rice, E.L., 1986. Allelopathic growth stimulation. In: Putnam, A.R., Chang, C.S. (Eds.), The Science of Allelopathy. J. Wiley & sons, New York, pp. 23–42. Rice, E.L., 1992. Allelopathic effects on nitrogene cycling. In: Rivzi, S.J.H., Rizvi, V. (Eds.), Allelopathy: Basic and Applied Aspects. Chapman & Hall, London, pp. 31–58. Seal, A.N., Halg, T., Pratley, J.E., 2004. Evaluation of putative allelochemicals in rice root exudates for their role in the suppression of arrowhead root growth. J. Chem. Ecol. 30, 1663–1678. Singhvi, N.R., Sharma, K.D., 1984. Allelopathic effects of Ludwigia adscendens L. and Ipomoea aquatica Forsk. on seedling growth of pearl millet (Pennisetum typhoideum Rich.). Trans. ISDT UCDS 9, 95– 100. S-Plus 6 for Windows, 2001. Guide to Statistics, vol. 1. Insightful Corp., Seattle, WA, USA. Verlaque, R., Médail, F., Aboucaya, A., 2001. Valeur prédictive des types biologiques pour la conservation de la flore méditerranéenne. C. R. Acad. Sci. III-Vie 324, 1157–1165. Wooten, J.W., Elakovich, S.D., 1991. Comparisons of potential allelopathy of seven freshwater species of spikerushes (Eleocharis). J. Aquat. Plant Manage. 29, 12–15.