Pablo Demelo-Rodríguez, a,∗ María Olmedo Samperio, Daniel
Anuncio

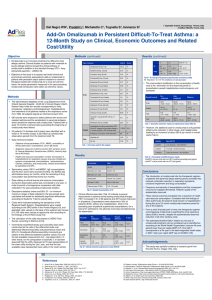
Documento descargado de http://www.archbronconeumol.org el 20/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. Letters to the Editor / Arch Bronconeumol. 2015;51(12):656–665 Pablo Demelo-Rodríguez,a,∗ María Olmedo Samperio,a Daniel Germán Gaitán Tocora,a Juan Carlos Cano Ballesteros,a Juan Antonio Andueza Lillob 659 b Servicio de Urgencias, Hospital General Universitario Gregorio Marañón, Madrid, Spain ∗ Corresponding a Departamento de Medicina Interna, Hospital General Universitario Gregorio Marañón, Madrid, Spain author. E-mail address: pbdemelo@hotmail.com (P. Demelo-Rodríguez). Paraneoplastic cerebellar degeneration associated with small cell neuroendocrine mediastinal carcinoma夽 Degeneración cerebelosa paraneoplásica asociada a carcinoma neuroendocrino mediastínico de células pequeñas To the Editor, We report the case of a 69-year-old woman admitted with a 7-month history of anorexia and asthenia, along with unsteadiness, vomiting, tremor and double vision. She also reported a 1-month history of dyspnea and voice changes. On examination, she presented a bitonal voice, ataxic gait and dysmetria, with intention tremor in the left arm. Magnetic resonance imaging (MRI) of the head revealed a small lacunar infarction, which did not explain the clinical symptoms. Blood work, biochemistry, proteins, immunoglobulins, thyroid profile, vitamin B12 , vitamin E and folate were normal, and serologies for HIV, hepatitis, cytomegalovirus, Epstein–Barr virus, syphilis and Borrelia were all negative. Cerebrospinal fluid cytochemistry was also normal. Carcinoembryonic antigen and neurospecific enolase tumor markers were elevated at 30.4 ng/ml (0–5 ng/ml) and 21.7 ng/ml (0–16 ng/ml), respectively. Computed tomography (CT) of the chest and abdomen revealed a mass in the upper mediastinum, measuring 70 mm×40 mm, suggestive of clustered lymphadenopathy. An immune-mediated process was suspected, so antinuclear antibodies and onconeuronal antibodies were tested, but these were negative, with anti-glutamic acid decarboxylase antibodies (anti-GAD) 2.4 U/ml (0–5 U/ml) and anti-acetylcholine receptor antibodies 0.01 nmol/l (0–0.1 nmol/l). F-18 fluorodeoxyglucose uptake on a positron emission tomography scan suggested malignancy, so a biopsy was obtained by mediastinoscopy. The pathology report confirmed small cell neuroendocrine carcinoma of the thymus, positive for chromogranin and synaptophysin (Fig. 1). A scintigraphy (octreoscan) was then performed to complete the examination, showing 111 indium-pentetreotide uptake in the tumor and supraclavicular lymph node metastases. The patient underwent surgical resection and received chemotherapy with carboplatin and etoposide. She achieved full remission of the cancer and almost complete resolution of her neurological symptoms, although tremor at rest did persist. Neuroendocrine tumors (NET) are rare cancers, generally found in the gastrointestinal tract, although they may occur in the lung, thymus, ovaries and non-parenchymatous tissue. Thymic NETs account for less than 5% of mediastinal tumors.1 Approximately 30% are malignant, although this rate rises to 82% if they are located in the thymus.1 They present as a mediastinal mass mainly in 夽 Please cite this article as: Serrano-Martínez JL, Zamora-Pasadas M, Redondo-Orts M. Degeneración cerebelosa paraneoplásica asociada a carcinoma neuroendocrino mediastínico de células pequeñas. Arch Bronconeumol. 2015;51:659–660. Fig. 1. Histology slide at 30× amplification with chromogranin immune staining, showing tumor cell nests separated by fibrovascular tracts. patients aged 30–50 years, and are 3 times more common in men.2 Local clinical manifestations can range from dysphonia or dyspnea to superior vena cava syndrome, and one-third of patients have endocrine symptoms associated with multiple endocrine neoplasia syndrome. CT, MRI and 123 I-metaiodobenzylguanidine scintigraphy are useful, but the octreoscan is the most sensitive procedure (71%–100%) for detecting NETs, depending on the somatostatin receptors expressed by the tumors. Histology examination shows cell nests with fibrovascular tracts, positive for neuroendocrine markers such as chromogranin, synaptophysin and neuroenolase. Paraneoplastic cerebellar degeneration (PCD) is the most common paraneoplastic neurological syndrome. In 18%–50% of cases, no antibodies are identified,3 and in early stages of the disease, MRI is normal.4 It is associated with several neoplastic processes, including thymic NETs.5 In view of the favorable response to specific cancer treatment, we classified our case as seronegative PCD associated with thymic NET. References 1. Duh QY, Hybarger CP, Geist R, Gamsu G, Goodman PC, Gooding GA, et al. Carcinoids associated with multiple endocrine neoplasia syndromes. Am J Surg. 1987;154:142–8. 2. Suster S, Moran CA. Neuroendocrine neoplasms of the mediastinum. Am J Clin Pathol. 2001;115:17–27. 3. Ducray F, Demarquay G, Graus F, Decullier E, Antoine JC, Giometto B, et al. Seronegative paraneoplastic cerebellar degeneration: the PNS Euronetwork experience. Eur J Neurol. 2014;21:731–5. 4. Pfiffner TJ, Jani R, Mechtler L. Neuro-oncological disorders of the cerebellum. Neurol Clin. 2014;32:913–41. 5. Bataller L, Valero C, Díaz R, Froufe A, Garcia-Zarza A, Ribalta T, et al. Cerebellar ataxia associated with neuroendocrine thymic carcinoma and GAD antibodies. J Neurol Neurosurg Psychiatry. 2009;80:696–7. Documento descargado de http://www.archbronconeumol.org el 20/11/2016. Copia para uso personal, se prohíbe la transmisión de este documento por cualquier medio o formato. 660 Letters to the Editor / Arch Bronconeumol. 2015;51(12):656–665 José Luis Serrano-Martínez,a,∗ Mónica Zamora-Pasadas,a María Redondo-Ortsb b Servicio de Cuidados Críticos y Urgencias, Hospital Universitario Virgen de las Nieves, Granada, Spain a Servicio de Medicina Interna, Hospital Universitario Virgen de las Nieves, Granada, Spain ∗ Corresponding author. E-mail address: jlserranomi@gmail.com (J.L. Serrano-Martínez). Cortico-dependent Asthma: Our Clinical Experience夽 Table 1 Clinical Progress and Treatment Modifications in the 10 Patients Diagnosed With Corticosteroid-dependent Asthma. Asma corticodependiente: nuestra experiencia clínica To the Editor, Corticosteroid-dependent asthma is defined as the need for daily administration of oral corticosteroids.1 This definition, however, is ambiguous, since it includes both patients who receive this treatment and experience little improvement, and those who benefit from it (with a varying degree of response). The GOAL study showed that only 7% of patients who were uncontrolled at maximum doses of fluticasone/salmeterol achieved control with an oral steroid regimen.2 Few studies have been conducted in this specific patient group, despite their clinical relevance. We report the case of a patient with corticosteroid-dependent asthma, and review the management and progress of all corticosteroid-dependent asthmatics seen in our specialist clinic (10 of a total of 475 patients). A 69-year-old woman, non-smoker, with a diagnosis of lateonset, non-allergic asthma, IgE 770 kU/l, eosinophils 900/mm3 , FENO 31 ppb. Asthma was initially poorly controlled with a combination of maximum doses of budesonide/formoterol, as indicated by asthma control test (ACT) 15, a severe exacerbation in the previous year, FEV1 64%, and positive bronchodilator challenge. Significant comorbidities included rhinosinusitis and obesity. Treatment with deflazacort 30 mg for 3 weeks increased ACT to 23 and FEV1 to 67%, but when it was withdrawn, ACT returned to 16 and FEV1 to 67%. Tiotropium (18 g/day) was added to the combination of fluticasone/salmeterol (500/50 g), but ACT remained unchanged, while FEV1 rose to 70%. Treatment was subsequently switched to inhaled fluticasone (1000 g/day), tiotropium (18 g/day) and indacaterol (150 g/day). The patient is currently free of exacerbations, her ACT is 24 and FEV1 is 79%. In our opinion, a patient who is uncontrolled and presents bronchial obstruction despite treatment with a combination of maximum dose inhaled corticosteroids (IC)-long-acting -2 agonist (LABA) has corticosteroid-dependent asthma. In the case of our patient, her FEV1 normalized (at least to >70%) and ACT rose to ≥20 after 3–4 weeks of treatment with deflazacort 30 mg. Subsequently, when the oral corticosteroid was discontinued, her clinical and functional status returned to the previous situation. ACT Exacerbations/patient/year FEV1 % Oral corticosteroids Omalizumab LAMA Indacaterol Initial Final 16.9±3.8 0.32 53.5±14.2 0 0 0 0 22.5±2.7 0 76.4±13.3 0 2 10 7 ACT: asthma control tests; FEV1 %: forced expiratory volume in 1 second; LAMA: long-acting anticholinergics. sity, rhinosinusitis, and polyposis). Despite the correct use of IC/LABA at maximum doses, these patients remained symptomatic with signs of bronchial obstruction, yet only 2 developed 2 or more severe exacerbations in a period of 1 year. It seems that in most cases, standard treatment can prevent exacerbations, while failing to provide full control of symptoms or normalization of lung function. This was achieved in all cases when an oral corticosteroid was added, although this treatment was unacceptable due to adverse events. These 10 patients were managed by the same pulmonologist. For the 2 patients in whom severe exacerbations persisted, the treatment strategy consisted, firstly, of adding omalizumab to the regimen. The response of the patients who received omalizumab confirm its efficacy in reducing exacerbations, but also its lack of effect on lung function.3 Persisting bronchial obstruction may explain why optimal control of symptoms is elusive in many patients. The next step for all patients consisted of adding a long-acting muscarinic antagonist, a medication that has already demonstrated its efficacy in this clinical setting.4 This intervention helped improve patients’ lung function and symptoms, but to an insufficient degree in 7 cases. We decided to add indacaterol to these 7 patients’ regimens: this potent bronchodilator has demonstrated efficacy in chronic obstructive pulmonary disease, but little experience is available in asthma.5 This combined therapeutic strategy resulted in a significant improvement in lung function and symptoms (Table 1) among corticosteroid-dependent asthma patients, while avoiding the use of oral steroids. Authorship Clinical characteristics, treatment, and progress of patients with corticosteroid-dependent asthma Ten of our patients (of 475 regularly seen in our consulting rooms) had corticosteroid-dependent asthma. They were typically middle-aged (49.2±15.1 years), with late onset of symptoms (7/10 cases), intense peripheral eosinophilia (eosinophils 565.0±286.8/mm3 ), elevated IgE (379.7±357.3 kU/l), FENO (31.7±13.2 ppb), and significant comorbidities (particularly obe- 夽 Please cite this article as: Pérez de Llano LA, García Rivero JL, Pallares A, Mengual N, Golpe R. Asma corticodependiente: nuestra experiencia clínica. Arch Bronconeumol. 2015;51:660–661. Study concept and design, data collection, analysis of results, interpretation of findings, and drafting the manuscript: Pérez de Llano. Study design and data collection: García Rivero and Pallares. Data collection: Mengual. Data analysis and interpretation of results: Golpe. Conflict of Interests Dr Pérez de Llano has received payment from Novartis, Boehringer, Chiesi, Almirall, Esteve and Ferrer, for presentations at medical congresses, consultancy, and coordination or participation