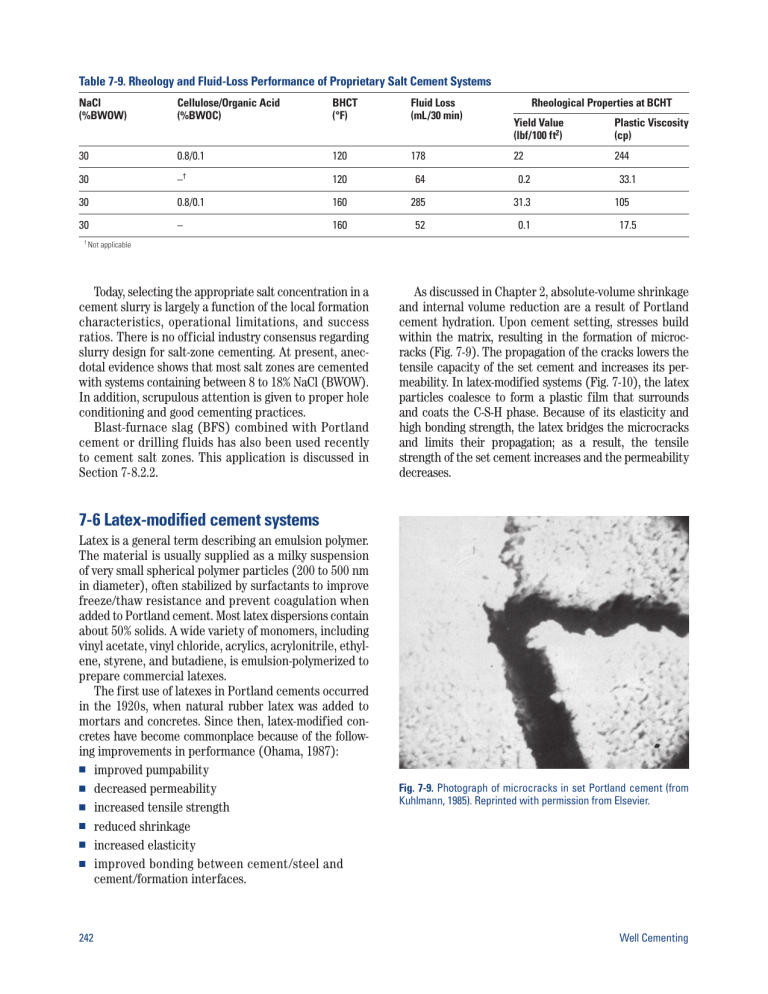
Table 7-9. Rheology and Fluid-Loss Performance of Proprietary Salt Cement Systems NaCl (%BWOW) Cellulose/Organic Acid (%BWOC) BHCT (°F) Fluid Loss (mL/30 min) 30 0.8/0.1 120 178 30 –† 120 64 0.2 30 0.8/0.1 160 285 31.3 30 – 160 52 0.1 † Not Rheological Properties at BCHT Yield Value (lbf/100 ft2) Plastic Viscosity (cp) 22 244 33.1 105 17.5 applicable Today, selecting the appropriate salt concentration in a cement slurry is largely a function of the local formation characteristics, operational limitations, and success ratios. There is no official industry consensus regarding slurry design for salt-zone cementing. At present, anecdotal evidence shows that most salt zones are cemented with systems containing between 8 to 18% NaCl (BWOW). In addition, scrupulous attention is given to proper hole conditioning and good cementing practices. Blast-furnace slag (BFS) combined with Portland cement or drilling fluids has also been used recently to cement salt zones. This application is discussed in Section 7-8.2.2. As discussed in Chapter 2, absolute-volume shrinkage and internal volume reduction are a result of Portland cement hydration. Upon cement setting, stresses build within the matrix, resulting in the formation of microcracks (Fig. 7-9). The propagation of the cracks lowers the tensile capacity of the set cement and increases its permeability. In latex-modified systems (Fig. 7-10), the latex particles coalesce to form a plastic film that surrounds and coats the C-S-H phase. Because of its elasticity and high bonding strength, the latex bridges the microcracks and limits their propagation; as a result, the tensile strength of the set cement increases and the permeability decreases. 7-6 Latex-modified cement systems Latex is a general term describing an emulsion polymer. The material is usually supplied as a milky suspension of very small spherical polymer particles (200 to 500 nm in diameter), often stabilized by surfactants to improve freeze/thaw resistance and prevent coagulation when added to Portland cement. Most latex dispersions contain about 50% solids. A wide variety of monomers, including vinyl acetate, vinyl chloride, acrylics, acrylonitrile, ethylene, styrene, and butadiene, is emulsion-polymerized to prepare commercial latexes. The first use of latexes in Portland cements occurred in the 1920s, when natural rubber latex was added to mortars and concretes. Since then, latex-modified concretes have become commonplace because of the following improvements in performance (Ohama, 1987): ■ improved pumpability ■ decreased permeability ■ increased tensile strength ■ reduced shrinkage ■ increased elasticity ■ improved bonding between cement/steel and cement/formation interfaces. 242 Fig. 7-9. Photograph of microcracks in set Portland cement (from Kuhlmann, 1985). Reprinted with permission from Elsevier. Well Cementing 7-6.2 Early latex-modified well cement systems In 1958, Eberhard and Park patented the use of vinylidene chloride latex in well cements. Improved performance was observed for systems containing up to 35% latex solids BWOC. Later, polyvinyl acetate latex was identified as a suitable material (Woodard and Merkle, 1964). The preferred concentration of latex solids varied from 2.5% to 25% BWOC. The polyvinyl acetate system has been used successfully for many years; however, it is limited to applications at temperatures less than 122°F [50°C] bottomhole static temperature (BHST). 7-6.3 Styrene butadiene latex systems Fig. 7-10. Photograph of latex-modified Portland cement, magnified 1,200 times (from Kuhlmann, 1985). Reprinted with permission from Elsevier. 7-6.1 Behavior of latexes in well cement slurries The use of latex in well cements occurred much later. In 1957, Rollins and Davidson reported improved performance when latex was added to the mix water. In addition to the attributes mentioned above, adding latex provided the following additional benefits: ■ better bonding to oil-wet and water-wet surfaces ■ less shattering when perforated ■ increased resistance to contamination by well fluids ■ lowered fluid-loss rate ■ improved durability. When latex is added as part of the liquid phase of a Portland cement system, the resulting slurry has a normal color and consistency; however, because of the solids content of the latex, such slurries contain 20% to 35% less water. After curing, the set product consists of hydrated cement particles connected by a film of latex particles (Kuhlmann, 1985). The film of latex particles imparts the physical and chemical properties described above (Parcevaux and Sault, 1984; Drecq and Parcevaux, 1988). While the slurry is still liquid, the latex particles impart excellent rheological properties because of a lubricating action. In addition, the latex particles provide excellent fluid-loss control by physically plugging small pores in the cement filtercake (Drecq and Parcevaux, 1988) (Chapter 3). An improvement in latex cement technology occurred when Parcevaux et al. (1985) identified styrene butadiene latex as an effective additive for the prevention of annular gas migration (Chapter 9). Additional refinements were made by Sault et al. (1986). Styrene butadiene latexes impart the same beneficial effects described above; however, they are effective at temperatures as high as 350°F [176°C]. Fig. 7-11 is a plot of API/ISO fluid-loss rate versus latex concentration for various well cement slurries. The results illustrate that normal-density neat slurries require less latex to achieve a given fluid-loss rate. More latex is required for slurries containing extenders or weighting agents, especially those with a lower solids content (extended with sodium silicate). Figure 7-12 illustrates the decreased volumetric shrinkage observed with a latex-modified Portland cement system cured at 158°F [70°C]. 3.0 Neat: 15.8 lbm/gal Barite: 18 lbm/gal Bentonite: 13.3 lbm/gal Fly ash: 13.6 lbm/gal Sodium silicate: 13 lbm/gal 2.5 Styrene butadiene latex (gal/sk) 2.0 1.5 1.0 0.5 0 50 100 150 200 250 300 API/ISO fluid loss (mL/30 min) Fig. 7-11. Fluid-loss rates of latex-modified cement slurries (185°F [85°C]). Chapter 7 Special Cement Systems 243 7 Neat cement 6 5 Absolute volume shrinkage (%) 4 3 Latex-modified cement 2 1 0 0 6 12 18 24 Time (hr) Fig. 7-12. Absolute volume shrinkage of normal-density Portland cements (from Parcevaux and Sault, 1984). Reprinted with permission of SPE. Portland cement systems are not compatible with strong inorganic acids such as sulfuric, hydrochloric, and nitric acid. In such environments, organic polymer cements, usually epoxy-based, must be used to provide sufficient chemical resistance (Cole, 1979). Such systems are also known as “synthetic cements.” Epoxy cements are prepared by mixing an epoxy resin such as bisphenol-A (Fig. 7-13) with a hardening agent. Depending upon the desired end properties, the hardening agent can be an anhydride, aliphatic amine, or polyamide (Sherman et al., 1980). A solid filler such as silica flour is often used to build density, reduce cost, and act as a heat sink for the exothermic reaction that occurs during the curing period. Depending upon the circulating and static well temperatures, various catalysts and accelerators can also be added to control the placement and setting times. 7-7 Cements for corrosive environments Set Portland cement is a remarkably durable and forgiving material, but it has its limits. In a wellbore environment, Portland cement is subject to chemical attack by certain formations, migrating fluids, and substances injected from the surface. As discussed in Chapter 10, saline geothermal brines containing CO2 are particularly deleterious to the integrity of the set cement. Cement durability is also a key consideration in wells for chemical waste disposal and for enhanced oil recovery by CO2 flooding. 7-7.1 Cements for chemical waste disposal wells Zonal isolation is of paramount importance in chemical waste disposal wells. If not properly confined, injected waste fluids could contaminate freshwater aquifers and corrode the exterior of the casing. To ensure zonal isolation throughout the life of such wells, the cement and the tubular hardware in the well must be chemically resistant to the waste fluids (Runyan, 1974). The chemically resistant casings used in waste disposal wells include modified polyester and epoxy fibercast, or metal alloys such as Carpenter 20,† Incoly 825,‡ and Hastalloy G.§ The cement systems are chosen for compatibility with the injected waste material. Modified Portland cements are generally appropriate for disposal wells involving weak organic acids, sewage waters, or solutions having a pH of 6 or above. The durability of the set cement is improved by adding pozzolans, increasing the density by addition of dispersants, employing controlled particle-size technology, or adding liquid latexes to the slurry. These methods substantially reduce the permeability of the set cement. O CH2 CHCH2 O CH3 C CH3 OH OCH2CHCH2 O n CH3 C O OCH2CH CH2 CH3 Fig. 7-13. Chemical structure of bisphenol-A. Epoxy resin cement systems are characterized by their corrosion resistance and high compressive and shear-bond strength. They are compatible with strong acids and bases (up to 37% HCl, 60% H2SO4, and 50% NaOH) at temperatures up to 200°F [93°C] during extended exposure periods. Epoxies are also resistant to hydrocarbons and alcohols, but not to chlorinated organics or acetone. Typically, the compressive strengths range between 8,000 and 10,000 psi [56 to 70 MPa], and shear-bond strengths can be as much as 9 times higher than those of Portland cement (R.A. Bruckdorfer, unpublished data, 1985). Until recently, to prevent premature setting and to enhance cement bonding, nonaqueous spacers such as gelled oil, diesel, or alcohol were required on all epoxy cement jobs. The use of such preflushes is costly and time consuming. In 2001, Eoff et al. introduced a watercompatible epoxy cement system. Instead of bisphenol-A, the epoxy sealant is the diglycidyl ether of cyclohexane dimethanol. The hardening agent is a mixture of diethyltoluenediamine and tris(dimethylaminomethylphenol). The performance of this system is unaffected by water contamination, up to 25% by volume. † Trademark of CRS Holdings, Inc. of International Nickel Co. § Trademark of Haynes International, Inc. ‡ Trademark 244 Well Cementing