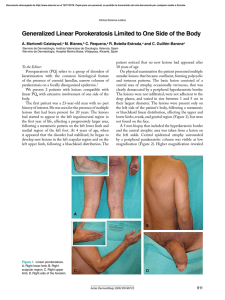
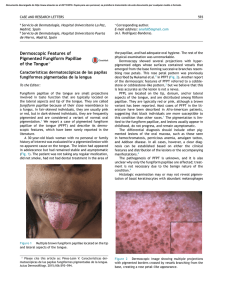
Veterinary Microbiology 148 (2011) 368–371 Contents lists available at ScienceDirect Veterinary Microbiology journal homepage: www.elsevier.com/locate/vetmic Research article Geotrichum candidum as etiological agent of horse dermatomycosis Luciana A. Figueredo, Claudia Cafarchia, Domenico Otranto * Dipartimento di Sanità Pubblica e Zootecnia, Facoltà di Medicina Veterinaria, Università degli Studi di Bari, Str. prov.le per Casamassima Km 3, 70010 Valenzano, Bari, Italy A R T I C L E I N F O A B S T R A C T Article history: Received 23 July 2010 Received in revised form 21 September 2010 Accepted 24 September 2010 Fungi of the genus Geotrichum are commonly found in the environment and, in some circumstances, they may cause diseases in humans and animals. Although these fungi have been isolated from skin lesions of some animal species, their pathogenic role in horses remains uncertain. With the aim to investigate the role of Geotrichum candidum as etiological agent of dermatomycoses, a retrospective study of 64 horses presenting skin lesions and suspected to have fungal infections was carried out. For each animal, anamnestic data were recorded and fungal culture were performed using hair. Out of 64 cases, 18 (28.1%) were positive for G. candidum and only two (3.1%) for dermatophytes (i.e., one for Microsporum equinum and the other for Microsporum canis). Alopecia, desquamation, and pruritus localized mainly on head and neck were frequently observed in G. candidum infected animals. Most of G. candidum infections were recorded during spring (44.4%). Out of the 18 animals presenting G. candidum infections, eight were treated using a disinfectant with antifungal proprieties. After one month of treatment, the clinical lesions were healed and fungal cultures resulted negative. The high prevalence of G. candidum in skin lesions of horses and the clinical recover following antifungal treatment indicated that these yeast-like fungi might play a role as etiological agents of horse cutaneous mycoses. ß 2010 Elsevier B.V. All rights reserved. Keywords: Geotrichosis Geotrichum candidum Horses Skin lesions 1. Introduction Yeast-like fungi of the genus Geotrichum are easily found in the environment, and in some circumstances they may cause disseminated or localized diseases of humans and animals, also known as geotrichoses (Ishikawa et al., 1996; Pal, 2005; Etienne et al., 2008). The genus Geotrichum includes 13 species of filamentous yeast-like fungi which represent the anamorphic state of species of the genera Dipodascus Lagerheim and Galactomyces Redhead and Malloch (de Hoog and Smith, 2004; Pimenta * Corresponding author at: Dipartimento di Sanità Pubblica e Zootecnia, Facoltà di Medicina Veterinaria, Str. prov.le per Casamassima Km 3, 70010 Valenzano, Bari, Italy. Tel.: +39 080 467 9839; fax: +39 080 467 9839. E-mail address: d.otranto@veterinaria.uniba.it (D. Otranto). 0378-1135/$ – see front matter ß 2010 Elsevier B.V. All rights reserved. doi:10.1016/j.vetmic.2010.09.025 et al., 2005; Suh and Blackwell, 2006; Wuczkowski et al., 2006). Geotrichum species are worldwide distributed and Geotrichum candidum is one of the most spread species causing infections. In humans, cases of geotrichoses have been reported in medical literature in immunocompromised patients (Mahapatra, 2005; Henrich et al., 2009), with prevalence up to 2.5% in patients presenting bronchomycosis (Reeves, 1941). Geotrichoses have also been reported in many animal species, including dogs (Gambale et al., 1987; Rhyan et al., 1990; Sidhu et al., 1993; Reppas and Snoeck, 1999), bovine (Chahota et al., 2001; Conti Dı́az et al., 2003), birds (Spanoghe et al., 1976), turtle (Ruiz et al., 1980), snakes (McKenzie and Green, 1976), primates (Dolensek et al., 1977; Migaki et al., 1982) and horses (Mós et al., 1978; Santos et al., 1983). Geotrichum spp. mainly cause respiratory diseases in humans whereas in animals it affects mainly the skin (Ruiz L.A. Figueredo et al. / Veterinary Microbiology 148 (2011) 368–371 et al., 1980; Santos et al., 1983; Gambale et al., 1987; Reppas and Snoeck, 1999), and the digestive tracts (McKenzie and Green, 1976; Dolensek et al., 1977; Mós et al., 1978; Migaki et al., 1982; Pal, 2005). Miconazole lotion, clorexidine gluconate and lotion of 2% gentian violet have been successfully employed for the treatment of cutaneous geotrichosis (Santos et al., 1983; Holzworth et al., 1987; Sidhu et al., 1993). Additionally, Virkon1 S (Farmaceutici Gellini, Aprilia, Italy) is an acid peroxygen system based disinfectant used as environmental disinfectant as well as therapeutic lotion against fungal and bacterial microorganisms (Marchetti et al., 2006). Horses skin lesions caused by G. candidum have been described once (Santos et al., 1983), but since then, no other reports of cutaneous geotrichosis are available. Thus, this study aims to assess the role of G. candidum as etiological agent of dermatomycoses in horses with skin lesions. Table 1 Prevalence of Geotrichum candidum isolated from the hair coats of skin lesions of horses according to sex, age, breed, habitat and season. P values calculated using Fisher test and x2 are also reported. Anamnesis data Gender Age Clinical signs Location of lesions Season 2. Material and methods Selection of the study population. From January 2000 to March 2010 a retrospective survey was conducted including clinical data of horses presenting skin lesions suspected to be caused by dermatophytes, for which microbiological and parasitic infections were previously excluded. A total of 64 riding horses from Apulia region (southern Italy) were selected and anamnestic data (i.e., age, sex, clinical signs, sampling periods and treatment) recorded in individual files. Hair and skin scraping samples were collected from lesions using a sterile scalpel or pliers and sent to the Laboratory of Mycology (Faculty of Veterinary Medicine at the University of Bari, Italy). Microscopic examination and sample culture. Hair and skin scraping samples were examined microscopically using 10% potassium hydroxide (KOH) and cultured onto Sabouraud dextrose agar with chloramphenicol (0.4 mg/ mL), and cycloheximide (0.5 mg/mL) (MIC) and onto MIC supplemented with nicotinic acid (2 mg/mL) at 25 8C for 7– 14 days. Fungi were isolated and identified on the basis of macroscopic and microscopy features (de Hoog and Guarro, 1995). Api 20C AUX test (bioMérieux, Marcyl’Etoile, France) were used according to the manufacturer’s instructions to confirm the morphological identification of the yeast-like species. Treatment. Therapy using a topic solution (5% of concentration) of an acid peroxygen system based disinfectant (Virkon1 S - Farmaceutici Gellini, Aprilia, Italy), twice a day was recommended in all horses positive to G. candidum, However, only for eight horses a complete follow up was obtained until the remission of symptoms associated to negative culture results. Statistical analysis. Epidemiological data from horses were analysed separately by using the x2 and fisher test to evaluate possible risk factors associated with the presence of G. candidum. A value of P 0.05 was considered to be statistically significant. 3. Results Out of the 64 cases of suspected fungal infection, 18 (28.1%) were positive for G. candidum and only two (3.1%) 369 Female Male <3 year 3–17 years >17 years Pruritus Alopecia Desquamation Erythema Hyperkeratosis Head and neck Body Legs and tail Generalized Spring Summer Fall Winter Positive/total (%) P-value 5/12 (41.7%) 7/12 (58.3%) 2/9 (22.2%) 6/9 (66.7%) 1/9 (11.1%) 4/12 (33.3%) 11/12 (91.7%) 5/12 (47.7%) 1/12 (8.3%) 1/12 (8.3%) 8/12 (66.7%) 1/12 (8.3%) 4/12 (33.3%) 1/12 (8.3%) 8/18 (44.4%) 1/18 (5.6%) 5/18 (27.8%) 4/18 (22.2%) P > 0.05 P < 0.05 P < 0.001 P < 0.01 P > 0.05 for dermatophytes (one for Microsporum equinum and the other for Microsporum canis) at fungal culture. No samples were positive on direct microscopic examination. Most of G. candidum infections were recorded during spring and fall seasons, followed by winter (Table 1). Only one case was registered during the summer, even though no statistical significant association between G. candidumpositive horses and seasons was found. G. candidum positivity was associated to horse age being the adults (66.7%) more affected than the young animals (P 0.05). The skin lesions were characterized by a dry, erythematous, well-defined circular alopecia. Alopecia was the most frequent symptom (91.7%), followed by desquamation (47.7%) and pruritus (33.3%), localized mainly on the head and neck regions (P 0.05; Table 1). Out of the eight animals treated with Virkon1 S (Farmaceutici Gellini, Aprilia, Italy) the complete remission of symptoms was observed following one month of treatment (Fig. 1) resulting the culture negative. 4. Discussion In the present study G. candidum was often isolated (28.1%) from samples collected on lesioned skin for which microbiological and parasitic infections were excluded. Our results, along with previous investigations in which G. candidum was only occasionally isolated from healthy tissues of horses (Ishikawa et al., 1996; Keller et al., 2000; Rosa et al., 2003), might suggest the pathogenic role this fungus plays in the occurrence of horse skin lesions. Predisposing factors for localized geotrichosis (e.g., the prolonged contact of the skin with pond water), or for disseminated geotrichosis (e.g., senescence, immunosuppressive therapy and long use of antibacterial drugs) were previously described (Spanoghe et al., 1976; Reppas and Snoeck, 1999). In this study, two out of the positive females gave birth recently and, at least in one case, the owner reported the long-term use of corticosteroids, which, in turn, could have compromised the host immune system. [()TD$FIG] 370 L.A. Figueredo et al. / Veterinary Microbiology 148 (2011) 368–371 diseases affecting horses, laboratory test are required to confirm the clinical diagnosis. Conflict of interest statement All authors declare to have no conflict of interest. Acknowledgment Thanks to Romina Paradies for her contribution on the individual files selection. References Fig. 1. (A) Horse presenting with miliary skin lesions that had positive cultures for G. candidum. (B) The same horse after one month of treatment. Furthermore, since animals included in the study were riding horses, they underwent routine prophylactic therapies (e.g., benzimidazoles, tetrahydropyrimidines, macrocyclic lactones), which, in turn, could have favoured geotrichosis (Santos et al., 1983). Even thought not statistically significant, the highest frequency of geotrichosis detected in spring (i.e., 44.4%) could be related to the high incidence of females giving birth in this season. The cutaneous lesions by G. candidum were characterized by circular alopecia mainly localized on the head and neck and they resembled those caused by dermatophytes, which appear initially on the trunk and spreading over the rump throughout the neck, head and limbs (Soltys and SumnerSmith, 1969). The localization of lesions on the horse body and their general presentation require a differential diagnosis with lesions caused by dermatophytes. In addition, the negativity of hair and skin scraping samples at the microscopic observation suggests that cultural examination has to be performed for diagnosing of geotrichoses. Further studies regarding the diagnosis using cytology and histopathological examination are encouraged. The results of this study also indicate that Virkon1 S, was useful in resolving skin lesions in the eight treated horses. Indeed, all clinical signs disappeared one month after the treatment and no adverse reactions were observed. Finally, these results suggest that G. candidum should be considered as an etiological agent of cutaneous mycosis in horses and thus geotrichosis should be included in the differential diagnosis of skin infections. Because the clinical signs of geotrichosis are similar to other skin Chahota, R., Katoch, R., Mahajan, A., Verma, S., 2001. Clinical bovine mastitis caused by Geotrichum candidum. Vet. Arhiv. 71, 197–201. Conti Dı́az, I.A., Vargas, R., Apolo, A., Moraña, J.A., Pedrana, G., Cardozo, E., Almeida, E., 2003. Mycotic bovine nasal granuloma. Rev. Inst. Med. Trop. São Paulo 45, 163–166. de Hoog, G.S., Guarro, J., 1995. Atlas of Clinical Fungi. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, p. 720. de Hoog, G., Smith, M., 2004. Ribosomal gene phylogeny and species delimitation in Geotrichum and its teleomorphs. Stud. Mycol. 50, 489–515. Dolensek, E.P., Napolitano, R.L., Kazimiroff, J., 1977. Gastrointestinal geotrichosis in six adult gorillas. J. Am. Vet. Med. Assoc. 171, 975– 976. Etienne, A., Datry, A., Gaspar, N., Morel, V., Delabesse, E., Lmimouni, B., Vernant, J.P., Dhédin, N., 2008. Successful treatment of disseminated Geotrichum capitatum infection with a combination of caspofungin and voriconazole in an immunocompromised patient. Mycoses 51, 270–272. Gambale, W., Correa, B., Rodrigues, P.C., Purchio, A., Larsson, C.E., 1987. Ocorrência de fungos em lesões superficiais de cães na cidade de São Paulo, Brasil. Rev. Fac. Med. Vet. Zootec. Univ. São Paulo 24, 187–191. Henrich, T.J., Marty, F.M., Milner Jr., D.A., Thorner, A.R., 2009. Disseminated Geotrichum candidum infection in a patient with relapsed acute myelogenous leukemia following allogeneic stem cell transplantation and review of the literature. Transpl. Infect. Dis. 11, 458–462. Holzworth, J., Blouin, P., Conner, M.W., 1987. Mycotic diseases. In: Holzworth, J. (Ed.), Deseases of the Cat. Medicine and Surgery. Saunders, Philadelphia, pp. 320–358. Ishikawa, M.M., Lucas, R., Larsson, C.E., Gambale, W., Fernandes, W.R., 1996. Isolamento e identificação da microbiota fúngica de dermatófitos da pele de eqüinos hı́gidos e daqueles afetados por dermatofitose. Braz. J. Vet. Res. Anim. Sci. 33, 170–175. Keller, M., Krehon, S., Stanek, C., Rosengarten, R., 2000. Keratinopathogenic mould fungi and dermatophytes in healthy and diseased hooves of horses. Vet. Rec. 147, 619–622. Mahapatra, A., 2005. Coinfection of Cryptosporidium and Geotrichum in a case of AIDS. Indian J. Pathol. Microbiol. 48, 25–27. Marchetti, V., Mancianti, F., Cardini, G., Luchetti, E., 2006. Evaluation of fungicidal efficacy of benzalkonium chloride (Steramina G u.v.) and Virkon-S against Microsporum canis for environmental disinfection. Vet. Res. Commun. 30, 255–261. McKenzie, R.A., Green, P.E., 1976. Mycotic in captive in carpet snakes. J. Wildlife Dis. 12, 405–408. Migaki, G., Schmidt, R.E., Toft, J.D.2nd., Kaufmann, A.F., 1982. Mycotic infections of the alimentary tract of nonhuman primates: a review. Vet. Pathol. Suppl. 7, 93–103. Mós, E.N., Macruz, R., Santos, M.R.S., Porto, E., 1978. Geotricose em puro sangue ingles. Rev. Fac. Med. Vet. Zootec. Univ. São Paulo 15, 93. Pal, M., 2005. Role of Geotrichum candidum in canine oral ulcers. Rev. Iberoam. Micol. 22, 183. Pimenta, R.S., Alves, P.D.D., Correa Jr., A., Lachance, M.-A., Prasad, G.S., Rajaram, Sinha, B.R.R.P., Rosa, C.A., 2005. Geotrichum silvicola sp. nov. a novel asexual arthroconidial yeast species related to the genus Galactomyces. Int. J. Syst. Evol. Microbiol. 55, 497–501. Reeves, R.J., 1941. The incidence of bronchomycosis in the South. Am. J. Roentgenol. 45, 513. Reppas, G.P., Snoeck, T.D., 1999. Cutaneous geotrichosis in a dog. Aust. Vet. J. 77, 567–569. Rhyan, J.C., Stackhouse, L.L., Davis, E.G., 1990. Disseminated geotrichosis in two dogs. J. Am. Vet. Med. Assoc. 197, 358–360. L.A. Figueredo et al. / Veterinary Microbiology 148 (2011) 368–371 Rosa, M., Cardozo, L.M., da Silva Pereira, J., Brooks, D.E., Martins, A.L., Florido, P.S., Stussi, J.S., 2003. Fungal flora of normal eyes of healthy horses from the State of Rio de Janeiro, Brazil. Vet. Ophthalmol. 6, 51– 55. Ruiz, J.M., Arteaga, E., Martinez, J., Rubio, E.M., Torres, J.M., 1980. Cutaneous and renal geotrichosis in a giant tortoise (Geochelone elephantopus). Sabouraudia 18, 51–59. Santos, M.R.S., Portugal, M.A.S.C., Athaide, A.J., Ferraz, E.F., 1983. Geotricose cutânea em eqüinos. Biológico 49, 75–79. Sidhu, R.K., Singh, K.B., Jand, S.K., Joshi, D.V., 1993. Cutaneous geotrichosis in a dog and its handler-a case report. Indian J. Anim. Health 32, 75. 371 Soltys, M.A., Sumner-Smith, G., 1969. Dermatophytes in veterinary practice. Can. Vet. J. 10, 111–116. Spanoghe, L., Devos, A., Viaene, N., 1976. Cutaneous geotrichosis in the red flamingo (Phoenicopterus ruber). Sabouraudia 14, 37–42. Suh, S.-O., Blackwell, M., 2006. Three new asexual arthroconidial yeasts, Geotrichum carabidarum sp. nov., Geotrichum histeridarum sp. nov. and Geotrichum cucujoidarum sp. nov. isolated from the gut of insects. Mycol. Res. 110, 220–228. Wuczkowski, M., Bond, C., Prillinger, H., 2006. Geotrichum vulgare sp. nov., a novel asexual arthroconidial yeast. Int. J. Syst. Evol. Microbiol. 56, 301–303.