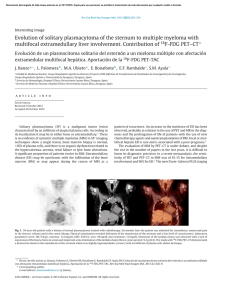
J Radioanal Nucl Chem (2014) 299:741–757 DOI 10.1007/s10967-013-2823-1 Nanomaterial-based adsorbents: the prospect of developing new generation radionuclide generators to meet future research and clinical demands Rubel Chakravarty • Ashutosh Dash Received: 5 August 2013 / Published online: 14 November 2013 Akadémiai Kiadó, Budapest, Hungary 2013 Abstract Nanostructured materials by virtue of huge surface to volume ratios, altered physical properties, tailored surface chemistry, favorable adsorption characteristics, and enhanced surface reactivity resulting from the nanoscale dimensions, have attracted considerable attention as a new class of adsorbent material in column chromatographic separation. This emerging class of adsorbent represents an innovative paradigm and is expected to play an important role in the development of radionuclide generators for nuclear medicine. The optimal combination of suitable nanomaterial and appropriate parent/daughter radionuclide pair forms the basis of such generators. Development of such generators is currently under intensive investigations and the utility of such systems is expected to pave the way for broad panoply of diagnostic and therapeutic applications in nuclear medicine. While nanomaterial-based radionuclide generator is still in its infancy, the use of such novel class of adsorbents is expected to have potential impact on shaping the radionuclide generator technology of future generation. This review provides a comprehensive summary on the utility of nanomaterials as effective adsorbents in the development column chromatographic radionuclide generators for medical applications. This overview outlines a critical assessment of role of the nanosorbents, recent developments, the contemporary status, and key challenges and apertures to the near future. Keywords Adsorbent Column chromatographic separation Nanomaterials Radionuclidic generator Radiopharmaceuticals R. Chakravarty A. Dash (&) Isotope Applications and Radiopharmaceuticals Division, Bhabha Atomic Research Centre, Mumbai 400 085, India e-mail: adash@barc.gov.in Introduction The role of radionuclide generator in nuclear medicine in providing radionuclides for both research and clinical applications has been well demonstrated and recognized [1–4]. Generator produced radionuclides find extensive utility in nuclear medicine, oncology and interventional specialties. Widespread interest in the use of radionuclide generators to obtain a variety of diagnostic and therapeutic radionuclides to meet the needs of nuclear medicine have stimulated considerable research leading to the development of several innovative strategies [4–6]. The current importance and success of diagnostic radionuclide imaging using nuclear medicine techniques is primarily due to the availability of 99Mo/99mTc generator system [7–10]. The selection of an appropriate separation technology to isolate the daughter radionuclide of required purity with appreciable yield has been the cornerstone in the success of a radionuclide generator [11]. While numerous separation technologies have been investigated and effectively used over the years for achieving the separation of radionuclide of interest, the column chromatography technology has dominated the field significantly due to the following advantages. • • • • • • Simple and user friendly process, requiring minimum time for operation. Reproducible performance in terms of daughter radionuclide elution yields on continual use. High radionuclidic (RN), radiochemical (RC) and chemical purity of the daughter radionuclide are attainable. Generate insignificant quantity of radioactive waste. Negligible operational constraints. Offer the possibility of regeneration and recharging. 123 742 For these reasons, radionuclide generators making use of column chromatography technique have been in use for several years and have offered substantial benefits for nuclear medicine applications by providing short-lived radionuclides for the onsite formulation of radiopharmaceuticals for a variety of diagnostic and therapeutic applications [2, 12]. The tremendous prospects along with the challenges associated with the development of radionuclide generator of desired attributes have thus led to a considerable amount of fascinating research and innovative strategies. Among the various separation technologies available, the operational simplicity of the well established chromatographic-type generator using a solid parent adsorption material is the preferred method of choice for radionuclide generator from which the daughter radionuclide formed from the decay of its generic parent radionuclide is selectively eluted. Within the heart of column chromatography separation technique lays the adsorbent and the ability of the adsorbent to separate the daughter radionuclide constitutes the pillars for its success in radionuclide generator. With substantial efforts by both academia and the industry, a wide variety of adsorbents with appropriate physicochemical properties have been successfully developed and used in a wide range of radionuclide generators [4, 12, 13]. The required volume for quantitative elution of daughter radionuclide from a chromatographic-based generator apparently depends on the size of the column which, in turn, is inversely proportional to the specific activity of the parent. In view of the limited adsorption capacity of adsorbents derived from bulk material, the use of high specific activity parent radionuclide is not only an interesting prospect, but may be viewed as a necessary one owing to the requirement to obtain high radioactive concentration (RAC) or specific volume of the daughter radionuclide to prepare radiopharmaceuticals. Despite the potential advantages of column chromatography separation technique, the use of low specific activity parent radionuclide poses numerous challenges and hurdles. In light of the perceived need to adsorb required amount of activity in a generator, the use of low specific activity parent radionuclide necessitates large amount of adsorbent which in turn increases the size of the column. Elution of the daughter radionuclide from such generator requires a large volume of eluent and results in a solution of low specific volume activity (low RAC) which is unsuitable for subsequent chemical attachment of daughter radionuclide to an agent for clinical use. While post elution concentration (PEC) of generator eluate [13–22] has tangible benefits to render them useful for radiopharmaceuticals applications, use of high capacity adsorbent in a small size chromatography column is a trustworthy proposition. The development of high capacity adsorbent represents an important challenge and can only be 123 J Radioanal Nucl Chem (2014) 299:741–757 overcome by technical breakthroughs in areas of material science. The past few decades have witnessed unprecedented endeavors for developing a wide variety of high capacity adsorbent materials in attempts to improve the specific volume activity of the generator eluate [12, 23]. Yet, in spite of many impressive advances in the development and use of high capacity adsorbent, the promise to develop a practical high capacity adsorbent suitable for radionuclide generator application has not been fulfilled. While the attempt made in this direction constitutes a step in the right direction, all the efforts turned out futile as the adsorption capacity of bulk material cannot be improved beyond certain degree owing to the limitation posed by the density of surface active sites, the activation energy of adsorptive bonds and the mass transfer rate to the adsorbent surface. A more prudent approach to mitigate these unforeseen difficulties is to identify the prospect of using nanomaterial as a new generation adsorbent. Nanomaterials differ from microsized and bulk materials not only in the scale of their characteristic dimensions, but also in the fact that they possess new physical properties and offer new possibilities for developing high capacity adsorbent. The enhancement in specific surface area and associated improved surface energy offers a great deal of advantages and renders some very important properties. The use of nanoparticles as effective adsorbents requires careful synthesis, structural characterization, detailed adsorption properties analysis, and reproducible scale-up and manufacturing process to achieve a consistent product with the intended physicochemical characteristics. The recent surge of interest in the use of nanomaterialbased adsorbent in the preparation of column chromatography radionuclide generators has been the motivation to provide this detailed review on this emerging field of technology. It is not a comprehensive review but rather discusses key developments and applications. In the following sections, the radionuclide generator principle, role of nanomaterial-based adsorbent in the development of radionuclide generators, different types of nanomaterialbased radionuclide generators, current status, survey of major strategies for enhancing their utilities, and future prospective are discussed. Principle of a radionuclide generator system A radionuclide generator (Fig. 1) is a self-contained system housing an equilibrium mixture of a parent/daughter radionuclide pair. The system is designed to separate and provide the daughter radionuclide formed by the decay of a parent radionuclide [1, 4, 11, 23, 24]. The parent–daughter nuclear relationships offer the possibility to provide J Radioanal Nucl Chem (2014) 299:741–757 743 Radioactive equilibrium The daughter radionuclide formed from the decay of a parent radionuclide. The decay of parent radionuclide can be mathematically expressed by the Eq. (1): dN ¼ k1 N1 ; dt where N1 ¼ N10 ek1 t ; ð1Þ where k1 is the decay constant for the parent radionuclide and N1 represents the number of atoms of parent at time t. The N10 term indicates the corresponding quantity when t = 0. The daughter radionuclide formed from the decay of the parent, k1N1, also decays at a rate, k2N2 and thus the net production rate of the daughter radionuclide is given by Eq. (2): dN2 ¼ k1 N1 k2 N2 ¼ k1 N10 ek1 t k2 N20 ek2 t : dt ð2Þ Solution of this linear differential equation leads to: k1 N2 ¼ N10 ek1 t ek2 t þ N20 ek2 t : ð3Þ k2 k1 For radionuclide generator application, the half life of the parent radionuclide should be greater than that of the daughter (t1/2,1 [ t1/2,2, i.e. k1 \ k2) to allow repeat elution of the daughter radionuclide over a reasonable shelf-life period. Thus, ek2 t is negligible compared with ek1 t after t becomes sufficiently large and the N20 ek2 t term also becomes negligible and therefore the Eq. (2) can be simplified as below: Fig. 1 Schematic diagram of a chromatography radionuclide generator, a generator assembly and b generator column radionuclide generators to separate the short-lived daughter at suitable time intervals. Advantages of radionuclide generators are as follows: • • • • Ensures onsite availability of short lived daughter radionuclides on demand for diagnostic and therapeutic applications without reliance on local accelerator or reactor production capabilities. Ease of availing short lived daughter radionuclides in a cost-effective way for the onsite formulation of radiopharmaceuticals. Availability of the daughter radionuclide in a high specific activity, no-carrier-added (NCA) form. Provide the scope of performing diagnosis and therapy in places far from isotope production facilities. However, the radionuclide generators intended for medical applications must meet regulatory and quality control requirements. N2 ¼ k1 N 0 ek1 t : k2 k1 1 ð4Þ Since, N1 ¼ N10 ek1 t ; the ratio of the number of atoms of the two radionuclides can be expressed as: N1 k2 k1 ¼ ; N2 k1 N1 k1 k2 k1 A1 ðk2 k1 Þ k1 ¼ ¼ ¼ ¼1 : or k2 N2 k2 k2 A2 k2 ð5Þ A1 and A2 refer to the activities of the parent and daughter radionuclide, respectively. The daughter activity reaches the state of equilibrium or maximum activity, when its formation is exactly compensating those which are decaying: When A1 ¼ A2 or k1 N1 ¼ k2 N2 or dN2 ¼ 0: dt ð6Þ This condition of a constant ratio for the parent and daughter activity is known as radioactive equilibrium, and the maximum activity of the daughter which occurs at the time of equilibrium t is given by: 123 744 J Radioanal Nucl Chem (2014) 299:741–757 activity is at its maximum as calculated using Eq. (7). Owing to the decrease of the parent radionuclide activity within the time between two elution steps, the radionuclide generator is expected to provide a reduced level of the daughter radionuclide activity by each subsequent elution as shown in Fig. 3. In-growth and isolation of daughter radionuclide Fig. 2 Schematic diagram of secular and transient equilibrium. a Transient equilibrium is a condition when the t1/2(phys) of the parent is approximately 10 times greater than the t1/2(phys) of the daughter. b Secular equilibrium is a condition when the t1/2(phys) of the parent is many times greater than the t1/2(phys) of the daughter (100–1,000 times greater or more) t¼ 1 k2 ln : k2 k1 k1 ð7Þ Radioactive equilibrium between the parent and daughter is referred to as transient or secular equilibrium, depending on the relative half lives of the parent and daughter pair. In transient equilibrium, the ratio between the half lives of the parent and daughter is less than 10. For a secular equilibrium, the parent half life is far higher than the daughter half life (t1/2,1 t1/2,2 or k1 k2), the Eqs. (5) and (6) reduce to N1/N2 = k2/k1 and A1 = A2. Hence, in secular equilibrium, the daughter activity is equal to the parent activity. Illustration of the relative growth and decay of the parent and daughter radionuclide pair is illustrated in Fig. 2a (transient equilibrium) and Fig. 2b for secular equilibrium. The daughter radionuclide can be repeatedly extracted from parent/daughter radionuclide pair, which is the main benefit of using these systems for medical and industrial applications. For practical considerations, radionuclide generators are eluted at periodic intervals depending on the daughter activity requirements. Often the separation of the daughter from the parent may not occur at the time the daughter 123 The growth and separation of the daughter radionuclide can be continued as long as there are useful activity levels of the parent radionuclide available. Elution of the generator is performed either manually or with an automated system. The activity eluted from the generator typically follows a Gaussian distribution with the maximum activity being eluted in the intermediate fractions. The eluent can also be ‘fractionated’ by discarding those fractions with low activity if the activity concentration is a critical issue. Separation may be performed any time before equilibrium is reached, and the activity levels of daughter recovered will depend on the time elapsed since the last separation. In-growth of the daughter species is continuous, and once the activity of the daughter is recovered from the mixture, the daughter activity increases until its activity level reaches a maximum and is in equilibrium with the parent radionuclide (Fig. 2). The growth of the daughter depends on the half life of the daughter radionuclide which also governs the frequency of its separation from the parent radionuclide. When the daughter radionuclide is relatively long-lived, periodic elution will take place prior to reaching the maximum equilibrium daughter activity levels and it is normal to use generators in this way. As an example, 50 % daughter activity in-growth is detected in one half life, 75 % in two half lives and the daughter activity reaches the activity of the parent radionuclide in five–six half lives (Fig. 4). Criteria for selection of parent/daughter pairs Although an examination of the chart of the radionuclide indicates that there are about 130 parent/daughter pairs of radionuclides that can be used for the preparation of radionuclide generators, only a few are of practical interest. While selecting a parent/daughter pair for making radionuclide generator, the following criteria need to be considered. • Availability of parent radionuclide: Cost-effective production of the parent radionuclides is important. Parent radionuclide which exhibits attractive characteristics but which lack a cost effective production route will constitute a major barrier for its utility in radionuclide generator. J Radioanal Nucl Chem (2014) 299:741–757 745 Fig. 3 Multiple growth and elution of daughter activity in a radionuclide generator • • • Fig. 4 The growth of daughter radionuclide in a radionuclide generator as a function of time (t1/2) • • • • Parent radionuclide half life: The physical half-life of the parent radionuclide should be long enough to provide long practical shelf-lives of the generator. Parent specific activity: The specific activity of the parent radionuclide should be high to retain required activity in a given mass of adsorbent. Daughter radionuclide half life: The physical half-life of the daughter radionuclide should be matched well with the in vivo pharmacokinetics of the radiolabeled targeting molecule. Emission and energy of the radiation of the daughter radionuclide: The daughter product of a radionuclide generator will decay by any of the decay modes (isomeric transition, b-, b?, electron capture, a decay) or by a combination of decay modes. Consequently, the applications of generators vary depending on the decay characteristics. c Emitters, with c energy within the range of 100–250 keV are suitable for SPECT imaging. Particle emitting radionuclides (a particle, b- particle or Auger electron emitters) are suitable for therapy. Positron emitting radionuclides are needed for positron emission tomography (PET) imaging. Decay of daughter radionuclide: The daughter radionuclide should preferably decay to a stable or very long-lived product to preclude radiation dose to the patient undergoing diagnosis or therapy. Purity of daughter radionuclide: The daughter radionuclide eluted from the generator should be of high purity (RN, RC, and elemental purity) and free from trace metal contamination. Chemical characteristics of daughter radionuclide: The daughter radionuclide should have chemistry amenable to its attachment with a broad class of carrier molecules and binding must exhibit high in vivo stability when attached to the radiopharmaceutical. Table 1 summarizes the characteristics of several key radionuclide generator systems that could be useful to provide daughter radionuclide for life science applications. While devising a radionuclide generator system, the following points should be taken into consideration. • • • • • Chemical or physical properties of the daughter radionuclide must be sufficiently different from that of the parent to permit easy and efficient separation using appropriate chemical or physical techniques. Separation must be performed rapidly to minimize decay losses of the daughter radionuclide. The daughter radionuclide should be separated with 0.9 % NaCl or in a chemical form amenable for the radiolabeling with a broad class of carrier molecules. Yield and purity of the daughter radionuclide intended for radiotracer investigation should be within the acceptable range. Physical intervention with the generator/elution system should be minimal to minimize radiation dose to operating staff. 123 746 J Radioanal Nucl Chem (2014) 299:741–757 Table 1 Radionuclide generators for biomedical applications Generator systems 42 Ar/42K Parent radionuclide Daughter radionuclide t1/2 Principal production route Main decay mode t1/2 Main decay mode Application 32.9 years R b- 12.36 h b- Chemistry 44 47.3 years A EC 3.93 h b? PET 52 Fe/52mMn 68 Ge/68Ga 8.28 h 270.8 days A A b? EC 21.1 min 1.14 h b? b? PET PET 72 8.4 days A EC 1.08 days b? PET 44 Ti/ Sc Se/72As 83 83m 86.2 days A EC 1.86 h c Chemistry/RPC 82 25.6 days A EC 1.27 min b? PET 90 28.5 years R, FP b- 2.67 days b- ERT 99 2.75 days R, FP b- 6.01 h c SPECT 103 16.97 days R, A EC 56.12 min c, Ae Chemistry 109 1.267 years A EC 39.6 s c FPRNA Rb/ Kr Sr/82Rb Sr/90Y Mo/99mTc Pd/103mRh Cd/109mAg 113 113m 115.1 days R EC 1.66 h c Chemistry/RPC 118 6.00 days A EC 3.6 min b? PET 132 3.26 days R, FP b- 2.28 h c, b- Therapy 137 30.0 years R, FP b- 2.55 min c In vivo diagnosis 140 12.75 days A b- 1.68 days c, b- Sn/ In Te/118Sb Te/132I Cs/137mBa Ba/140La 134 134 ? Chemistry/RPC 3.16 days A EC 6.4 min b PET Ce/144Pr 140 Nd/140Pr 284.9 days 3.37 days R, FP A bEC 17.3 min 3.39 min c b?, Ae Chemistry/RPC PET 166 3.40 days R b- 1.12 days b- ERT 9.24 days A EC 2.28 s c Chemistry/RPC Ce/ La 144 Dy/166Ho 167 167m Tm/ Er 172 1.87 years A EC 6.70 days c Chemistry/RPC 178 Hf/172Lu 21.5 days A EC 9.31 min c FPRNA 69.4 days R b- 16.98 h b- ERT 15.4 days R - b 4.94 s c FPRNA 194 6.0 years R b- 19.15 h c, b- ERT 226 1.6 9 103 years DC a 3.83 days a ERT 225 10.0 days A DC 45.6 min b-, a ERT W/178Ta 188 W/188Re 191 Os/ 191m Ir Os/194Ir Ra/222Rn Ac/213Bi A accelerator, DC decay chain, f fission, R reactor/neutron capture, FP fission product, Ae atomic electrons, EC electron capture, b1 if EC \50 %, ERT endoradiotherapy, FPRNA first pass radionuclide angiography, PET positron emission tomography, RPC radiopharmaceutical chemistry, SPECT single photon emission computed tomography • Processes for the production of radionuclide generator systems for clinical applications should follow good manufacturing practice standards. Nanomaterial-based adsorbent Nanomaterials are materials with a particle size of less than 100 nm in at least one dimension and have drawn the attention, imagination, and close scrutiny of scientists of diverse fields [24–32]. When the characteristic dimensions changes to nanoscale, they make up a new realm of matter that results in: • large fraction of surface atoms, 123 • • • high surface energy, spatial confinement, reduced imperfections. As a result, these materials possess unique physical properties and offer possibilities to develop new technologies and improve the existing ones. The high surface area to-mass ratio of nanomaterials can greatly improve the adsorption capacities of sorbent materials. Because of their reduced size and large radii of curvature, the nanomaterials have a reactive surface due to the high density of lowcoordinated atoms at the surface, edges and vortices. The surface atoms are unsaturated and can therefore bind with other atoms, possess highly chemical activity and can strongly chemisorb many substances. J Radioanal Nucl Chem (2014) 299:741–757 747 Table 2 Synthesis and structural characterization of nanosorbents for radionuclide generators Nanosorbents Synthesis method Structural characteristics Radionuclide generators reported using this material References Polymer embedded nanocrystalline titania (TiP) Controlled hydrolysis of TiCl4 in isopropyl alcohol medium Nanocrystalline, rutile phase, 5 nm crystallite size, surface area 30 m2/g 99 [43, 44] Mixed phase nanocrystalline zirconia (nanoZrO2) Controlled hydrolysis of ZrOCl28H2O in isopropyl alcohol medium Nanocrystalline, biphasic with monoclinic phase as major, 15 nm crystallite size, surface area 45 m2/g 188 [42] Tetragonal nanocrystalline zirconia (t-ZrO2) Controlled hydrolysis of ZrOCl28H2O in ammonical medium Nanocrystalline, tetragonal phase, 7 nm crystallite size, surface area 340 m2/g 99 [39, 41] Nanocrystalline alumina (c-Al2O3) Mechanochemical reaction of aluminum nitrate with ammonium carbonate Nanocrystalline, c-phase, 2 nm crystallite size, surface area 250 m2/g 99 Nanoceria– polyacrylonitrile composite (CeO2– PAN) Decomposition of cerium oxalate precursor followed by incorporation in polyacrylonitrile matrix Nanocrystalline, 10 nm crystallite size, surface area 72 m2/g 68 The advantages for using nanomaterials as adsorbent in column chromatographic radionuclide generator are due to • • • • • • Exceptional adsorption capabilities. Large surface area to attain high adsorption capacity. Fast adsorption kinetics due to absence of internal diffusion resistance to achieve rapid equilibrium conditions. Selectivity towards certain radionuclides. Favorable radiation, mechanical and thermal stabilities. Possibility for regeneration and reusability. The performance of adsorbent media in a fixed bed column depends mainly on two factors: the adsorption capacity of the media and its mass transport kinetics. Since both the factors can be limiting, fixed bed adsorption media can be designed using nanomaterials. This in turn would maximize the mass transport kinetics by providing radionuclides with rapid access to the sorption sites due to high surface area of the nanomaterial and by promoting internal mass transport due to its porosity. For successful use of nanomaterial-based adsorbents in radionuclide generator applications, a number of factors should be carefully considered with the most important amongst them being: • • • • • • Chemical composition, Morphology, Surface area and porosity of the material, Granular properties, Chemical durability, Adsorption characteristics in aqueous medium. While the adsorption of ionic species of radionuclides from solution on the nanoadsorbent is essentially Mo/99mTc, W/188Re 188 W/188Re Mo/99mTc, Ge/68Ga 68 Mo/99mTc, W/188Re [36, 38] 188 Ge/68Ga [35, 40] controlled by electrostatic forces, the primary interactions between radionuclide and sorbent are mainly due to the following parameters. • • • Physical adsorption or chemisorption of ion (dipole– dipole interaction). Ion-exchange process with the participation of surface groups or oxygen-containing surface groups. Formation of surface complexes and hydroxo complexes. In radionuclide generator applications, the parent radionuclide from solution is preferentially retained by the chromatographic column containing nanomaterial by adsorption. Nanomaterials provide almost unlimited combinations of various compositions, sizes and dimensions of materials, which can be tailored to develop a wide range of radionuclide generators with desired properties [24, 33]. Potentially useful radionuclide generator systems developed using nanomaterial-based sorbents Nanomaterial-based sorbents have been primarily utilized for preparation of three types of medically useful radionuclide generators, namely, 99Mo/99mTc, 68Ge/68Ga and 188 W/188Re generators. The characteristics of five nanomaterial-based sorbents, namely, polymer embedded nanocrystalline titania (TiP), mixed phase nano-zirconia (nano-ZrO2), tetragonal nano-zirconia (t-ZrO2), nanocrystalline alumina (c-Al2O3) and nano-ceria–polyacrylonitrile composite (CeO2–PAN) were evaluated for use in the preparation of the radionuclide generators [33–45]. The 123 748 methods of synthesis and structural characteristics of these nanosorbents and their applicability towards preparation of a particular type of radionuclide generator are briefly summarized in Table 2. The development of different types of radionuclide generators using nanosorbents is briefly described below. 99 Mo/99mTc generator The 99Mo/99mTc generators are extensively used for assessing 99mTc which is the ‘work-horse’ of diagnostic nuclear medicine [9, 46–49]. Every year, more than 30 million patient studies are performed worldwide using 99m Tc-based radiopharmaceuticals [9]. The most commonly used approach for assessing 99mTc from 99Mo is based on the use of column chromatography using acidic alumina as the sorbent matrix. However, the limited sorption capacity of bulk alumina (2–20 mg Mo/g) necessitates the use of high specific activity 99Mo produced through fission route [50]. The current production capability of fission 99Mo is confined to a limited number of aging nuclear reactors around the world, which have demonstrated numerous tribulations in the recent times [51–58]. Additionally, these reactors use highly enriched uranium targets (HEU) for production of 99Mo and currently there is an increasing global consensus to abandon the use of such weapons grade target materials for medical radioisotope production to thwart proliferation risks [51–58]. The supply chain of 99Mo is rather fragile and disruption in supply of this radioisotope might adversely affect the patient services in different parts of the world [51–58]. In order to reduce reliance on fission 99Mo, a number of alternative strategies for 99Mo production, such as aqueous homogeneous reactor concept, target fuel isotope reactor concept, direct cyclotron production of 99mTc, photo fission of 238U, photon-induced transmutation of 100Mo and accelerator driven subcritical assembly, have been proposed in the recent past [59]. However, completing the technology development and establishing the economics of these approaches as new potential sources of 99Mo for clinical applications will require several years. These approaches are balanced on a fine line, with technical breakthroughs on the one hand and long-term economic viability on the other. Devising an effective strategy from the conversion of HEU–LEU targets for 99Mo producers is challenging owing to concerns about logistical difficulties and economics. A more prudent and time-tested approach which is within the reach of most institutions having operating research reactors in the world, is to use 99Mo produced by (n,c) route for preparation of 99Mo/99mTc generators [56, 60, 61]. However, the relatively low specific activity [0.35–3.5 Ci/g (13–130 GBq/g)] of (n,c)99Mo is the major impediment for its utilization in the existing alumina based 123 J Radioanal Nucl Chem (2014) 299:741–757 generators, as large quantity of alumina would be required which in turn would result in elution of 99mTc with low RAC. Though several high capacity sorbents, such as hydrous manganese dioxide, hydrous titanium dioxide, hydrotalcite, cerium oxide, hydroxyapatite, polymeric zirconium compound (PZC), polytitanium oxychloride, alumina functionalized with a sulfate moiety, etc. have been proposed to circumvent this limitation for preparation of 99 Mo/99mTc column generators [62–69], the inherent drawbacks of these sorbent include loading of radioactive 99 Mo solution into the sorbent by batch process, difficulties in realizing optimum capacity owing to slow kinetics of sorption and requirement of a post purification column to achieve requisite 99mTc purity and RAC. In this context, the utility of nanomaterial-based sorbents which do not constitute a radical break with current generator technology, has been the centre of much effort in the quest for sorbents with enhanced capacity as well as selectivity. In order to tap the potential of nanomaterials as a sorbent for the preparation of clinical-scale 99Mo/99mTc generators, the utility of TiP, t-ZrO2 and c-Al2O3 have not only been profusely explored but also the feasibility of making of 99Mo/99mTc generators using (n,c)99Mo has been demonstrated [33, 36, 41, 44]. The 99Mo/99mTc generators developed using these sorbents and their performance characteristics are summarized in Table 3. It is seen from the result that the adsorption capacity of this class of sorbent are several fold greater than alumina and all these nanosorbents are suitable for preparation of 99Mo/99mTc generators for biomedical applications. During the process demonstration step, it was observed that the 99mTc elution yields were appreciably high and the level of RN, RC and chemical impurities in 99mTc obtained from all these generators were well within the acceptable limits prescribed in the pharmacopoeias [70]. As might have been expected, for all the sorbents, the sorption capacity under dynamic conditions was much less than that under equilibrium (static) conditions. This might be attributed to mass transfer limitations, such as incomplete external film diffusion and/or intra-particle transfer and slow kinetics of sorption. In view of the difficulties in handling of radioactive material and to reduce the radiation exposure, loading of 99Mo solution under dynamic (column-flow) condition seems to be an appealing protocol for the preparation of chromatographic generators despite the lower attainable sorption capacity and therefore recommended. Among the three sorbents studied, the use cAl2O3 provides the scope for the preparation of clinicalscale generator for routine use in nuclear medicine departments owing to highest dynamic sorption capacity. For the preparation of the generators, the authors have utilized low specific activity 99Mo (*18.5 GBq/g) produced by neutron irradiation of natural MoO3 target in a J Radioanal Nucl Chem (2014) 299:741–757 Table 3 Summary of Sorbents 99 Mo/99mTc generators developed using nanosorbents Sorption capacity (mg Mo/g) Static 749 Activity of Mo loaded GBq (mCi) Elution yield of 99m Tc (%) Maximum radioactive concentration of 99mTc achieved GBq (mCi)/mL Level of 99Mo impurity in 99m Tc (%) Radiochemical purity of 99m TcO 4 (%) Consistency in generator performance reported 99 Breakthrough TiP 110 75 3.74 (100) [75 0.74 (20) \10-3 [99 Consistent for at least 7 days t-ZrO2 250 80 9.25 (250) [80 1.85 (50) \10-4 [99 c-Al2O3 205 150 13.0 (350) [82 2.59 (70) \10-3 [99 Consistent for at least 10 days Consistent for at least 10 days medium flux reactor (u * 1 9 1014 neutrons/cm2/s) for 1 week [33, 36]. Using c-Al2O3, 99Mo/99mTc generators of activity up to 13 GBq (350 mCi) have been reported, which is appreciably high for routine clinical use in hospital radiopharmacies. Using the same sorbent, it should be possible to make column generators of [65 GBq (1.75 Ci) by producing the 99Mo in reactors with [5 9 1014 neutrons/cm2/s as is the case with the Missouri University Research Reactor in USA. The capacity of the generator could be increased to *130 GBq (3.5 Ci) by using a reactor having a neutron flux of 1 9 1015 neutrons/cm2/s such as the Oak Ridge National Laboratory (ORNL) reactor in USA or the SM Reactor at Dmitrovgrad in the Russian Federation. Recently, with a view to realize the scope of developing clinical scale 99Mo/99mTc generator using (n,c)99Mo produced in medium flux reactors, a novel tandem column generator concept was proposed [71]. This approach utilized two generator columns containing mesoporous alumina loaded with 99Mo, connected in series. The same eluent (0.9 % NaCl solution) was used for elution for both the columns. The major advantages of this approach over conventional single column generators are (a) higher elution yield of 99mTc and (b) sharper elution profile resulting in higher RAC of 99mTc eluate. The use of enriched 98Mo would be a positive step because target enrichment of C96 % augments the production yield and the specific activity of 99Mo by a factor of *4. In view of the precious nature of enriched 98Mo, the viability of recovering and reusing the enriched 98Mo from the spent generator columns merits attention. It is pertinent to point out that more than 80 % of Mo could be desorbed from the spent generators prepared from this class of sorbent with 5 M NaOH solution containing H2O2 (15 mL of 5 M NaOH solution ? 1 mL of 30 % H2O2; [33, 36]). One important point to note is that irrespective of the specific activity of 99Mo used, the 99mTc is always NCA and has essentially the same specific activity. The suitability of 99mTcO 4 eluate obtained from these generators in preparation of standard 99mTc labeled formulations were evaluated and the complexation yields of the radiolabeled complexes were estimated to be [98 %, in all the cases. The nanosorbents were stable to radiation and the 99 Mo/99mTc generators demonstrate satisfactory performance for [ 7 days, which is normally the shelf-life of 99 Mo/99mTc generators. An examination of the utility nanomaterial-based sorbent in the development of 99Mo/99mTc generators clearly indicates the impact of nanotechnology. This strategy deserves greater attention not only because a greater range of 99Mo/99mTc generators can be produced, but also for the adaptability to use 99Mo produced by (n,c) route with a wide range of specific activities. The concept of preparing nanomaterial-based 99Mo/99mTc generators is inexpensive, realistic, can be implemented in a very short period of time and capable of producing pharmaceutical grade 99mTc to a reasonable extent. The IAEA [72] data base provides a summary of approximately 251 research reactors currently operate worldwide; of these, approximately 134 have sufficient thermal neutron flux, target volume, and operational capabilities for routine production of (n,c)99Mo. Fifty of these research reactors have thermal neutron flux [1 9 1014 neutrons/cm2/s, and the thermal flux of an additional 85 reactors ranges from 1 9 1012 to 1 9 1014 neutrons/cm2/s. Seventy-eight of the above reactors are already involved in radioisotope production, and these reactors have a good geographic distribution. Many of these reactors could be used for production of (n,c)99Mo. A country that acquires 99Mo production as well as 99 Mo/99mTc generators fabrication capability will be better able to meet its domestic needs and may have a quantity for export. To ensure that all countries are well served, it is advantageous to spread 99Mo/99mTc generators production facilities throughout the world. The more widely the facilities are distributed, the more effectively used will be the 99Mo/99mTc generators because losses from decay will be diminished. 123 750 J Radioanal Nucl Chem (2014) 299:741–757 188 W-sorption capacity compared to alumina. More recently, several sorbents with higher capacity for W such as gel– metal oxide composite, synthetic alumina, polymeric titanium oxychloride and PZC, have been developed and exploited for the preparation of 188W/188Re generators [92–98]. However, the 188W/188Re generators prepared using these sorbents could only be loaded under static conditions, which is not preferred due to the difficulty in handling the long-lived 188W (t1/2 = 69 days), radiation exposure and expected deteriorating performance of the generator when eluted under dynamic conditions. All these generators demonstrated significant breakthrough of 188 W in 188Re and required a secondary column for purification of the eluate. At the intersection of column chromatography technique and nanomaterial-based sorbents lays a thriving world of possibilities which is beneficial to the advancement of existing or pave the way for the exploration and development of new 188W/188Re generators [33]. Undoubtedly, the impetus for the use of nanomaterialbased sorbents has come mainly from their potential to realize high adsorption capacity resulting from the nanoscale dimensions, as a way to circumvent the limited specific activity of 188W. The ability of three nanomaterialbased sorbents including TiP, t-ZrO2 and c-Al2O3 have been comprehensively studied and profusely explored for the development of 188W/188Re generators [33, 38, 42, 43], the results of which are summarized in Table 4. Taking advantage of the new physical properties uniquely associated with nanomaterials, 188W/188Re generators were prepared using 88W produced in high flux reactors (/ * 1015 neutrons/cm2/s). A scrutiny of the results depicted in Table 4 reveals that all these sorbents are suitable for preparation of 188W/188Re generators and can easily be scaled up to 37 GBq (1 Ci) activity level which is more than adequate for routine clinical use. Among all, the cAl2O3 was found to exhibit the highest sorption capacity under dynamic conditions as compared to TiP and t-ZrO2 and is therefore the most appropriate sorbent for the preparation of clinical-scale 188W/188Re generator preparation. The performances of these generators were studied for more than 6 months and reported to be satisfactory. The 188Re elution yields were not only appreciably high but also the level of RN, RC and chemical impurities in 188 Re obtained from all these generators were well within the acceptable limits prescribed in the pharmacopoeias [70]. In order to examine the suitability of 188ReO 4 obtained from the generators for radiolabeling studies, it was complexed with standard ligands such as DMSA and HEDP with [98 % RC purity. Spurred by the perceived need to recycle the precious enriched 186W target for subsequent irradiation, effective W/188Re generator The 188W/188Re generator using an acidic alumina column is the most popular source for availing NCA 188Re suitable for targeted radiotherapy [73]. The widespread interest in use of 188Re in therapeutic nuclear medicine is due to its reasonable half-life (16.9 h), high energy beta radiation (Ebmax = 2.118 MeV), emission of a 155 keV c-ray (15 %) suitable for imaging, potential availability in NCA form from a generator, and chemistry similar to 99mTc which is suitable for preparation of a wide variety of radiopharmaceuticals [74–85]. Despite such favorable attributes, 188Re is not routinely used in therapeutic applications primarily due to the unavailability of cost-effective 188W/188Re generators. Tungsten-188 can only be produced by double neutron capture with low neutron absorption cross-sections 187 [186W(n,c)187W (r = 37.9 ± 0.6b), W(n,c)188W (r = 64 ± 10b)] [86]. Further, due to the long half-life of 188 W (t1/2 = 69 days), relatively long irradiation periods are required even for the production of 188W of modest specific activity [86]. Consequently, 188W from the high flux reactors (/ * 1015 neutrons/cm2/s) such as the High Flux Isotope Reactor in ORNL, USA or SM Reactor in Dmitrovgrad, Russian Federation or BR3 Reactor in Belgium can only be used to make 188W/188Re generators suitable for clinical use. The specific activity of 188W, produced in the high flux reactors, ranges from 150 to 190 GBq/g of W [86]. While therapeutic applications of 188Re in nuclear medicine ‘‘lives’’ at the interface between many disciplines, its dependence on 188W/188Re generator is considered to be one of the strongest. Due to the limited sorption capacity of alumina (*50 mg W/g), 188Re obtained from the alumina based 188W/188Re generators is of low RAC, even while using 188W produced in high flux reactors for preparation of the generators. Before undertaking the preparation of 188Re radiopharmaceuticals, it is considered appropriate to concentrate the generator eluate using suitable PEC technique [14, 17–21]. Often PEC of the 188Re eluate results in a fairly complex system, addition of chemical impurities, high dose rates and low reliability. Recently, automated systems for the concentration of 188Re eluate have also been developed [87]. However, the high cost involved in the operation of the complex automation systems, further increases the production cost of 188Re and renders it cost-ineffective for routine therapeutic use. To overcome these limitations, several alternate sorbents like hydroxyapatite, the hydrous oxides of zirconium, titanium, manganese, tin(IV), and cerium, silica gel, the AG 1-X12 and AG 50 W-X12 ion-exchange resins and activated charcoal have been studied to determine their suitability for the preparation of 188W/188Re generators [88–91]. Unfortunately, none of these materials exhibited an improved 123 J Radioanal Nucl Chem (2014) 299:741–757 Table 4 Summary of Sorbents 751 188 W/188Re generators developed using nanosorbents Sorption capacity (mg W/g) Activity of 188 W loaded GBq (mCi) Elution yield of 188 Re (%) Maximum radioactive concentration of 188Re achieved GBq (mCi)/mL Level of 188W impurity in 188 Re (%) Radiochemical purity of 188 ReO4- (%) Consistency in generator performance reported Static Breakthrough TiP 325 100 1.85 (50) [80 0.37 (10) \10-3 [99 Consistent for at least 4 months NanoZrO2 c-Al2O3 300 120 1.85 (50) [78 0.33 (9) \10-4 [99 512 326 11.1 (300) [80 1.85 (50) \10-3 [99 Consistent for at least 6 months Consistent for at least 6 months method has been developed to recover W from the spent 188 W/188Re generator column [33, 38, 42, 43]. This step is equally important from the perspective of waste management point of view, as the exhausted generator columns cannot be directly discarded without removing the sorbed 188 W from the column. More than 80 % of W could be effectively desorbed from the spent generator columns by passing 5 M NaOH solution containing H2O2 (15 mL of 5 M NaOH solution ? 1 mL of 30 % H2O2) through them [33, 38, 42, 43]. There appears to be enticing interest among the user’s community to consider the use of 188W/188Re generators without the aid of PEC step. In this context, the scope of using nanomaterial-based sorbent not only constitutes an innovative concept but also pave the way for preparing clinical scale 188W/188Re generators using high specific activity (*150 GBq/g) 188W produced in high flux reactors akin to the convention alumina based 99Mo/99mTc generators with fission 99Mo. Rhenium-188 obtained from such 188W/188Re generators can provide the scope for preparation of 188Re-based radiopharmaceuticals without any post-elution purification or PEC requirement. Although the scope for realizing clinical scale 188 W/188Re generators using 188W produced in high flux reactors (1 9 1015 neutrons/cm2/s) without post elution set up constitute as a step in the right direction, devising an effective strategy to prepare generators using 188W obtained from neutron irradiation of enriched (96 %) 186W in medium flux (*1014 neutrons/cm2/s) research reactors is challenging owing to the concerns about lower specific activity of 188W. The specific activity of 188W from irradiation in medium flux reactors (1.5 GBq/g) would be far lesser (*100 folds) than the product from high flux (*1015 neutrons/cm2/s) reactors using same irradiation time. This would have a profound influence on the amount of sorbent required and in turn the size of the column to be used. Therefore, only small-scale generators (of activity *3.7 GBq) suitable for R&D purpose can be prepared using low specific activity 188W and the present generation of nanosorbents. The specific activity of 188W can be augmented by enhancing the irradiation time. Irradiation of enriched (*96 %) 186W targets for a longer time period (*6 months) in medium neutron flux (*1014 neutrons/ cm2/s) reactors would result in the production of 188W of specific activity *6.7 GBq/g [99]. With this specific activity of 188W, it is possible to prepare 11 GBq (300 mCi) 188W/188Re generators using 5–6 g of c-Al2O3, which is quite reasonable for clinical use. Although this strategy is technically challenging compared to the use of high flux reactors research reactors, this alternative option has merits due to the good distribution of medium flux (*1014 neutrons/cm2/s) research reactors throughout the world [72]. It would offer immediate benefits, with the smallest practical hurdles for implementation. While the use of nanomaterial-based sorbent unveiled many possibilities in devising effective strategies to prepare 188W/188Re generators, the principal challenges for widespread pursuit of this novel paradigm dependent upon the production of 188W of appreciable specific activity in medium neutron flux (*1014 neutrons/cm2/s) reactors. This source of 188W is independent of existing supply chains and would provide redundancy as well as emergency backup. Yet, the ultimate implementation of this strategy in a country with operating medium neutron flux research reactors purely relies on organizational preferences rather than technical considerations. It is envisaged that the nanosorbents based 188W/188Re generators (particularly c-Al2O3) will not replace existing commercial alumina column chromatographic generators (along with post processing set-up), and certainly not in the near future, but will find a complementary role in ensuring the availability of 188Re for therapeutic use. 68 Ge/68Ga generators The 68Ge/68Ga generator has emerged as a convenient source to provide 68Ga in a hospital radiopharmacy for PET-based molecular imaging. The introduction of 68Ga 123 752 into clinical practice provided a significant breakthrough in the ongoing developments in functional and metabolic imaging using PET, which is independent of the availability of a cyclotron [100, 101]. Gallium-68 decays by positron emission (89 %) having a maximum energy 1.92 MeV with a short half-life of 68 min, which is compatible with the pharmacokinetics of many peptides and other small molecules [102]. The impressive success of 68 Ga-labeled peptides, namely 68Ga-DOTATOC, 68GaDOTATATE, and 68Ga-DOTANOC, paved the way not only towards clinical acceptance of this particular tracer for PET imaging of neuroendocrine tumors, but to the realization of the great potential of the 68Ge/68Ga generator for modern nuclear medicine in general. Beside DOTAoctreotide derivatives, a large number of 68Ga-labeled molecules have been developed and many others are used in pre-clinical studies [103, 104]. Myocardial perfusion, pulmonal blood flow, bone imaging, or membrane receptor status can be expected to be observable with sophisticated 68 Ga-PET analogues [100–104]. Though the 68Ge/68Ga radionuclide generators have been the object of development and investigation for almost 50 years, their proper and relevant clinical use has started only recently [6, 105, 106], due to lack of proper sorbents, as one of the reasons. The past few years have witnessed unprecedented endeavors by both academia and the industry for developing a wide variety of 68Ge/68Ga using a wide variety of sorbents such as Al2O3, CeO2, SnO2, TiO2, ZrO2 and organic matrices in attempts to obtain ionic 68Ga [105]. The 68Ge/68Ga generator technology has undergone several stages of modification and sophistication and presently, these generators are supplied by three commercial manufacturers, namely, Cyclotron Ltd., Obninsk, Russian Federation, the Eckert and Ziegler Isotope Products (EZIPs) and iThemba, Republic of South Africa [107–110]. For preparation of 68Ge/68Ga generators, the Cyclotron Ltd. uses a modified TiO2 phase [107], the EZIPs uses titanium dioxide (IGG 100 [108]) and iThemba, Republic of South Africa, uses a SnO2 [109, 110]. While the commercial availability of a range of 68Ge/68Ga generators constitute a step in the right direction for providing ionic 68Ga for PET, the low RAC, high acidity, unacceptable 68Ge breakthrough, and the presence of potential metal ion impurities in the generator eluate have emerged as the major deterrents in the path of direct preparation of 68Ga based radiopharmaceuticals [105]. Also, most of the commercially available generators demonstrate deteriorating performance in terms of increased 68Ge breakthrough and reduced 68Ga elution yield on repeated elutions over a prolonged period of time [105]. In order to circumvent these issues, attempts were made to perform post-elution processing of 68Ga eluate from the 68 Ge/68Ga generator and a number of purification strategies 123 J Radioanal Nucl Chem (2014) 299:741–757 using anion and cation exchange chromatography and fractionation have evolved which provide 68Ga in acceptable pH free from metallic impurities as well as 68Ge breakthrough [105, 111–115]. However, these additional manipulations not only make the studies with 68Ga more time consuming which results in decay-loss of this shortlived radioisotope but also quite expensive. It is pertinent to point out that 68Ge/68Ga generator must be accepted not just as a good science but also to ensure that quality of the 68Ga3? ion obtained from the generator is amenable for the routine direct radiolabeling of a gamut of pharmaceutical compounds at high specific activity and RC purity for clinical purposes. While the utilization of automatic post-elution purification as well as concentration systems [116] has tangible benefits, use of 68Ge/68Ga generators which could be directly used for the synthesis of 68 Ga radiotracers in the hospital radiopharmacy is a trustworthy proposition. The basis for the success of 68Ga radiopharmacy lies in the development of 68Ge/68Ga generators systems providing the 68Ga not only in ionic form but also in an acceptable RAC free from 68Ge breakthrough as well as of potential metal ion impurities. Recently, efforts were resorted towards preparation of 68 Ge/68Ga generators using nanomaterial-based sorbents which can be directly used in the hospital radiopharmacy similar to 99Mo/99mTc generators and considerable success has been achieved in this direction [33]. Two nanosorbents, namely, t-ZrO2 and CeO2–PAN have been utilized for preparation of the 68Ge/68Ga generators (each of 740 MBq activity; [33, 35, 39, 40]) and the results are summarized in Table 5. Since, preparation of a single patient-dose of 68Ga-radiopharmaceuticals that are commonly used in nuclear medicine departments requires *74–185 MBq (2–5 mCi) of 68Ga activity, a 740 MBq (20 mCi) generator would be adequate for regular clinical use in a medium-scale hospital radiopharmacy. It can be seen from the table that the sorption capacity of t-ZrO2 is much higher than that of CeO2–PAN. Since 68Ge is available in NCA form, 500 mg of either of these sorbents would be adequate for preparation of 18.5 GBq (500 mCi) 68 Ge/68Ga generator. The performance of both these generators were evaluated for 1 year in terms of 68Ga yield, 68 Ge breakthrough, RAC of the 68Ga solution and suitability of the 68Ga for the preparation of 68Ga-labeled radiotracers. The elution yields of 68Ga from these generators varied between 70 and 85 % and the breakthrough of 68Ge in 68Ga eluate was negligibly low (\10-4 %). The presence of metal ion impurities such as Fe3?, Cu2?, Al3?, Zn2?, Sn2?, Ti4?, Mn2?, etc., in 68Ga eluate was negligibly low (\0.1 ppm) as determined by ICP-AES analysis. Gallium-68 obtained from these generators could directly be utilized for preparation of various radiopharmaceuticals with high radiolabeling yield J Radioanal Nucl Chem (2014) 299:741–757 Table 5 Summary of Sorbents 68 Ge/68Ga generators developed using nanosorbents Elution yield of 68 Ga (%) Maximum radioactive concentration of 68Ga achieved GBq (mCi)/ mL Level of 68 Ge impurity in 68 Ga (%) Level of chemical impurities in 68Ga (%) Breakthrough Activity of Ge loaded GBq (mCi) Consistency in generator performance reported Sorption capacity (mg Ge/g) Static 753 68 CeO2– PAN 40 20 0.74 (20) [70 0.37 (10) \10-5 Ce4?, Fe3?, and Mn2? ions were \1 lg/mL. No other metal ion impurity detected Consistent for at least 1 year t-ZrO2 135 70 0.74 (20) [80 0.44 (12) \10-5 Co2?, Cu2?, Cd2?, Ni2? ions, etc., were \5 ng/mL. No other metal ion impurity detected Consistent for at least 1 year ([98 %) and appreciably high specific activity of the radiolabeled conjugates. The performance of both these generators remained consistent over the period of 1 year. It is anticipated that the realization of this paradigmchanging approach for preparation of 68Ge/68Ga generator would represent a cost-effective proposition for assessing 68 Ga to meet future research and clinical demands. The availability of such reliable and easy-to-handle 68Ge/68Ga generators would facilitate more research on new 68Ga radiopharmaceuticals for PET imaging. The generator systems are amenable for automation which can be successfully used in hospital radiopharmacies. The nanosorbent based generators have got very high potential for use in institutions, where commercial sources of PET radioisotopes are not readily available or are too expensive. Summary and conclusions This article highlights the importance of nanomaterialbased adsorbents in the development of radionuclide generators. Nanomaterial-based adsorbents have proven to be effective in enhancing the performance of the column chromatography radionuclide generators owing to novel adsorption characteristics such as large surface area, reactive surface and high specificity. A review of the research indicates that significant strides have been made in this fascinating field of research. Although this class of adsorbent has been relatively less explored compared to conventional bulk material adsorbents, generators using nanomaterial-based adsorbents is evolving, and essentially represents a story of a technique awaiting technology realization. The advances made so far are exciting and there are no technical barriers for their adoption. With the appropriate selection of suitable adsorbent and parent/ daughter radionuclide pair, it would be possible to envision a future where a wide range of radionuclide generators, commensurate with hospital radiopharmacy can be prepared to address the need of nuclear medicine community. Despite the huge potential of nanomaterial-based adsorbents as chromatographic supports, wide scale availability of the nanomaterial is one of the major challenges which currently obstruct the path for their widespread progress. Establishing cost effective large-scale production method for a constant and reliable supply of this class of adsorbents is an appealing vision for the ongoing efforts to create a foundation as well as advancement. This would not only ensure a sustained growth and future expansion but would also empower future developments. This is the area in which innovation is required and where significant novel developments can be expected to occur in near future. There is no doubt that in the near future, efforts will be focused to unlock the secrets for undertaking sustainable large-scale, cost-effective production of nanomaterialbased adsorbents. As applications of nanomaterial-based adsorbents and use of generator prepared from this class of adsorbent increases, development of automated generators is crucial. Automation offers several advantages, including reducing the radiation exposure to personnel, minimize the probability of human errors, offering consistent generator performance, and provides an elution record. Such automation strategies must be hastened to expand the scope as well to revolutionize radionuclide generators technology. It is clear that nanomaterial-based radionuclide generator is still in its infancy, has to grow more in terms of productivity and utility. We have passed few roadblocks but without doubt the end of the road stretches into the distance and nanomaterial stonemasons are going to be busy for the foreseeable future as there is plenty of room for high level research. With continuous research efforts, we have every reason to believe that huge steps forward will be made during the coming decade. While merging nanomaterial-based adsorbents and column chromatography separation technique unveil innovations in radionuclide generator technology, and made substantial inroads, regulatory approval of generator produced radionuclide as an approved pharmaceutical 123 754 J Radioanal Nucl Chem (2014) 299:741–757 ingredient, would of course, be a prerequisite for clinical use. However, we must not forget that nanomaterial-based radionuclide generator is required to be customized to local legislative, regulatory and institutional conditions. It is the responsibility of all the stakeholders, including scientists, engineers, clinicians, radiopharmacists, hospitals, industries and federal agencies to share a common platform not only to harness the immense potential of nanomaterial-based radionuclide generator but also to create this technology a mainstream for hospital radiopharmacy practice. Acknowledgments Research at the Bhabha Atomic Research Centre is part of the ongoing activities of the Department of Atomic Energy, India and is fully supported by government funding. The authors are grateful to Dr. Gursharan Singh, Associate Director, Radiochemistry and Isotope Group (I), Bhabha Atomic Research Centre for his valuable support to the isotope program. Conflict of interest financial interest. The authors have declared no conflicting References 1. Lambrecht RM (1983) Radionuclide generators. Radiochim Acta 34:9–24 2. Knapp FF Jr, Mirzadeh S (1994) The continuing important role of radionuclide generator systems for nuclear medicine. Eur J Nucl Med 21:1151–1165 3. Mirzadeh S, Knapp FF Jr (1996) Biomedical radioisotope generator systems. J Radioanal Nucl Chem 203:471–488 4. Chakravarty R, Dash A (2013) Development of radionuclide generators for biomedical applications. Lambert Academic Publishing, Saarbrücken 5. Knapp Jr FF, Butler TA (eds) (1984) Radionuclide generators: new systems for nuclear medicine applications. ACS symposium series, vol 241. American Chemical Society 6. Rosch F, Knapp FF Jr (2003) Radionuclide generators. In: Vertes A, Nagy S, Klenscar Z (eds) Radiochemistry and radiopharmaceutical chemistry in life sciences: handbook of nuclear chemistry. Kluwer Academic Publisher, Dordrecht, pp 81–118 7. Jones AG (1995) Technetium in nuclear medicine. Radiochim Acta 71:289–297 8. Richards P, Tucker WD, Srivastava SC (1982) Technetium99m: an historical perspective. Int J Appl Radiat Isot 33:793–799 9. Eckelman WC (2009) Unparalleled contribution of technetium99m to medicine over 5 decades. J Am Coll Cardiol Imaging 2:364–368 10. Banerjee S, Pillai MR, Ramamoorthy N (2001) Evolution of Tc99m in diagnostic radiopharmaceuticals. Semin Nucl Med 31:260–277 11. Dash A, Knapp FF Jr, Pillai MRA (2013) Industrial radionuclide generators: a potential step towards accelerating radiotracer investigations in industry. RSC Adv. doi:10.1039/C3RA41639A 12. Mushtaq A (2004) Inorganic ion-exchangers: their role in chromatographic radionuclide generators for the decade 1993–2002. J Radioanal Nucl Chem 262:797–810 13. Therapeutic radionuclide generators: 90Sr/90Y and 188W/188Re generators (2009). IAEA Technical Report Series No. 470 123 14. Guhlke S, Beets AL, Oetjen K, Mirzadeh S, Biersack HJ, Knapp FF Jr (2000) Simple new method for effective concentration of 188 Re solution from an alumina based 188W–188Re generator. J Nucl Med 41:1271–1278 15. Blower PJ (1993) Extending the life of a Tc-99m generator: a simple and convenient method for concentrating generator eluate for clinical use. Nucl Med Commun 14:995–997 16. Guhlke S, Beets AL, Oetjin K (1998) Convenient concentration of rhenium-188 and technetium-99m eluates from tungsten-188/ rhenium-188 or (n,c)-produced molybdenum-99/technetium99m generators to high specific volumes. J Label Compd Radiopharm 40:294–297 17. Sarkar SK, Venkatesh M, Ramamoorthy N (2009) Evaluation of two methods of concentrating perrhenate (188Re) eluates from 188 W–188Re generator. Appl Radiat Isot 67:234–239 18. Mansur MS, Mushtaq A, Jehangir M (2006) Concentration of 99mTcpertechnate and 188Re-perrhenate. Radiochim Acta 94:107–111 19. Jackel B, Cripps R, Guntay S, Bruchertseifer H (2005) Development of semi-automated system for the preparation of 188Re aqueous solutions of high and reproducible activity concentrations. Appl Radiat Isot 63:299–304 20. Mushtaq A (2004) Concentration of 99mTcO4-/188ReO4- by a single, compact, anion exchange cartridge. Nucl Med Commun 25:957–962 21. Chakravarty R, Dash A, Pillai MRA, Venkatesh M (2010) Postelution concentration of 188Re by an electrochemical method. Appl Radiat Isot 68:2302–2305 22. Chakravarty R, Sarkar SK, Venkatesh M, Pillai MRA (2012) An electrochemical procedure to concentrate 99mTc availed from a zirconium [99Mo] molybdate gel generator. Appl Radiat Isot 70:375–379 23. Chakravarty R (2011) Development of radionuclide generators for biomedical applications. PhD Thesis, Homi Bhabha National Institute, Mumbai. http://www.hbni.ac.in/phdthesis/thesis_aug 2012/CHEM01200804008_RChakravarty.pdf. Accessed 8 Nov 2013 24. Chakravarty R, Shukla R, Tyagi AK, Dash A (2012) In: Ariga K (ed) Manipulation of nanoscale materials: an introduction to nanoarchitectonics. Royal Society of Chemistry, London, pp 259–301 25. Pradeep T (2008) Nano: the essentials understanding nanoscience and nanotechnology, 1st edn. Tata McGraw Hill Publishing Company Limited, New Delhi 26. Poole CP Jr, Owens FJ (2007) Introduction to nanotechnology, 1st edn. Wiley India Pvt. Ltd., New Delhi 27. Feynman RP (1961) There’s plenty of room at the bottom. In: Gilbert HD (ed) Miniaturization. Reinhold Publishing Corporation, New York, pp 282–296 28. Andrievski RA (2003) Modern nanoparticle research in Russia. J Nanopart Res 5:415–418 29. Cao G (2004) Nanostructures & nanomaterials: synthesis, properties & applications, 1st edn. Imperial College Press, London 30. Rao CNR, Thomas PJ, Kulkarni GU (2007) Nanocrystals: synthesis, properties and applications. Springer series on material science, vol 95 31. Rao CNR, Muller A, Cheetham AK (eds) (2004) The chemistry of nanomaterials: synthesis, properties and applications, 1st edn. Wiley-VCH Verlag, Weinheim 32. Rao CNR, Muller A, Cheetham AK (eds) (2007) Nanomaterials chemistry: recent developments and new directions, 1st edn. Wiley-VCH Verlag, Weinheim 33. Chakravarty R, Dash A (2013) Role of nanoporous materials in radiochemical separations for biomedical applications. J Nanosci Nanotechnol 13:2431–2450 34. Chakravarty R, Dash A (2013) Nano structured metal oxides as potential sorbents for 188W/188Re generator: a comparative study. Sep Sci Technol 48:607–616 J Radioanal Nucl Chem (2014) 299:741–757 35. Chakraborty S, Chakravarty R, Dash A, Pillai MRA (2013) The practicality of nanoceria–PAN-based 68Ge/68Ga generator toward preparation of 68Ga-labeled cyclic RGD dimer as a potential PET radiotracer for tumor imaging. Cancer Biother Radiopharm 28:77–83 36. Chakravarty R, Ram R, Dash A, Pillai MRA (2012) Preparation of clinical-scale 99Mo/99mTc column generator using neutron activated low specific activity 99Mo and nanocrystalline c-Al2O3 as column matrix. Nucl Med Biol 39:916–922 37. Chakravarty R, Ram R, Venkatesh M, Dash A (2012) Development of a 68Ge/68Ga generator to avail 68Ga in organic medium for industrial radiotracer applications. Sep Sci Technol 47:1673–1676 38. Chakravarty R, Shukla R, Ram R, Venkatesh M, Tyagi AK, Dash A (2011) Exploitation of nano alumina for the chromatographic separation of clinical grade 188Re from 188W: a renaissance of the 188W/188Re generator technology. Anal Chem 83:6342–6348 39. Chakravarty R, Shukla R, Ram R, Tyagi AK, Dash A, Venkatesh M (2011) Development of nano-zirconia based 68Ge/68Ga generator for biomedical applications. Nucl Med Biol 38:575–583 40. Chakravarty R, Shukla R, Ram R, Venkatesh M, Dash A, Tyagi AK (2010) Nano-ceria–PAN composite based advanced sorbent material: a major step forward in the field of clinical grade 68 Ge/68Ga generator. ACS Appl Mater Interfaces 2:2069–2075 41. Chakravarty R, Shukla R, Ram R, Tyagi AK, Dash A, Venkatesh M (2010) Practicality of tetragonal nano-zirconia as a prospective sorbent in the preparation of 99Mo/99mTc generator for biomedical applications. Chromatographia 72:875–884 42. Chakravarty R, Shukla R, Ram R, Tyagi AK, Dash A, Venkatesh M (2010) Nanocrystalline zirconia: a novel sorbent for the preparation of 188W/188Re generator. Appl Radiat Isot 68:229–238 43. Chakravarty R, Dash A, Venkatesh M (2009) Separation of clinical grade 188Re from 188W using polymer embedded nanocrystalline titania (TiP). Chromatographia 69:1363–1371 44. Chakravarty R, Shukla R, Gandhi S, Ram R, Dash A, Venkatesh M, Tyagi AK (2008) Polymer embedded nanocrystalline titania sorbent for 99Mo–99mTc generator. J Nanosci Nanotechnol 8:4447–4452 45. Chakravarty R, Chakraborty S, Dash A, Pillai MRA (2013) Long-term evaluation of ‘BARC 68Ge/68Ga generator’ based on the nanoceria–polyacrylonitrile composite sorbent. Cancer Biother Radiopharm 28:631–637 46. Osso JA Jr, Catanoso MF, Barrio G, Brambilla TP, Teodoro R, Dias CRBR, Suzuki KN (2012) Technetium-99m—new production and processing strategies to provide adequate levels for SPECT imaging. Curr Radiopharm 5:178–186 47. Boyd RE (1982) Molybdenum-99:technetium-99m generator. Radiochim Acta 30:123–145 48. Boyd RE (1987) Technetium generators: status and prospects. Radiochim Acta 41:59–63 49. Bremer KH, Aktiengesellschaft H (1987) Large scale production and distribution of Tc-99m generators for medical use. Radiochim Acta 41:73–81 50. Molinsky VJ (1982) A review of 99mTc generator technology. Int J Appl Radiat Isot 33:811–819 51. Cecchin D, Zucchetta P, Faggin P, Bolla E, Bui F (2010) 99 Mo/99mTc generator shortage. J Nucl Med 51:14N–15N 52. Gould P (2009) Medical isotope shortage reaches crisis level. Nature 460:312–313 53. Perkins AC, Vivian G (2009) Molybdenum supplies and nuclear medicine services. Nucl Med Commun 30:657–659 54. Webster P (2009) North America’s medical isotope crisis. Lancet 374:103 755 55. Ballinger JR (2010) 99Mo shortage in nuclear medicine: crisis or challenge? J Label Compd Radiopharm 53:167–168 56. Pillai MRA, Knapp FF Jr (2011) Overcoming the 99mTc shortage: are options being overlooked? J Nucl Med 52:15N–28N 57. Gould P (2008) Medical isotopes: time to secure supplies? Lancet Oncol 9:1027 58. Ruth T (2009) Accelerating production of medical isotopes. Nature 457:536–537 59. Pillai MRA, Dash A, Knapp FF Jr (2013) Sustained availability of 99mTc: possible paths forward. J Nucl Med 54:313–323 60. Pillai MRA, Knapp FF Jr (2012) Molybdenum-99 production from reactor irradiation of molybdenum targets: a viable strategy for enhanced availability of technetium-99m. Q J Nucl Med Mol Imaging 56:385–399 61. Dash A, Knapp FF Jr, Pillai MRA (2013) 99Mo/99mTc separation: an assessment of technology options. Nucl Med Biol 40:167–176 62. Hasegawa Y, Nishino M, Takeuchi T, Tatenuma K, Tanase M, Kurosawa K (1987) Mo adsorbent for 99Mo–99mTc generators and manufacturing thereof. US Patent 5,681,974, 28 Oct 1997 63. Lee JS, Han HS, Park UJ, Son J, Shin HY, Hong SB, Jang KD, Lee JS (2010) Adsorbents for radioisotopes, preparation methodology thereof, and radioisotope generators using the same. US Patent 2010/0,248,955 A1, 30 Sep 2010 64. Salehi H, Mollarazi E, Abbasi H, Zoghi M (2008) A new 99mTc generator using cerium(IV) oxide as an adsorbent for 99Mo. J Phys Theor Chem 4:245–249 65. Mushtaq A, Mansoor MS, Karim HMA, Khan MA (1991) Hydrated titanium dioxide as an adsorbent for 99Mo/99mTc generator. J Radioanal Nucl Chem 147:257–261 66. Serrano J, Bertin V, Bulbulian S (2000) 99Mo sorption by thermally treated hydrotalcites. Langmuir 16:3355–3360 67. Qazi QM, Mushtaq A (2011) Preparation and evaluation of hydrous titanium oxide as a high affinity adsorbent for molybdenum (99Mo) and its potential for use in 99mTc generators. Radiochim Acta 99:231–235 68. Gómez JS, Correa FG (2002) 99mTc generator with hydrated MnO2 as adsorbent of 99Mo. J Radioanal Nucl Chem 254:625–628 69. Tanase M, Tatenuma K, Ishikawa K, Kurosawa K, Nishino M, Hasegawa Y (1997) A 99mTc generator using a new inorganic polymer adsorbent for (n,c)99Mo. Appl Radiat Isot 48:607–611 70. British Pharmacopoeia Commission (2008) British pharmacopoeia. The Stationery Office, Norwich. www.pharmacopoeia. org.uk. Accessed 8 Nov 2013 71. Chakravarty R, Ram R, Mishra R, Sen D, Mazumder S, Dash A, Pillai MRA (2013) Mesoporous alumina (MA) based double column approach for development of clinical scale 99Mo/99mTc generator using (n,c)99Mo: an enticing application of nanomaterial. Ind Eng Chem Res 52:11673–11684 72. International Atomic Energy Agency. Operation research reactors in the world [database]. www.naweb.iaea.org/napc/physics/research_ reactors/database/RR%20Data%20Base/datasets/foreword_home. html. Accessed 20 July 2013 73. Knapp FF Jr (1998) Rhenium-188—a generator-derived radioisotope for cancer therapy. Cancer Biother Radiopharm 13:337–349 74. Jeong JM, Knapp FF Jr (2008) Use of the Oak Ridge National Laboratory Tungsten-188/Rhenium-188 generator for preparation of the Rhenium-188 HDD/Lipiodol complex for transarterial liver cancer therapy. Semin Nucl Med 38:S19–S29 75. Abram U, Alberto R (2006) Technetium and rhenium—coordination chemistry and nuclear medical applications. J Braz Chem Soc 17:1486–1500 76. Savio E, Gaudiano J, Robles AM, Balter H, Paolino A, López A, Hermida JC, De Marco E, Martinez G, Osinaga E, Knapp FF Jr 123 756 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 91. 92. 93. J Radioanal Nucl Chem (2014) 299:741–757 (2001) Rhenium-188 HEDP: pharmacokinetic characterization, clinical and dosimetric evaluation in osseous metastatic patients with two levels of radiopharmaceutical dose. BMC Nucl Med 1:2–10 Blower PJ, Kettle AG, O’Doherty MJ, Coakley AJ, Knapp FF Jr 99m Tc(V)-DMSA quantitatively predicts (2000) 188 Re(V) DMSA distribution in patients with prostate cancer metastatic to bone. Eur J Nucl Med 9:1405–1409 Liepe K, Hlises R, Kropp J, Gruning T, Runge R, Koch R, Knapp FF Jr, Franke WG (2000) Rhenium-188-HEDP in palliative treatment of bone metastases. Cancer Biother Radiopharm 15:261–265 Palmedo H, Guhlke S, Bender H, Sartor J, Schoeneich G, Grunwald F, Knapp FF, Biersack HJ (2000) Dose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastases. Eur J Nucl Med 27:123–130 Knapp FF Jr, Spencer R, Kropp J (2001) Intravascular radiation therapy with radioactive filled balloons for inhibition of restenosis after angioplasty: a new opportunity for nuclear medicine. J Nucl Med 42:1384–1387 Weinberger J, Giedd KN, Simon AD, Marboe C, Knapp FF Jr, Trichter F, Amols H (1999) Radioactive beta-emitting solution filled balloon treatment prevents porcine coronary restenosis. Cardiovasc Radiat Med 1:252–256 Tzanopoulou S, Sagnou M, Paravatou-Petsotas M, Gourni E, Loudos G, Xanthopoulos S, Lafkas D, Pelecanou M (2010) Evaluation of Re and 99mTc complexes of 2-(40 -aminophenyl) benzothiazole as potential breast cancer radiopharmaceuticals. J Med Chem 53:4633–4641 Chen Y, Xiong QF, Yang XQ, He L, Huang ZW (2010) Evaluation of 188Re–DTPA–deoxyglucose as a potential cancer radiopharmaceutical. Am J Roentgenol 194:761–765 Hafeli UO, Warburton MC, Landau U (1998) Electrodeposition of radioactive rhenium onto stents to prevent restenosis. Biomaterials 19:925–933 Pillai MRA, Dash A, Knapp FF Jr (2012) Rhenium-188: availability from the 188W/188Re generator and status of current applications. Curr Radiopharm 5:228–243 Knapp FF Jr, Callahan AP, Beets AL, Mirzadeh S (1994) Processing of reactor-produced 188W for fabrication of clinical scale alumina based 188W/188Re generators. Appl Radiat Isot 45:1123–1128 Luo TY, Lo AR, Hsieh BT, Lin WJ (2007) A design for automatic preparation of highly concentrated (Re-188)-perrhenate solutions. Appl Radiat Isot 65:21–25 Lewis RE (1996) Production of 70-day Tungsten-188 and development of a 17 hour Rhenium-188 radioisotope generator. J Nucl Med 7:804–805 Hayes RL (1966) Rhenium-188 as a possible diagnostic agent. Oak Ridge Associated Universities. Medical Division Report ORAU 101, pp 74–77 Monroy-Guzman F, Badillo-Almaraz VE, Flores De La Torre JA, Cosgrove J, Knapp FF (2007) Hydroxyapatite-based Mo-99/ Tc-99m and W-188/Re-188 generator systems. In: Proceeding of the international symposium on trends in radiopharmaceuticals, ISTR-2005, vol 1. IAEA, Vienna, pp 333–348 Knapp FF, Mirzadeh S (1992) Reactor capabilities for production of Tungsten-188 for the Tungsten-188/Rhenium-188 generator system. Oak Ridge National Laboratories, Report ORNL/ TM-12222, pp 6–9 So LV, Nguyen CD, Pellegrini P, Bui VC (2009) Polymeric titanium oxychloride sorbent for 188W/188Re nuclide pair separation. Sep Sci Technol 44:1074–1098 Iller E, Polkowska-Motrenko H, Wawszczak D, Konior M, Milczarek J (2007) Synthesis and testing of a gel metal oxide 123 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. 107. 108. 109. 110. 111. composites as filling materials for 188W–188Re generator column. Annual Report, Radioisotope Centre, POLATOM, vol 4, p 102 Iller E, Deptula A, Brykala M, Sypula M, Konior M (2007) Preliminary results of synthesis and investigations of new materials for packing of chromatographic columns of W-188/ Re-188 generators. Eur J Nucl Med Mol Imaging 34:S210 Monroy-Guzman F, Almaraz VEB, Gutierrez TR, Cohen LG, Cosgrove J, Knapp Jr FF, Nava PR, Rosales CJ (2009) Development of inorganic adsorbents as matrices of generators for therapeutic radionuclides. In: Therapeutic radionuclide generators: 90Sr/90Y and 188W/188Re generators. IAEA-TRS-470 Matsuoka H, Hasimoto K, Hishinuma Y, Ishikawa K, Terunuma H, Tatenuma K (2005) Application of PZC to 188W/188Re generator. J Nucl Radiochem Sci 6:189–191 Iller E, Polkowska-Motrenko H, Lada W, Wawszczak D, Sypula M, Doner K, Konior M, Milczarek J, Zoladek J, Ralis J (2009) Studies of gel metal–oxide composite samples as filling materials for W-188/Re-188 generator column. J Radioanal Nucl Chem 281:83–86 Lee JS, Lee JS, Park UJ, Son KJ, Han HS (2009) Development of a high performance 188W/188Re generator by using a synthetic alumina. Appl Radiat Isot 67:1162–1166 Dadachova MS, So LV, Lambrecht RM, Dadachova E (2002) Development of a titanium tungstate-based 188W/188Re gel generator using tungsten of natural isotopic abundance. Appl Radiat Isot 57:641–664 Rosch F (2013) 68Ge/68Ga Generators: past, present, and future. Recent Results Cancer Res 194:3–16 Rosch F (2013) Past, present, and future of 68Ge/68Ga generators. Appl Radiat Isot 76:24–30 Breeman WAP, Verbruggen AM (2007) The 68Ge/68Ga generator has high potential, but when can we use 68Ga-labelled tracers in clinical routine? Eur J Nucl Med Mol Imaging 34:978–981 Smith DL, Breeman WAP, Sims-Mourtada J (2013) The untapped potential of Gallium 68-PET: the next wave of 68Gaagents. Appl Radiat Isot 76:14–23 Banerjee SR, Pomper MG (2013) Clinical applications of Gallium-68. Appl Radiat Isot 76:2–13 Roesch F, Riss PJ (2010) The renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Curr Top Med Chem 10:1633–1668 Decristoforo C (2012) Gallium-68—a new opportunity for PET available from a long shelflife generator—automation and applications. Curr Radiopharm 5:212–220 Razbash AA, Sevastianov YG, Krasnov NN, Leonov AI, Pavlekhin VE (2005) Germanium-68 row of products. In: Proceedings of the 5th international conference on isotopes 5ICI, Brussels, Belgium, 25–29 April 2005. Medimond, Bologna, pp 147–151 IGG100 68Ge/68Ga generator product information Eckert and Ziegler AG, Berlin. http://www.radiustech.it/public/files/ 1d000044.pdf. Accessed 8 Nov 2013 Aardaneh K, van der Walt TN (2006) Ga2O for target, solvent extraction for radiochemical separation and SnO2 for the preparation of a 68Ge/68Ga generator. J Radioanal Nucl Chem 268:25–32 de Blois E, Sze Chan H, Naidoo C, Prince D, Krenning EP, Breeman WA (2011) Characteristics of SnO2-based 68Ge/68Ga generator and aspects of radiolabelling DOTA-peptides. Appl Radiat Isot 69:308–315 Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA (2007) Processing of generator-produced 68Ga for medical application. J Nucl Med 48:1741–1748 J Radioanal Nucl Chem (2014) 299:741–757 112. Mueller D, Klette I, Baum RP, Gottschaldt M, Schultz MK, Breeman WAP (2012) Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem 23:1712–1717 113. Loktionova NS, Belozub AN, Filosofov DV, Zhernosekov KP, Wagner T, Turler A, Rosch F (2011) Improved column-based radiochemical processing of the generator produced 68Ga. Appl Radiat Isot 69:942–946 757 114. Rösch F (2013) Post-processing via cation exchange cartridges: versatile options. Recent Results Cancer Res 194:33–42 115. Mueller D, Klette I, Baum RP (2013) Purification and labeling strategies for 68Ga from 68Ge/68Ga generator eluate. Recent Results Cancer Res 194:77–87 116. Boschi S, Lodi F, Malizia C, Cicoria G, Marengo M (2013) Automation synthesis modules review. Appl Radiat Isot 76:38–45 123