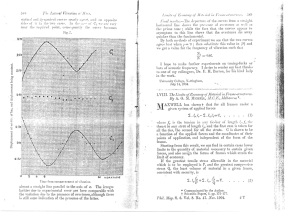
(Clark) Cannabinoids for pain management (Revista++++)
Anuncio

Pain10(SA)_Cov_spread.qxd 8/30/2005 4:22 PM Page 1 Volume 10 Supplement A • Autumn 2005 Pain Research &Management Cannabinoids for Pain Management Guest Editor: Dr Alexander J Clark Publication of this supplement sponsored by an unrestricted educational grant from Valeant The Journal of the Canadian Pain Society Journal de la société canadienne pour le traitement de la douleur Pulsus Group Inc, 2902 South Sheridan Way, Oakville, Ontario, Canada L6J 7L6 Telephone 905-829-4770, fax 905-829-4799, pulsus@pulsus.com, www.pulsus.com PM 40062595 p u l s u s . c o m p u l s u s . c o m MH10(SA).qxd 8/30/2005 10:39 AM Page 1 EDITOR-IN-CHIEF PUBLISHER’S OFFICE Harold Merskey (London) ASSOCIATE EDITORS Anaesthesia Cancer Pain Dentistry Headache Health Care Studies Infant Pain Medical Biophysics Neurology Neuroscience Neurosurgery Nursing Palliative Care Pediatric Pain Pharmacology Physical Medicine & Rehabilitation Psychiatry Psychology Rheumatology RH Catchlove (Montreal) A John Clark (Halifax) Allen Finley (Halifax) Patricia Morley-Foster (London) Ellen N Thompson (Ottawa) Dwight Moulin (London) Ralph Brooke (London) Miriam Grushka (Toronto) Barry Sessle (Toronto) Marek J Gawel (Scarborough) Patricia McGrath (London) Celeste Johnston (Montreal) Alex W Thomas (London) C Peter N Watson (Toronto) Fernando Cervero (Montreal) Terrence Coderre (Montreal) James Henry (Montreal) Michael Salter (Toronto) Ronald Tasker (Toronto) Mary Ellen Jeans (Ottawa) Judith Watt-Watson (Toronto) Bruno Gagnon (Montreal) Patrick McGrath (Halifax) Jana Sawynok (Halifax) Robert Teasell (London) Harvey Moldofsky (Toronto) Eldon R Tunks (Hamilton) Kenneth D Craig (Vancouver) Joel Katz (Toronto) Ronald Melzack (Montreal) Gary B Rollman (London) Manfred Harth (London) INTERNATIONAL CONSULTANTS Frank Adams (USA) Allan I Basbaum (USA) Jean-Marie Besson (France) Nikolai Bogduk (Australia) Michael J Cousins (Australia) Torbjørn Fredriksen (Norway) Bob Large (New Zealand) Ulf Lindblom (Sweden) George Mendelson (Australia) Frederick Wolfe (USA) David Niv (Israel) Bogdan Radanov (Zurich) I Jon Russell (USA) Yair Sharav (Israel) Ottar Sjaastad (Norway) Bengt Sjölund (Sweden) Lars Jacob Stovner (Norway) Dennis Turk (USA) William Willis (USA) CANADIAN PAIN SOCIETY OFFICERS President Gary Rollman Department of Psychology, The University of Western Ontario, London, Ontario President-Elect Roman Jovey (2003-2005) Alcohol & Drug Treatment Program, Credit Valley Hospital, Mississauga, Ontario Past-President Celeste Johnston (2003-2005) School of Nursing, McGill University, Montreal, Quebec Secretary Allen Finley (2002-2005) Anesthesia & Psychology, Dalhousie University, Halifax, Nova Scotia Treasurer Brian Knight (2001-2004) University of Alberta, Edmonton, Alberta Coordinator Ellen Maracle-Benton Publisher Vice-President Robert B Kalina Ann LeBlanc EDITORIAL Managing Editor Sara Miller Editorial Project Manager Associate Editor Assistant Editors Production Coordinator Editorial Coordinators Production Artist Cathy Dusome Jill Toffoli Ben Carino, Will Lakusta Lynne Mumford Donna Kennedy, Jen Parkes Alex Haren SALES Director of Advertising Lisa Robb (905-829-4770 ext 143) Director of Projects Jadzia Ronald (514-945-2170) Account Representative Kathleen Pratt (905-829-4770 ext 125) ADMINISTRATION Director of Administration Accounts Receivable Administrative Assistant Andrea Holter Ursula Noriega Lauren Crompton IT DEPARTMENT IT Manager Systems Administrator Web Developer Mary Shanahan Deval Parikh Ali Kalbali OFFICES Pulsus Group Inc 2902 South Sheridan Way Oakville, Ontario, Canada L6J 7L6 Telephone 905-829-4770 Fax 905-829-4799 pulsus@pulsus.com www.pulsus.com Instructions to Authors are published regularly in the Journal. They are also available on-line at www.pulsus.com (2003-2005) SEND MANUSCRIPTS TO Dr Harold Merskey Editor-in-Chief, Pain Research & Management 71 Logan Avenue London, Ontario N5Y 2P9 Telephone: 519-679-1045 Fax: 519-679-6849 E-mail harold.merskey@sympatico.ca (2004-2005) CANADIAN PAIN SOCIETY CENTRAL OFFICE 701 Rossland Road East, Suite 373, Whitby, ON L1N 9K3 Telephone 905-668-9545, fax 905-668-3728 Web site www.canadianpainsociety.ca E-mail ellen.maracle-benton@sympatico.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 Publications Mail Agreement 40062595. Return Undeliverable Canadian Addresses to: Pulsus Group Inc, 2902 South Sheridan Way, Oakville, Ontario L6J 7L6 1A MH10(SA).qxd 8/30/2005 10:39 AM Page 2 GENERAL INFORMATION Pain Research & Management – the official journal of the Canadian Pain Society – is published four times a year by Pulsus Group Inc and is printed on recycled paper in Canada. Circulation: 15,500. © 2005 Pain Research & Management. All rights reserved. This Journal is wholly owned by the publisher, Pulsus Group Inc, and the contents may not be reproduced without the consent of the publisher. All editorial matter published in Pain Research & Management represents the opinions of the authors and not necessarily those of the Publisher or the Canadian Pain Society. Statements and opinions expressed in Pain Research & Management do not represent the official policy of the Canadian Pain Society unless so stated. No responsibility is assumed by the Canadian Pain Society or the Publisher for any injury and/or damage to persons or property arising from any errors or omission or from the use of any information or advice contained in Pain Research & Management including articles, editorials, studies, reports, letters and advertisements. Discussions, views and recommendations as to medical procedures, choice of drugs and drug dosages are the responsibility of the authors. All drug advertisements have been cleared by the Pharmaceutical Advertising Advisory Board; however, inclusion in Pain Research & Management does not constitute a guarantee or endorsement by the Canadian Pain Society or the Publisher of the quality or value of products or of claims made of them by their manufacturers. Subscriptions/Changes of address Please direct orders for subscriptions, single orders and back issues, changes of address and claims for missing issues to: Pulsus Group Inc, 2902 South Sheridan Way, Oakville, Ontario, Canada L6J 7L6, fax 905-829-4799, e-mail pulsus@pulsus.com Reprints Requests for single reprints should be directed to individual authors. Inquiries regarding multiple reprints (100 or more) should be made to the Publisher. Instructions to Authors Manuscripts should be submitted in triplicate to the Editor-in-Chief. Full Instructions to Authors are available from the Publisher and are published regularly in the Journal. Display/Classified advertising For information regarding advertising, please contact the Media Representative at Pulsus Group Inc. Please send announcements for classified advertising at least two months before the desired publication date. Claims Pulsus Group Inc will honour claims for missing issues within six months of the issue date. Claims submitted after this period will be subject to the full issue price plus shipping and handling, and applicable taxes (subject to availability). Payment is required prior to shipment. ISSN 1203-6765 Date of issue: September 2005 Canadian publications mail product sales agreement number 40062595 Postage paid at Winnipeg, Manitoba Indexed by Index Medicus/MEDLINE, EMBASE/Excerpta Medica, PsycINFO Abstracts Abstracts of articles published in the Journal are available online at www.pulsus.com Mail subscription form and payment to: Pulsus Group Inc, 2902 South Sheridan Way, Oakville, Ontario, Canada L6J 7L6 (telephone 905-829-4770, fax 905-829-4799, e-mail pulsus@pulsus.com) or subscribe online at www.pulsus.com K Canada – CDN$95 (personal); CDN$125 (institutional) (includes 7% GST – #R100761253) Pain Research & Management is a ‘Canadian Periodical’ as defined by section 19 of the Income Tax act. The deduction of advertising costs for advertising in this periodical is therefore not restricted. Please enter my subscription for 2005 (four issues plus all supplements) Name ____________________________________________ Address ____________________________________________ K US subscriptions – US$95 (personal); US$125 (institutional) ____________________________________________ K Other countries – US$120 (personal); US$160 (institutional) ____________________________________________ K Cheque enclosed (made payable to Pulsus Group Inc) OR ____________________________________________ Please charge to: K Mastercard ____________________________________________ K Visa K AMEX E-mail ____________________________________________ Signature ____________________________________________ Card No: _____________________________________________ Expiry Date: __________________________________________ 2A Pain Res Manage Vol 10 Suppl A Autumn 2005 TOC_10(SA).qxd 8/30/2005 10:39 AM Page 3 Autumn 2005 Volume 10 Supplement A EDITORIAL Cannabinoids for pain management: What is their role? 5A Alexander J Clark, Mary E Lynch REVIEWS Preclinical science regarding cannabinoids as analgesics: An overview 7A ME Lynch Modern pharmacology of cannabinoids began in 1964 with the isolation and partial synthesis of delta-9tetrahydrocannabinol, the main psychoactive agent in herbal cannabis. Since then, potent antinociceptive and antihyperalgesic effects of cannabinoid agonists in animal models of acute and chronic pain; the presence of cannabinoid receptors in pain-processing areas of the brain, spinal cord and periphery; and evidence supporting endogenous modulation of pain systems by cannabinoids has provided support that cannabinoids exhibit significant potential as analgesics. This article presents an overview of the preclinical science. Pharmacokinetics of cannabinoids 15A Iain J McGilveray Delta-9-tetrahydrocannabinol (∆-9-THC) is the main psychoactive ingredient in cannabis (marijuana). This review focuses on the pharmacokinetics of THC, but also includes known information for cannabinol and cannabidiol, as well as the synthetic marketed cannabinoids, dronabinol (synthetic THC) and nabilone. Toxic effects of cannabis and cannabinoids: Animal data 23A Pierre Beaulieu This article reviews the main toxic effects of cannabis and cannabinoids in animals. Toxic effects can be separated into acute and chronic classifications. Animal toxicity data, however, are difficult to extrapolate to humans, and one should be very careful when interpreting the results and appying them to humans. The best approach to human toxicology rests on the study of human data. Cannabinoids for the treatment of pain: An update on recent clinical trials 27A Mark Ware, Pierre Beaulieu Over the past five years, there has been a considerable increase in clinical research on cannabinoid use for a range of pain syndromes. Cannabinoid products are becoming available for research and clinical use, and pharmaceutical industry interest in the potential for cannabinoids in therapeutics is also gaining momentum. This article summarizes recent clinical trial data in the field of pain management and suggests that the potential for cannabinoid therapy for chronic pain states is encouraging. Continued on page 4A Pain Res Manage Vol 10 Suppl A Autumn 2005 3A TOC_10(SA).qxd 8/30/2005 10:39 AM Page 4 Autumn 2005 Volume 10 Supplement A REVIEWS – CONTINUED Safety issues concerning the medical use of cannabis and cannabinoids 31A Mark A Ware, Vivianne L Tawfik Safety issues are a major barrier to the use of cannabis and cannabinoid medications for clinical purposes. In this article, the evidence behind major safety issues related to cannabis use is summarized, with the aim of promoting informed dialogue between physicians and patients in whom cannabinoid therapy is being considered. Caution is advised in interpreting these data, because clinical experience with cannabinoid use is in the early stages. Addiction and pain medicine 38A Douglas Gourlay The adequate cotreatment of chronic pain and addiction disorders is a complex and challenging problem for health care professionals. There is great potential for cannabinoids in the treatment of pain; however, the increasing prevalence of recreational cannabis use has led to a considerable increase in the number of people seeking treatment for cannabis use disorders. Guidelines for the use of cannabinoid compounds in chronic pain 44A AJ Clark, ME Lynch, M Ware, P Beaulieu, IJ McGilveray, D Gourlay The objective of this paper was to provide clinicians with guidelines for the use of cannabinoid compounds in the treatment of chronic pain. Publications indexed from 1990 to 2005 in the National Library of Medicine Index Medicus were searched through PubMed. A consensus concerning these guidelines was achieved by the authors through review and discussion. A practical approach to the treatment of chronic pain with cannabinoid compounds is presented. DEPARTMENTS Instructions to Authors 4A 47A Pain Res Manage Vol 10 Suppl A Autumn 2005 clark_ed_8900.qxd 8/30/2005 10:57 AM Page 5 EDITORIAL Cannabinoids for pain management: What is their role? Alexander J Clark MD FRCPC1, Mary E Lynch MD FRCPC2 n the popular press, many Canadians claim to be using herbal cannabis (marijuana) to control symptoms such as pain, nausea, muscle spasm, anorexia and anxiety that may be associated with diseases like AIDS/HIV, multiple sclerosis, epilepsy and chronic pain. Surveys of patients with chronic pain, multiple sclerosis and epilepsy suggest that approximately 10% to 12% of these patients are active cannabis users, and up to 36% have tried using herbal cannabis or its derivatives to control symptoms they experience with these diseases (1-3). On the other hand, the prescription cannabinoids available have, to date, only limited use in the management of pain. Neither nabilone (Cesamet, Valeant Canada limitée/Limited) nor dronabinol (Marinol, Solvay Pharma Inc, Canada) have pain as an indication according to the Canadian Compendium of Pharmaceuticals and Specialties (4), while Sativex (GW Pharma Ltd, United Kingdom) is only indicated for neuropathic pain in multiple sclerosis. Although there is a large and growing preclinical literature regarding potential therapeutic uses of cannabinoids, there are few studies on the clinical efficacy of natural and synthetic cannabinoids as analgesics. Previous available studies have involved only small numbers of subjects and were often of limited quality (5). An overview of the rapidly developing basic science relevant to potential analgesic actions of cannabinoids (6), which includes a review of the endogenous cannabinoid system; evidence of antinociceptive, antihyperalgesic and anti-inflammatory actions; and a review of the pharmacokinetics of cannabinoids (7) is presented in this special issue. Reviews of the current literature assist in understanding the possible toxic effects (8), safety (9) and risks of addiction (10) of cannabinoids. As with all pharmacologically active agents, currently available cannabinoids are associated with adverse effects, and it is important to be aware of these effects as clinicians begin to consider these agents in the treatment of pain. Examples include effects on drowsiness, attention and cognition, the possibility of exaggerating existing psychoses or provoking others long-term, postural hypotension and tachycardia. I Further understanding the roles of the cannabinoid receptors (CB1 and CB2) and the development of new cannabinoid receptor agonists will allow us to maximize the analgesic efficacy and minimize the side effects caused by the cannabinoids. Development of novel agonists, which do not cross the bloodbrain barrier, alternative methods of delivery (ie, transdermal, buccal, rectal, etc), and further elaboration of ways to manipulate the endogenous cannabinoid system are all exciting areas of research that will lead to compounds exhibiting greater efficacy, and potentially fewer adverse effects. However, it will likely be several years before such compounds will be clinically available and, right now, we need to be familiar with the currently available compounds. The purpose of the present supplement to Pain Research & Management is to provide Canadian clinicians and researchers with comprehensive reviews of the current basic and clinical science regarding cannabinoids, along with up-to-date reviews of the literature relevant to safety, toxicity and the risk of addiction related to cannabinoids. The authors of the present supplement have developed an algorithm for use by clinicians in the assessment and treatment of chronic pain when cannabinoid compounds are considered. In addition, guidelines for the use of the two oral cannabinoid compounds that are currently available by prescription in Canada are provided. It is not possible to make recommendations about the buccal administered cannabinoid compound because information is limited. The use of smoked herbal cannabis should be discouraged; there is significant controversy regarding this route of delivery. There is no question that smoking is toxic, but can patients limit their risk by minimizing the dose? Do the benefits outweigh the risks in some cases? The answers to these questions await further study and, until inexpensive, safer and efficient methods of delivery are available, it is probable that some patients with pain and other symptoms will seek relief by smoking cannabis. Others may receive existing preparations for off-label use. It is the goal of the present supplement to assist clinicians, researchers and policy makers with these issues. 1Chronic Pain Centre, Calgary Health Region, and Department of Anesthesia, University of Calgary, Calgary, Alberta; 2Pain Management Unit, Queen Elizabeth II Health Sciences Centre, and Departments of Psychiatry and Anesthesia, Dalhousie University, Halifax, Nova Scotia Correspondence and reprints: Dr Alexander J Clark, Chronic Pain Centre, Calgary Health Region, 160–2210 2nd Street SW, Calgary, Alberta T2S 3C3. Telephone 403-943-9900, fax 403-209-2955, e-mail john.clark@calgaryhealthregion.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 ©2005 Pulsus Group Inc. All rights reserved 5A clark_ed_8900.qxd 8/30/2005 10:57 AM Page 6 Editorial DECLARATION OF INTEREST: This series of articles has been supported by an unrestricted grant from Valeant Canada limitée/Limited. The grant enabled a symposium to be held in Montreal, Quebec on June 4, 2004 and facilitated preparation of the manuscripts. The Editor-in-Chief of the Journal, Dr Harold Merskey, participated in the symposium. The participants and authors wish to acknowledge the support provided. REFERENCES 1. Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain 2003;102:211-6. 2. Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004;62:2098-100. 3. Gross DW, Hamm J, Ashworth NL, Quigley D. Marijuana use and epilepsy: Prevalence in patients of a tertiary care epilepsy center. Neurology 2004;62:2095-7. 6A 4. Compendium of Pharmaceuticals and Specialties. Ottawa: Canadian Pharmacists Association, 2005. 5. Ware M, Beaulieu P. Cannabinoids for the treatment of pain: An update on recent clinical trials. Pain Res Manage 2005;10(Suppl A):27A-30A. 6. Lynch ME. Preclinical science regarding cannabinoids as analgesics: An overview. Pain Res Manage 2005;10(Suppl A):7A-14A. 7. McGilveray IJ. Pharmacokinetics of the cannabinoids. Pain Res Manage 2005;10(Suppl A):15A-22A. 8. Beaulieu P. Toxic effects of cannabis and cannabinoids: Animal data. Pain Res Manage 2005;10(Suppl A):23A-26A. 9. Ware MA, Tawfik VL. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manage 2005;10(Suppl A):31A-37A. 10. Gourlay D. Addiction and pain medicine. Pain Res Manage 2005;10(Suppl A):38A-43A Pain Res Manage Vol 10 Suppl A Autumn 2005 lynch_8901.qxd 8/30/2005 4:23 PM Page 7 REVIEW Preclinical science regarding cannabinoids as analgesics: An overview ME Lynch MD FRCPC ME Lynch. Preclinical science regarding cannabinoids as analgesics: An overview. Pain Res Manage 2005;10(Suppl A): 7A-14A. Survol des données précliniques sur les propriétés analgésiques des cannabinoïdes Modern pharmacology of cannabinoids began in 1964 with the isolation and partial synthesis of delta-9-tetrahydrocannabinol, the main psychoactive agent in herbal cannabis. Since then, potent antinociceptive and antihyperalgesic effects of cannabinoid agonists in animal models of acute and chronic pain; the presence of cannabinoid receptors in pain-processing areas of the brain, spinal cord and periphery; and evidence supporting endogenous modulation of pain systems by cannabinoids has provided support that cannabinoids exhibit significant potential as analgesics. The present article presents an overview of the preclinical science. C’est en 1964 qu’est née la pharmacologie moderne des cannabinoïdes, quand on a isolé et partiellement synthétisé le delta-9-tétrahydrocannabinol, principal agent psychoactif de la plante appelée cannabis. Depuis lors, les puissants effets antinociceptifs et antihyperalgésiques des agonistes des cannabinoïdes dans des modèles animaux de douleur aiguë et chronique, la découverte de récepteurs des cannabinoïdes dans les zones du cerveau, de la moelle épinière et du système nerveux périphérique responsables de la perception de la douleur et les preuves à l’appui d’une modulation endogène des stimuli douloureux par les cannabinoïdes confirment leur important potentiel analgésique. Le présent article propose une vue d’ensemble des données précliniques. Key Words: Cannabinoid opioid interactions; Cannabinoid receptors; Cannabinoids; Chronic pain; Endocannabinoids erbal cannabis has been used for centuries for medicinal and recreational purposes, but it has only been in the past 40 years that scientists have been able to elucidate the molecular basis of cannabinoid action. Modern pharmacology of cannabinoids began in 1964 when the major psychoactive constituent of cannabis, delta-9tetrahydrocannabinol (∆-9-THC), was isolated in pure form, its structure elucidated and later synthesized (1). Since then, the endogenous cannabinoid (endocannabinoid) system has been described, stimulating the development of a range of novel cannabinoid receptor agonists and antagonists (2). These developments have attracted renewed interest in the cannabinoids as potential therapeutic agents. In less than five years there have been over 1500 citations on MEDLINE regarding cannabinoids, and the rate of publications in the field is growing rapidly (Figure 1). Areas of inquiry include the potential role of cannabinoids in pain, antiemesis, appetite modulation, antispasticity, neuroprotection, anti-inflammatory action, tumour suppression, antioxidant activity, immune modulation, glaucoma, sexual dysfunction and addiction control. The present paper will focus on the preclinical literature regarding cannabinoids and pain. There are already a number of excellent reviews on this topic (2-7), and the current article will present an overview of the field. H ENDOCANNABINOID SYSTEM The first crucial step in elucidating the molecular basis of cannabinoid action was achieved in 1988. At that time, a radiolabelled potent synthetic cannabinoid was found to bind to brain membranes in a highly specific and selective manner, exhibiting features that were characteristic of receptor binding (8). Within a short time, the cannabinoid receptor (now called CB1) was discovered and cloned from rat and human brain (9). Three years later, a second cannabinoid receptor (CB2) was discovered and cloned (10). Since then, researchers around the world have mapped the location of CB1 and CB2 receptors, identified a probable third receptor, and described additional endocannabinoids as well as mechanisms and sites of action. Distribution of cannabinoid receptors CB1 receptors are found in particularly high concentrations within the central nervous system; indeed, CB1 receptors are 10 times more abundant than mu opioid receptors in the brain (11). CB1 receptors are also present in peripheral neurons and in non-neuronal tissues. The distribution of cannabinoid receptors has been examined by several methods (12-14). High levels of CB1 receptors have been found in the hippocampus, basal ganglia, hypothalamus, cerebellum, areas of the cerebral cortex and the nucleus accumbens, with implications for memory, coordination, feeding, higher cognitive function and reward. Most important for pain are moderately abundant concentrations located within the periaqueductal gray (PAG) of the midbrain, the rostral ventrolateral medulla (RVM), superficial layers of the spinal dorsal horn and dorsal root ganglion, from which they are transported to the peripheral and central terminals of the primary afferent neuron (Figure 2). These locations are important in descending pain modulation, spinal processing of pain and peripheral pain perception. Additional areas include the hypothalamus and the pituitary gland (temperature regulation, endocrine and reproductive function), the Pain Management Unit, Queen Elizabeth II Health Sciences Centre, Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia Correspondence: Dr Mary Lynch, Pain Management Unit, Queen Elizabeth II Health Sciences Centre, 4th Floor Dickson Centre, Room 4086, Halifax, Nova Scotia B3H 1V7. Telephone 902-473-6428, fax 902-473-4126, e-mail mary.lynch@dal.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 ©2005 Pulsus Group Inc. All rights reserved 7A lynch_8901.qxd 8/30/2005 4:23 PM Page 8 Lynch Figure 1) Number of PUBMED citations per five-year period regarding ‘cannabinoid’ and ‘cannabis’ research amygdala (emotional response and fear), the brainstem (arousal) and the nucleus of the solitary tract (nausea and vomiting) (2,12,15-21) (Figure 2). There are low levels of cannabinoid receptors in brainstem cardiopulmonary centres, which probably accounts for the high safety margin of the cannabinoids (5). The identification of receptors in the areas described above is consistent with the behavioural effects produced by cannabinoids. The first CB2 receptors were cloned not from brain, but from a human immune cell line (12); thus, it was apparent from the beginning that the cannabinoid system extended beyond the nervous system. Since that time, studies have demonstrated the presence of CB2 receptors throughout the immune system (22,23). This work has established the current model for cannabinoid receptors, with CB1 primarily located in brain and associated structures such as the pituitary gland and peripheral nervous tissues, and CB2 primarily located in the reproductive and immune systems (2,23). Recently, CB2 receptor-like immunoreactivity has been described in the rat brain in neuronal patterns supporting possible broader central nervous system roles for the CB2 receptor (24). Endocannabinoids Until the end of the 20th century, only two major endocannabinoids, anandamide (N-arachidonoyl-ethanolamine [AEA]) and 2-arachidonylglycerol (2-AG) had been discovered (25-27). Since then, additional endocannabinoids have been identified (28). These include noladin ether, virodhamine (O-arachidonoyl-ethanolamine) and N-arachidonoyl dopamine (NADA) (29-31), as well as others that are in the process of being identified. To qualify as an endocannabinoid, the agent must exhibit activity at cannabinoid receptors. The endocannabinoids vary in their activity at the receptor depending on the type of intracellular event measured (32). AEA, NADA and noladin are more selective for CB1, virodhamine appears to prefer CB2 and 2-AG is equipotent for both CB1 and CB2 (28). In addition to CB1 agonist activity, AEA binds to the vanilloid receptor (29). NADA also exhibits activity at vanilloid receptors (now called transient receptor potential vanilloid 1 receptors) and appears to be pronociceptive (28). Palmitoylethanolamide (PEA) is not strictly an endocannabinoid, but has cannabinomimetic properties, including analgesic effects, which in vivo are antagonized by the CB2 receptor antagonist SR144528 (7) (Table 1). 8A Figure 2) Location of cannabinoid receptors (CB1) in the areas of the nervous system that are important for pain transmission and modulation. PAG Periaqueductal gray; RVM Rostral ventrolateral medulla Biosynthesis and inactivation of endocannabinoids Endocannabinoids are biosynthesized via a phospholipiddependent pathway (Figure 3). The metabolic pathway for AEA and 2-AG have been identified; the detailed biosynthesis of the more recently discovered endocannabinoids is currently being worked out (28). The balance of evidence supports that AEA and 2-AG are synthesized and released on demand following physiological and pathological stimuli such as neuronal depolarization and the presence of bacterial lipopolysaccharides, possibly depending on calcium-dependent remodelling of phospholipid precursors. After biosynthesis, AEA and 2-AG are immediately released into the extracellular space. The release, disposition and potential recycling of endocannabinoids is not well understood. Research groups are pursuing various lines of inquiry, including identification of a putative transporter, uptake via caveolin-mediated endocytosis and passive diffusion. Inactivation of AEA and 2-AG occurs via fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase, respectively (4,6,28,33). CANNABINOID PHARMACOLOGY, MECHANISMS AND SITES OF ACTION There are several chemical classes of cannabinoid receptor agonists. These are the ‘classical’ cannabinoid ∆-9-THC, the ‘nonclassical’ cannabinoid CP55,940, the aminoalkylindole WIN55,212-2, the ‘eicosanoid’ cannabinoid AEA, and additional fatty acid ethanolamides and esters that act as endocannabinoids (2,4). As with the endocannabinoids, there is variability regarding the activity of cannabinoid ligands at the receptor. For example, ∆-9-THC and CP55,940 exhibit equal affinity for CB1 and CB2, whereas WIN55,212-2 exhibits modest selectivity for CB2 (2). Table 1 presents further detail regarding endogenous, naturally occurring and synthetic cannabinoids and their activity at receptors known to date. Signal transduction at the CB1 receptor Both cannabinoid receptor types are embedded in the cell membrane and are coupled to G proteins, negatively to adenylyl Pain Res Manage Vol 10 Suppl A Autumn 2005 lynch_8901.qxd 8/30/2005 4:23 PM Page 9 Cannabinoids as analgesics: An overview TABLE 1 Cannabinoid agonists and antagonists* Naturally occurring cannabinoids Endogenous cannabinoids Agent Action ∆-9-THC CB1 and CB2 agonist Comments Main psychoactive constituent of cannabis Cannabidiol Unknown mode of action Nonpsychoactive constituent of cannabis Anandamide CB1 partial agonist Also binds to TRPV1 2-Arachidonylglycerol CB1 and CB2 agonist Noladin CB1 N-arachidonoyl dopamine CB1 and TRPV1 agonist Virodhamine CB2 partial agonist Pronociceptive CB1 antagonist Acts like a CB2 agonist with analgesic Palmitoylethanolamide effects antagonized by CB2 antagonist but does not bind to CB2 receptors Synthetic cannabinoids Nabilone CB1 and CB2 agonist Available by prescription in Canada Synthetic ∆-9-THC (dronabinol; CB1 and CB2 agonist Available by prescription in Canada Marinol [Solvay Pharma Inc, Canada]) CP55,940 CB1 and CB2 agonist WIN55,212-2 CB1 and CB2 agonist AM1241 CB2 agonist HU-210 CB1 and CB2 agonist High-potency agonist HU-211 Not active at cannabinoid receptors Neuroprotective SR141716A CB1 antagonist Inverse agonist activity SR144528 CB2 antagonist Inverse agonist activity AM251 CB1 antagonist AM630 CB2 antagonist *This is not an exhaustive list. ∆-9-THC Delta-9-tetrahydrocannabinol; CB1 Cannabinoid receptors found primarily in the nervous system; CB2 Cannabinoid receptors found primarily in peripheral tissues and immune system; TRPV1 Transient receptor potential vanilloid 1 cyclase and positively to mitogen-activated protein kinase (2,6,7). CB1 receptors are coupled to ion channels through G proteins, positively to A-type and inwardly rectifying potassium channels and negatively to N-type and P/Q-type calcium channels and to D-type potassium channels (2). Activation of either receptor will result in inhibition of adenylyl cyclase activity resulting in a decrease in the production of cyclic AMP (cAMP) and cellular activities dependent on cAMP, with opening of inwardly rectifying potassium channels resulting in decreased cell firing and closing of calcium channels resulting in decreased release of neurotransmitters (Figure 4). The overall effect is that of cellular inhibition. This is very much like the mechanism of action of the opioids. The cannabinoids and opioids have similar actions but involve different systems. The CB1 receptor antagonist SR141716A prevents the analgesic effects of THC but not of morphine (34), whereas naloxone, an opioid antagonist, blocks the analgesic effect of morphine but not of THC and its analogues (35). Thus, with regard to signal transduction at the CB1 receptor, cannabinoids exhibit actions very much like the morphine group of drugs, but are able to act independently. The cannabinoid system is larger and occupies more areas than the opioid system, with the implication that the cannabinoid system may have wider potential therapeutic applications. Endocannabinoid signalling in the brain In contrast to classical neurotransmitters, investigators have identified that endocannabinoids are able to function as retrograde synaptic messengers (36). In this case, the endocannabinoid is Pain Res Manage Vol 10 Suppl A Autumn 2005 Tissue damage Phospholipids Phosphatidic acid phospholipase A2 phospholipase C Arachidonic acid phosphohydrolase Dopamine diacylglycerol lipase cyclo-oxygenase (COX 1, 2, 3) N-acyltransferase lipo-oxygenase 2-AG N-acylphosphatidyl-ethanolamine specific phospholipase D Leukotrienes Anandamide AEA Prostaglandins Prostacyclin Thromboxane NADA Noladin Virodhamine Biosynthetic pathway known Biosynthetic pathway details to be identified Figure 3) Biosynthesis of endocannabinoids in the context of the arachidonic acid pathway following tissue damage. 2-AG 2-arachidonylglycerol; AEA N-arachidonoyl-ethanolamine; NADA N-arachidonoyl dopamine synthesized and released from the postsynaptic neurons to travel backwards across the synapse, activating CB1 on presynaptic axons and then suppressing neurotransmitter release. This capacity for ‘working backwards’ is directly relevant to pain modulation. 9A lynch_8901.qxd 9/8/2005 1:26 PM Page 10 Lynch Figure 4) Signal transduction mechanisms at the cannabinoid receptor found primarily in the nervous system (CB1). Activation of the receptor stimulates coupling to the G protein with activation of mitogen-activated protein (MAP) kinase and inhibition of adenylyl cyclase with decreased production of cyclic (c)AMP. The G protein also directly couples the CB1 receptor negatively to N- and Q/P-type voltage-dependent calcium (Ca2+) channels and positively to A-type and inwardly rectifying potassium channels (K+A and K+ir, respectively). Thus, there is enhanced outward K+ current resulting in decreased cell firing, and closing of Ca2+ channels resulting in decreased release of neurotransmitters, resulting in an overall effect of cellular inhibition. PKA Protein kinase A. Adapted from reference 80 Supraspinal sites of action It has been demonstrated that cannabinoids act at multiple levels in the modulation of nociceptive or pain-related transmission (2,4,5). Intracerebroventricular administration of cannabinoids (37) suppresses tail-flick responses and spinal nociceptive responses (17). Direct brain injections into areas involved in descending inhibition of spinal nociceptive neurons elicits antinociceptive effects; these areas include the PAG in the midbrain, the RVM and the noradrenergic nucleus A5 in the medulla (38-40). Furthermore, microinfusion with the cannabinoid agonist WIN55,212-2 directly into the RVM in rats leads to increased ‘off-cell’ activity with increased tail-flick latencies, indicating that cannabinoids act directly within the RVM to affect off-cell activity (41). Additionally, cannabinoids have been shown to decrease noxious stimulus-evoked firing of nociceptive neurons in the ventral posterolateral nucleus of the thalamus as well as the RVM, with the latter being a demonstrated CB1 effect (4). Through a series of experiments involving animal behaviour (tail flick) and extracellular single unit recordings from RVM neurons, along with administration of specific cannabinoid and opioid agonists and antagonists, it has been demonstrated that cannabinoids produce analgesia through the same brainstem circuit used for opioid analgesia. The use of an opioid is not required for the cannabinoid to produce this effect (42). In addition, both systemic and intracerebroventricular administration of cannabinoids have been shown to decrease noxious heat-evoked activity of wide dynamic range (WDR) neurons in a manner sensitive to spinalization, indicating a supraspinal site of action and descending modulation of WDR neurons (17). 10A Spinal sites of action Several investigators have demonstrated that cannabinoids also inhibit pain by a direct spinal action (16,43-49). These observations are consistent with labelling studies exhibiting the presence of cannabinoid receptors in the dorsal horn of the spinal cord. For example, cannabinoids can act at spinal CB1 receptors to inhibit capsaicin-sensitive fibres in lumbar dorsal horn slices and to decrease noxious stimulus-evoked firing of WDR neurons (16,48). Additional evidence supports that activation of the spinal CB1 receptor can decrease N-methylD-aspartate receptor activation, potentially by inhibiting glutamate release into the spinal cord (49). Intrathecal injection of (methyl-6-phenylethynyl) pyridine, a selective metabotropic glutamate-5 receptor antagonist, reversed the antihyperalgesic effect of intrathecal WIN55,212-2 in a rat loose ligation sciatic nerve model (50). These data suggest that the antihyperalgesic effect of cannabinoid agonist WIN55,212-2 is mediated through an interaction with spinal metabotropic glutamate-5 receptors (50). In addition, there is growing support that cannabinoids modulate spinal noradrenergic and opioid systems (see ‘Opioid system’ section) (4). Peripheral cannabinoid action Cannabinoids also act in the periphery. The endocannabinoids AEA and PEA have been found in the skin in concentrations five- to 10-fold higher than in brain or plasma in the rat (50). Evidence supports the presence of CB1 receptors on central and peripheral terminals of primary afferent neurons (2). A number of studies have demonstrated a peripheral antinociceptive action for cannabinoid agonists in preclinical models (50-53); both CB1 (51,53) and CB2 receptor agonists (52) exhibit peripheral antinociceptive action. Peripheral application of cannabinoids has also been demonstrated to reduce hyperalgesia and inflammation in preclinical models of neuropathic and inflammatory pain (51). Furthermore, it has been demonstrated that topical application of cannabinoid agonist (WIN55,212-2) enhances the antinociceptive effect of topical morphine via a CB1-mediated effect; in addition, spinally ineffective doses of WIN55,212-2 potentiate the antinociceptive effects of topical morphine (54). ENDOGENOUS PAIN MODULATION There is growing support that endocannabinoids participate in endogenous pain modulation. Supraspinally, in the PAG, it has been demonstrated that administration of cannabinoid antagonists produces hyperalgesia and blocks the analgesia produced by electrical stimulation of the dorsal PAG (47,55). Furthermore, using microdialysis in the PAG along with liquid chromatography/mass spectrometry, it was established that the analgesia produced by electrical stimulation or by injection of the chemical irritant formalin into the hind paws of anesthetized rats was associated with the release of AEA in the PAG (56), supporting that either pain itself or electrical stimulation leads to the release of AEA, which then acts on cannabinoid receptors in the PAG to inhibit nociception. Spinally, it has been demonstrated that hypoactivity of the spinal cannabinoid system has been associated with N-methylD-aspartate-dependent hyperalgesia (49). There is also support for peripheral control of pain initiation by endocannabinoids. Gas chromatography/mass spectrometry measurements indicate that the levels of AEA and Pain Res Manage Vol 10 Suppl A Autumn 2005 lynch_8901.qxd 8/30/2005 4:23 PM Page 11 Cannabinoids as analgesics: An overview PEA in the skin are enough to cause tonic activation of local cannabinoid receptors. Furthermore, the CB1 antagonist SR141716A and the CB2 antagonist SR144528 prolong and enhance pain behaviour produced following formalin injection. This work supports participation of endocannabinoids in the intrinsic control of pain initiation at peripheral sites (50). CANNABINOIDS AND INTERACTIONS WITH OTHER SYSTEMS Monoaminergic/noradrenergic systems There is evidence suggesting the involvement of monoaminergic systems in cannabinoid-induced antinociception. The serotoninergic neurotoxin 5,7-dihydroxytryptamine and the dopaminergic neurotoxin 6-hydroxydopamine both reduce the antinociceptive effect of cannabinoids in animal models. In these studies, noradrenergic involvement could not be ruled out due to the lack of pretreatment with a noradrenergic uptake inhibitor. Intrathecal administration of yohimbine (an alpha-2adrenergic antagonist) blocked antinociceptive effects of ∆-9THC. In contrast, intrathecal injection of the nonspecific serotonin antagonist, methysergide, did not reduce ∆-9THC-induced antinociception, nor did serotonin depletion by p-chlorophenlyalanine, suggesting a lack of serotonin involvement in cannabinoid antinociception. Similarly, the alpha1-antagonist phenoxybenzamine failed to block cannabinoid antinociception. Taken together, these data support a role for the spinal noradrenergic system in cannabinoid-induced antinociception (3). Opioid system Studies have determined that the analgesic effect of THC is, at least in part, mediated through delta and kappa opioid receptors. THC administered intrathecally has been shown to release endogenous opioids that stimulate delta and kappa receptors (57). Delta antagonists do not interfere with cannabinoid antinociception. Dynorphin antisera and the selective kappa antagonist nor-binaltorphimine block THC-induced antinociception; this antagonism is specific to antinociception and occurs at the spinal level. Furthermore, dynorphin A (1-8) antiserum and antisense to the kappa-1 receptor antagonized the effect (2,3). In addition, a bidirectional cross tolerance of ∆-9THC and CP55,940 to kappa agonists has been demonstrated in the tail-flick test (58). Thus, the preponderance of data supports a role for kappa and delta opioid receptors in the mediation of a component of cannabinoid antinociception (57). There is also some evidence supporting a possible role for mu opioid receptors in the enhancement of morphine antinociception by THC. Both naloxone and SR141716A (CB1-specific antagonist) block the enhanced antinociception due to the combination of low-dose THC and morphine, supporting both CB1 and mu opioid roles in the synergy (57). Thus, the current literature supports the possible involvement of all three major opioid receptor subtypes involved in some part in the enhancement of opioids by THC (57). It has been demonstrated that cannabinoids can act synergistically with the opioid receptor agonists in the production of antinociception in animal models of acute pain (2,4). This synergy has been demonstrated in numerous studies, using several routes of administration (4), and the synergy works both ways, with cannabinoids enhancing opioid antinociception and morphine enhancing cannabinoid antinociception. Full Pain Res Manage Vol 10 Suppl A Autumn 2005 isobolographic analysis has substantiated the greater than additive effect necessary to identify synergy (57). Following chronic dosing, upregulation of opioid receptor protein in the spinal cord has been observed in combinationtreated animals and may play a role in retention of efficacy of the drug combination. Short-term administration of low-dose THC with morphine in mice attenuated opioid tolerance without the loss of the antinociceptive effect. Further prolonged exposure to a cannabinoid agonist failed to result in downregulation of delta opioid receptors in vitro. Taken together, these results support that cannabinoids can alter opioid tolerance (57). Thus, data support a synergistic effect of cannabinoids and opioids and a possible role for cannabinoids in situations of opioid tolerance. CANNABINOIDS AND PAIN Cannabinoids exhibit antinociceptive and antihyperalgesic effects in models of acute and chronic pain Preclinical work reveals that cannabinoids block pain responses in virtually every pain model tested. One of the earliest studies was performed by Dixon (59), who demonstrated that cannabis was able to suppress canine reactions to pinpricks. In models of acute or physiological pain, cannabinoids are effective against thermal, mechanical and chemical pain and are comparable with opioids in potency and efficacy (5). In models of chronic pain, cannabinoids exhibit greater potency and efficacy in both inflammatory (60) and neuropathic pain (61). Because cannabinoids are also able to affect motor systems, it is important to establish that the slowed reactions of animals in pain tests are not because of slowed motor activity rather than pain inhibition. In electrophysiological studies, it has been concluded that cannabinoids produce profound suppression of cellular nociceptive responses with no suppression of the low threshold mechanoreceptive neurons (5). These experiments include suppression of neurophysiological responses to all types of nociceptive stimuli tested, suppression of windup (a model of central sensitization observed in chronic pain) and suppression of increased spontaneous firing following injection of the inflammatory agent complete Freund’s adjuvant (2,5,17,18,62-65). Thus, there is significant evidence that cannabinoids exhibit antinociceptive and antihyperalgesic effects in models of acute and chronic pain. Of further importance to chronic pain is the fact that upregulation of CB1 receptors (within the ipsilateral superficial dorsal horn of the spinal cord in rats following chronic constriction injury of the sciatic nerve) has been demonstrated. This enhanced the effects of a cannabinoid agonist (WIN55,212-2) on both thermal hyperalgesia and mechanical allodynia, supporting that upregulation of spinal cannabinoid receptors following peripheral nerve injury may contribute to the effects of exogenous cannabinoids in neuropathic pain (66). Furthermore, repeated administration of WIN55,212-2 given subcutaneously reversed the development of hyperalgesia that normally develops in chronic constriction of the sciatic nerve in rats (67), supporting that cannabinoids may play a role in prevention of neuropathic pain if given early after nerve injury. Nonpsychoactive cannabinoids targeting pain There is significant interest in the development of synthetic cannabinoids without psychotropic activity (68-70). Ajulemic acid (also called CT-3) is a synthetic analogue of ∆8-THC-11-oicacid, one of the endogenous transformation products of THC. 11A lynch_8901.qxd 8/30/2005 4:23 PM Page 12 Lynch Figure 5) Cannabinoid influences on peripheral nerve activity with tissue injury and inflammation. 5-HT 5-hydroxytryptamine; CB1 Cannabinoid receptors found primarily in nervous system; CB2 Cannabinoid receptors found primarily in peripheral tissues/immune system; CGRP Calcitonin gene-related peptide; NGF Nerve growth factor; NO Nitric oxide; trk A High affinity NGF receptor. Figure adapted from reference 71 In preclinical studies, ajulemic acid has been found to exhibit potent analgesic, antiallodynic and anti-inflammatory activity; however, it binds to CB1 receptors and has been found to cause sedation in mice (70). Cannabidiol (CBD) is a nonpsychoactive cannabinoid present in cannabis that does not bind to cannabinoid receptors; it has also been demonstrated that CBD inhibits FAAH and blocks the reuptake of AEA, thus enhancing extracellular levels of AEA (71). Investigators have developed synthetic analogues to CBD in a search for a nonpsychoactive, nonsedating agent. HU-320 (CBD-dimethyl-heptyl-7oic acid) is a novel synthetic cannabinoid acid that has been demonstrated to exhibit strong anti-inflammatory and immunosuppressive properties while demonstrating no psychoactive effects (70). Anti-inflammatory and peripheral antihyperalgesic effects of cannabinoids Following tissue injury or inflammation with disruption in normal tissue integrity and migration of various cells (eg, immune and mast cells, platelets), a diversity of chemical mediators are produced or released locally. These mediators then activate peripheral sensory nerve endings. Some will activate the sensory nerve directly; others will sensitize the nerve to other stimuli or exert regulatory effects on the sensory neuron, inflammatory cells and adjacent sympathetic nerves (Figure 5) (72). There is evidence that CB1 and CB2 receptors are present peripherally, and the mechanisms for synthesizing, releasing and inactivating endocannabinoids are present during inflammation (7). CB1 agonists exhibit a direct effect on the sensory nerve terminal itself to inhibit release of calcitonin gene-related peptide (51) and inhibit sensitizing effects of nerve growth factor 12A (NGF) (7). Peripheral administration of AEA attenuates hyperalgesia and edema via a CB1 receptor mechanism and inhibits capsaicin-evoked plasma extravasation into the hindpaw (51). Local analgesic actions of directly and indirectly acting agonists for CB2 receptors, expressed on mast cells and inhibiting mast cell function, have also been demonstrated (50,52). CB2 receptor mechanisms may play a particularly prominent role in inflammatory pain (7). Both CB2 and high-affinity NGF receptors (trkA) have been identified on mast cells, and mast cells amplify the NGF signal during inflammation (7). There is increasing evidence that PEA (a CB2 agonist) attenuates this amplification. PEA accumulates in inflamed tissue, is synthesized by leukocytes, prevents mast cell degranulation and suppresses inflammatory hyperalgesia and edema (7). Furthermore, it has been demonstrated that neutrophil migration is diminished by endocannabinoids in models of inflammatory pain. In addition, cannabinoids attenuate nitric oxide production from stimulated macrophages via a CB2 receptor-mediated action (7), and have also been demonstrated to have profound and complex effects on cytokine production (73). A CB2 selective agonist (AM1241, administered intraperitoneally) suppressed development of intradermal capsaicininduced thermal and mechanical hyperalgesia and allodynia; this was reversed by a CB2 antagonist (SR144528) but not by a CB1 antagonist (SR141716A). Also, AM1241 suppressed thermally and mechanically evoked hyperalgesia and allodynia following local administration to the capsaicin ipsilateral paw but had no effect on the contralateral (untreated) paw. These data provide evidence that actions at CB2 receptors are sufficient to normalize nociceptive thresholds and produce antinociception in persistent pain states (74). In animal models of inflammmatory pain, local administration of AEA, PEA and synthetic cannabinoids have been repeatedly demonstrated to attenuate behavioural responses to proinflammatory substances including subcutaneous formalin, capsaicin and complete Freund’s adjuvant (7). A recent study (75) found that nabilone, a cannabinoid agonist available by prescription in Canada, reduced edema and associated hyperalgesia following carrageenan injection into the paw. It has also been demonstrated that AEA causes inhibition of interleukin-2 secretion in activated splenocytes via a mechanism involving both cyclooxygenase-1 and cyclooxygenase-2 (76). Old antiinflammatory analgesic drugs such as indomethacin and flurbiprofen activate CB1 receptors via a decrease in FAAH degradation and, therefore, an increase in AEA concentration, suggesting the potential for a cannabinoid mechanism of action contributing to their effects (77). Visceral pain conditions Manipulation of CB1 receptors can alter sensory processing from the gut; brain integration of the brain-gut axis; extrinsic control of the gut; and intrinsic control by the enteric nervous system (78). The upper gastrointestinal tract is strongly influenced by CB1 receptor activation on central vagal pathways, whereas intestinal peristalsis can be modified by CB1 receptor activation in the absence of extrinsic input (78). Endocannabinoids (AEA and PEA) attenuate viscera-visceral hyperreflexia, spinal Fos expression and the referred hyperalgesia in a model of cystitis that shares features of interstitial cystitis; the effects Pain Res Manage Vol 10 Suppl A Autumn 2005 lynch_8901.qxd 8/30/2005 4:23 PM Page 13 Cannabinoids as analgesics: An overview of AEA are predominantly CB1 receptor-mediated and the effects of PEA are predominantly CB2 receptor-mediated (7). CB1-deficient mice or wild-type mice administered CB1 antagonists exhibit increased inflammation following intrarectal administration of proinflammatory substances (eg, 2,4-dinitrobenzene sulphonic acid [DNBS]). Treatment with a cannabinoid agonist or genetic ablation of FAAH protected against the development of DNBS-induced colitis. Electrophysiological recordings from circular smooth muscle cells 8 h after the administration of DNBS revealed spontaneous action potentials in CB1-deficient mice but not in wildtype littermate colons, indicating early CB1-mediated control of inflammation-induced irritation of smooth muscle cells. DNBS treatment increased the percentage of myenteric neurons expressing CB 1 receptors, suggesting enhanced cannabinoid signalling during colitis. This work supports evidence that CB1 receptors mediate intrinsic protective signals that counteract proinflammatory responses and indicates the endocannabinoid system is a promising target for the treatment of gastrointestinal disorders with excessive inflammatory responses (79). The future of cannabinoid research The present review has focused on cannabinoid research relating to pain applications. As presented in the introduction, there are many other potential applications for cannabinoid agonist and antagonist molecules under development. Perhaps the most exciting area of research regarding cannabinoids is the identification of ways to manipulate the endocannabinoid system. Unlike endogenous opioids, endocannabinoids are synthesized by what appear to be relatively selective enzymes. Furthermore, there is also intense focus on the mechanism of reuptake and inactivation of the endocannabinoids. In the future, it may be possible to manipulate the endocannabinoid system for the treatment of pain in much the same way as the monoaminergic system is targeted for the treatment of depression. CONCLUSION The potent antinociceptive and antihyperalgesic effects of cannabinoid agonists, the presence of cannabinoid receptors in pain-processing areas of the brain, spinal cord and periphery, and the endogenous modulation of pain systems by cannabinoids support that cannabinoids exhibit significant potential as analgesics. REFERENCES 1. Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964;86:1646-7. 2. Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol 2001;63:569-611. 3. Martin BR, Lichtman AH. Cannabinoid transmission and pain perception. Neurobiol Dis 1998;5:447-61. 4. Richardson JD. Cannabinoids modulate pain by multiple mechanisms of action. J Pain 2000;1:2-14. 5. Walker JM, Strangman NM, Huang SM. Cannabinoids and pain. Pain Res Manag 2001;6:74-9. 6. Rice AS. Cannabinoids and pain. Curr Opin Investig Drugs 2001;2:399-414. 7. Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: Spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids 2002;66:243-56. 8. Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988;34:605-13. Pain Res Manage Vol 10 Suppl A Autumn 2005 9. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990;346:561-4. 10. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61-5. 11. Sim LJ, Selley DE, Xiau R, Childers SR. Differences in G-protein activation by mu- and delta-opioid, and cannabinoid, receptors in rat striatum. Eur J Pharmacol 1996;307:97-105. 12. Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci 1991;11:563-83. 13. Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: A comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 1992;48:655-68. 14. Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical localization of cannabinoid CB1 receptors in rat central nervous system. Neuroscience 1998;83:393-411. 15. Herkenham M. Localization of cannabinoid receptors in brain and periphery. In: Pertwee RG, ed. Cannabinoid Receptors. New York: Academic Press 1995:145-66. 16. Hohmann AG, Tsou K, Walker JM. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN 55,212-2. Neurosci Lett 1998;257:119-22. 17. Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res 1999;822:17-25.. 18. Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience 1999;92:1171-5. 19. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 1997;74:129-80. 20. Sanudo-Pena MC, Tsou K, Delay ER, Hohmann AG, Force M, Walker JM. Endogenous cannabinoids as an aversive or counterrewarding system in the rat. Neurosci Lett 1997;223:125-8. 21. Wenger T, Fernandez-Ruis JJ, Ramos JA. Immunohistochemical demonstration of CB1 cannabinoid receptors in the anterior lobe of the pituitary gland. J Neuroendocrinol 1999;11:873-8. 22. Klein TW. Cannabis and immunity. In: The Health Effects of Cannabis. Kalant H, Corregal W, eds. Toronto: Centre for Addiction and Mental Health, 1999:349-73. 23. Klein TW. Cannabinoids and the immune system. Pain Res Manage 2001;6:95-101. 24. Gong JP, Onaivi E, Uhl G. Cannabinoid CB2 receptors: Immunhistochemical localization in rat brain. ICRS abstracts. 15th Annual Symposium on the Cannabinoids, Clearwater, Florida, June 24-27, 2005. 25. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992;258:1946-9. 26. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995;50:83-90. 27. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 1995;215:89-97. 28. DePetrocellis L, Cascio MG, DiMarzo V. The endocannabinoid system: A general view and latest additions. Br J Pharmacol 2004;141:765-74. 29. Bisogno T, Melck D, Bobrov MY, et al. N-acyl-dopamines: Novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J 2000;351:817-24. 30. Huang SM, Bisogno T, Trevisani M, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid receptors. Proc Natl Acad Sci 2002;99:8400-5. 31. Porter AC, Sauer JM, Knierman MD, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther 2002;301:1020-4. 32. Mukhopadhyay S, Shim JY, Assi AA, Norford D, Howlett AC. CB(1) cannabinoid receptor-G-protein association: A possible mechanism for differential signaling. Chem Phys Lipids 2002;121:91-109. 33. DiMarzo V, Bifulco M, DePetrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004;3:771-84. 13A lynch_8901.qxd 8/30/2005 4:23 PM Page 14 Lynch 34. Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced nociception in rats. Pharmacol Biochem Behav 1997;57:7-12. 35. Howlett AC. Pharmacology of cannabinoid receptors. Ann Rev Pharmacol Toxicol 1995;35:607-34. 36. Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science 2002;296:678-82. 37. Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM. Antinociceptive actions of WIN 55,212-2 following intraventricular administration in rats. Brain Res 1993;629:300-4. 38. Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoidinduced antinociception in the rat. Life Sci 1995;56:2103-10. 39. Martin WJ, Tsou K, Walker JM. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci Lett 1998;232:33-6. 40. Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res 1999;822:237-42. 41. Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature 1998;395:381-3. 42. Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience 2004;124:685-93. 43. Yaksh TL. The antinociceptive effects of intrathecally administered levonantradol and desacetyllevonantradol in the rat. J Clin Pharmacol 1981;21:334S-40S. 44. Welch SP, Stevens DL. Antinociceptive activity of intrathecally administered cannabinoids alone and in combination with morphine in mice. J Pharmacol Exp Ther 1992;262:10-8. 45. Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther 1991;258:517-23. 46. Lichtman AH, Martin BR. Cannabinoid-induced antinociception is mediated by a spinal alpha 2-noradrenergic mechanism. Brain Res 1991;559:309-14. 47. Richardson JD, Aanonsen L, Hargreaves KM. SR141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur J Pharmacol 1997;319:R3-4. 48. Richardson JD, Aanonsen L, Hargreaves KM. Antihyperalgesic effects of spinal cannabinoids. Eur J Pharmacol 1998;345:145-53. 49. Richardson JD, Aanonsen L, Hargreaves KM. Hypoactivity of the spinal cannabinoid system results in NMDA-dependent hyperalgesia. J Neurosci 1998;18:451-7. 50. Calignano A, la Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature 1998;394:277-81. 51. Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain 1998;75:111-9. 52. Malan TP, Ibrahim MM, Deng H, et al. CB2 cannabinoid receptormediated peripheral antinociception. Pain 2001;93:239-45. 53. Dogrul A, Husamettin G, Akar A, Yildiz O, Bilgin F, Guzeldemir E. Topical cannabinoid antinociception: Synergy with spinal sites. Pain 2003;105:11-6. 54. Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain 2003;105:303-8. 55. Strangman NM, Patrick SL, Hohmann AG, Tsou K, Walker JM. Evidence for a role of endogenous cannabinoids in the modulation of acute and tonic pain sensitivity. Brain Res 1998;813:323-8. 56. Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Nat Acad Sci (USA) 1999;96:198-203. 57. Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci 2004;74:1317-24. 58. Smith PB, Welch SP, Martin BR. Interactions between delta-9tetrahydrocannabinol and kappa opioids in mice. J Pharmacol Exp Ther 1994;268:1381-7. 59. Dixon WE. The pharmacology of cannabis. Indica Br Med J 1899;2:1354-7. 60. Tsou K, Lowitz KA, Hohmann AG, et al. Suppression of noxious 14A 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. stimulus-evoked expression of fos-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience 1995;70:791-8. Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett 1997;221:157-60. Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus evoked activity of the spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sci 1995;56:2111-9. Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett 1998;252:13-6. Martin WJ, Hohman AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by the cannabinoid WIN 55,212-2: Correlation between electrophysiological and antinociceptive effects. J Neurosci 1996;16:6601-11. Strangman NM, Walker JM. The cannabinoid WIN 55,212-2 inhibits the activity-dependent facilitation of spinal nociceptive responses. J Neurophysiol 1999;82:472-7. Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain 2003;105:275-83. Costa B, Colleoni M, Conti S, et al. Repeated treatment with the synthetic cannabinoid WIN 55,212-2 reduces both hyperalgesia and production of pronociceptive mediators in a rat model of neuropathic pain. Br J Pharmacol 2004;141:4-8. Burstein SH, Audettte CA, Breuer A, et al. Synthetic nonpsychotropic cannabinoids with potent anti-inflammatory, analgesic, and leukocyte antiadhesion activities. J Med Chem 1992;35:3135-41. Burstein SH, Stebulis JA, Torres R, et al. Ajulemic acid, a nonpsychoactive cannabinoid acid, downregulates activation of human synovial cells. ICRS 2004, abstract 209. Sumariwalla PF, Gallily R, Tchilibon S, Fride E, Mechoulam R, Fledmann M. A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. Arthritis Rheum 2004;50:985-98. Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannnabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001;134:845-52. Sawynok J. Topical and peripherally acting analgesics. Pharm Rev 2003;55:1-20. Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med 2000;225:1-8. Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J Pharmacol Exp Ther 2004;308:446-53. Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br J Pharmacol 2002;135:181-7. Rockwell C, Kaminski N. Anandamide metabolites from both cyclooxygenase enzymes cause inhibition of Il-2 secretion in murine splenocytes. ICRS 2004 Abstract 210. Fowler CJ. Possible involvement of the endocannabinoid system in the actions of three clinically used drugs. Trends Pharmacol Sci 2004;25:59-61. Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol 2004;141:1335-45. Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004;113:1202-9. Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol 1999;58:315-48. Pain Res Manage Vol 10 Suppl A Autumn 2005 mcgilveray_8902.qxd 8/30/2005 4:26 PM Page 15 REVIEW Pharmacokinetics of cannabinoids Iain J McGilveray PhD IJ McGilveray. Pharmacokinetics of cannabinoids. Pain Res Manage 2005;10(Suppl A):15A-22A. Delta-9-tetrahydrocannabinol (∆-9-THC) is the main psychoactive ingredient of cannabis (marijuana). The present review focuses on the pharmacokinetics of THC, but also includes known information for cannabinol and cannabidiol, as well as the synthetic marketed cannabinoids, dronabinol (synthetic THC) and nabilone. The variability of THC in plant material (0.3% to 30%) leads to variability in tissue THC levels from smoking, which is, in itself, a highly individual process. THC bioavailability averages 30%. With a 3.55% THC cigarette, a peak plasma level of 152±86.3 ng/mL occured approximately 10 min after inhalation. Oral THC, on the other hand, is only 4% to 12% bioavailable and absorption is highly variable. THC is eliminated from plasma in a multiphasic manner, with low amounts detectable for over one week after dosing. A major active 11-hydroxy metabolite is formed after both inhalation and oral dosing (20% and 100% of parent, respectively). THC is widely distributed, particularly to fatty tissues, but less than 1% of an administered dose reaches the brain, while the spleen and body fat are long-term storage sites. The elimination of THC and its many metabolites (from all routes) occurs via the feces and urine. Metabolites persist in the urine and feces for several weeks. Nabilone is well absorbed and the pharmacokinetics, although variable, appear to be linear from oral doses of 1 mg to 4 mg (these doses show a plasma elimination half-life of approximately 2 h). As with THC, there is a high first-pass effect, and the feces to urine ratio of excretion is similar to other cannabinoids. Pharmacokineticpharmacodynamic modelling with plasma THC versus cardiac and psychotropic effects show that after equilibrium is reached, the intensity of effect is proportional to the plasma THC profile. Clinical trials have found that nabilone produces less tachycardia and less euphoria than THC for a similar antiemetic response. Key Words: Cannabinoids; Inhalation; Nabilone; Pharmacodynamics; Pharmacokinetics CHEMISTRY Marijuana is the common name for Cannabis, a hemp plant that grows throughout temperate and tropical climates in almost any soil condition. The plant yields cannabinoids such as delta-9-tetrahydrocannabinol (∆-9-THC; referred to as THC), which is the main psychoactive ingredient of cannabis. The flowering tops and leaves of this plant are used to produce cannabis for smoking. Marijuana is most commonly smoked in hand-rolled cigarettes (‘joints’) containing the plant material. Recent work (1) has suggested that the restrictive phytochemical definition of cannabinoids be changed to a broader definition to include “all ligands of the cannabinoid receptor(s) and related compounds, including endogenous ligands of the receptors and a large number of synthetic analogues”. The present review, however, will be restricted to the pharmacokinetics of Pharmacocinétique des cannabinoïdes Le delta-9-tétrahydrocannabinol (∆-9-THC) est le principal ingrédient psychoactif du cannabis (marijuana). Le présent article de synthèse s’attarde à la pharmacocinétique du THC, mais inclut également des données sur le cannabinol et le cannabidiol, de même que sur les cannabinoïdes de synthèse sur le marché, soit le dronabinol (THC synthétique) et le nabilone. La variabilité des taux de THC dans la substance végétale (0,3 % à 30 %) donne lieu à des taux tissulaires variables de THC après l’inhalation, qui en soi, est déjà un processus hautement individuel. La biodisponibilité du THC est en moyenne de 30 %. Avec une cigarette dont la teneur en THC est de 3,55 % un pic plasmatique de 152 ± 86,3 ng/mL s’obtient environ 10 minutes après l’inhalation. D’autre part, le THC oral n’est biodisponible que dans une proportion de 4 à 12 % et son absorption est très variable. Le THC est éliminé du plasma de façon multiphasique, de faibles quantités étant décelables encore dans les sept jours suivant la consommation. Un important métabolite 11-hydroxy actif est formé après l’inhalation ou la prise orale (20 % et 100 % de la molécule-mère, respectivement). Le THC est très largement distribué, particulièrement dans les tissus gras, mais moins de 1 % d’une dose administrée atteint le cerveau, alors que la rate et les graisses corporelles en sont les sites de réserve à long terme. L’élimination du THC et ses nombreux métabolites (peu importe la voie d’administration) se fait par les selles et l’urine. Les métabolites persistent dans l’urine et les selles pendant plusieurs semaines. Le nabilone est bien absorbé et sa pharmacocinétique, bien que variable, semble être linéaire avec des doses orales de 1 à 4 mg (qui ont une demi-vie d’élimination plasmatique d’environ deux heures). Comme avec le THC, on note un effet de premier passage important et le ratio selles-urine de l’excrétion est semblable à celui d’autres cannabinoïdes. Par rapport à ses effets cardiaques et psychotropiques, le modèle pharmacocinétiquepharmacodynamique plasmatique du THC révèle qu’après l’atteinte de l’état d’équilibre, l’intensité de l’effet est proportionnelle à son profil plasmatique. Les essais cliniques ont montré que le nabilone entraîne moins de tachycardie et moins d’euphorie que le THC, pour une réponse antiémétique semblable. the cannabinoids, including two synthetic compounds that have been subjected to extensive clinical studies. Although the leaves and flowering tops of Cannabis plants yield more than 60 different cannabinoids, the major active components are THC (Figure 1), cannabinol (CBN) and cannabidiol (CBD) (Figure 2) (2). Some reports mention the presence of ∆-8-THC, although this may be formed by the isomerization (2) of ∆-9-THC during isolation. ∆-9-THC is the principal psychoactive ingredient of cannabis, and the other components, such as CBN, CBD and ∆-8-THC, are present in smaller quantities and are not believed to make a significant contribution to the total effect of marijuana on behaviour or perception; however, CBD may have other pharmacological effects (3). THC is enantiomeric, with only the (–) enantiomers occuring in nature and the McGilveray Pharmacon Inc, and University of Ottawa, Ottawa, Ontario Correspondence and reprints: Dr McGilveray, 1 Stonehedge Park, Nepean, Ontario K2H 8Z1. Telephone 613-829-8551, fax 613-829-5175, e-mail mcgilver@magma.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 ©2005 Pulsus Group Inc. All rights reserved 15A mcgilveray_8902.qxd 8/30/2005 4:26 PM Page 16 McGilveray Figure 1) The structure of delta-9-tetrahydrocannabinol (∆-9-THC) using the common dibenzopyran numbering system. The ∆-9-THC analogue ∆-8-THC is also shown Figure 3) The structure of nabilone ABSORPTION Figure 2) The structure of cannabinol and cannabidiol synthetic (+) enantiomers being inactive. ∆-9-THC is sparingly soluble in water but has high lipid solubility (4). An oral form of synthetic ∆-9-THC ([–] enantiomer), dronabinol (2.5 mg, 5 mg or 10 mg capsules; dissolved in sesame oil), is marketed in the United States and Canada as Marinol (Solvay Pharma, Canada) (5). Structure-activity relationships, comprising the effects of alteration in the cannabinoid molecule, were studied extensively in the 1960s and 1970s. This led to one synthetic drug, nabilone (6), being marketed as Cesamet capsules (Valeant Canada limitée/Limited) (7) (Figure 3). Nabilone is a sparingly water-soluble, racemic (±) mixture that is crystalline, unlike the resinous cannabinoid oils from the Cannabis plant. Since the endogenous cannabinoid receptors were identified, there has been a resurgence in the study of the structure-activity relationship (8,9) of cannabinoids; however, while this research is promising, new receptor agonists are not yet in clinical use (10). Cannabis also contains variable amounts of the carboxylic acid analogues of ∆-9-THC (tetrahydrocannabinolic acid [THCA]), in which the carboxyl groups can be positioned on carbon 2 or 4 of the molecule (according to the numbering in the structure shown in Figure 1). These substances are not pharmacologically active, although they may be released by gastric acid (1) and readily degrade on heating (eg, smoking) to yield THC (11,12). The decarboxylation is said to occur within 5 min at a temperature of 200°C to 210°C and within seconds in smoked marijuana cigarettes. This is important because according to Agurell et al (13), the total amount of THC and its corresponding acids is almost always considered in potency content. This is crucial information relating to activity because the ratio of inactive ∆-9-THCA to active ∆-9-THC is reported to range from 2:1 in areas where cannabis is grown in warmer climates to 17:1 in plants grown in cooler climates (14). PHARMACOKINETICS The present section will be restricted to human pharmacokinetics, mainly of smoked cannabis and with some comparisons of oral THC, including dronabinol (Marinol). 16A Smoked cannabis The estimation of the dose administered by the smoking route is a major variable in assessing the absorption of cannabinoids (mainly THC) in humans. The source of the plant material and the composition of the cigarette, together with the efficiency of smoking by the subject, are additional uncontrolled factors. It is reasonable to consider approximately 10% to 13% as the average THC content in Canadian marijuana (Health Canada information) (15). Regarding smoking techniques, one research group (16) remarked, “it is incredible to see the variety of techniques marijuana users employ to smoke their cigarette”. It appears that habitual heavy marijuana smokers can increase the amount absorbed and this is attributed to more efficient smoking techniques (12,13). Table 1 indicates some of the variation found by various researchers (12,16-20) who investigated the amount of THC lost during smoking, with 69% regarded as the maximum available for absorption via mainstream smoke from a smoking machine (16). However, as much as 50% of the active drug in cigarettes can be lost due to pyrolysis. In one experiment, in which cigarettes containing approximately 19 mg of THC were smoked, it was reported that an average of 82% of the THC in the marijuana cigarette did not appear in the systemic circulation; an average of 6 mg (31%) was retained in the cigarette butts, with other losses due to pyrolysis and sidestream smoke during smoking (16,20). However, when the butt was smoked, it was estimated that 50% of the total THC dose was delivered. In experiments using a smoking machine, 16% to 19% of the THC was found in mainstream smoke, but when the cigarette was smoked in a single puff, avoiding sidestream smoke, 69% of the THC was in mainstream smoke (21); thus, approximately 30% of THC appears to be destroyed by pyrolysis (16). The United States National Institute of Drug Abuse (NIDA) group (16,20) reports that 20% to 37% of the THC is delivered in mainstream smoke, with pyrolytic destruction of 23% to 30% and sidestream losses of 40% to 50%. Less is known about the fate of smoked CBD and CBN, but it appears that the results are similar to THC, except that CBN plasma levels appear to be approximately twice as variable as other cannabinoids (13). In a very recent abstract (22), it was reported that from plant material containing up to 18% of THC and its corresponding acid (THCA), approximately 50% was pyrolyzed and, of the recovered amount, 50% of the THCA was converted to THC. It also appeared that some of the acid (or THC) pyrolyzed was converted to CBD, CBN and smaller Pain Res Manage Vol 10 Suppl A Autumn 2005 mcgilveray_8902.qxd 8/30/2005 4:26 PM Page 17 Pharmacokinetics of cannabinoids TABLE 1 Estimates of the percentage of delta-9-tetrahydrocannabinol flow during smoking TABLE 2 Relationship between cannabis potency and peak delta-9tetrahydrocannabinol (THC) plasma concentrations Plasma THC (ng/mL) ± SD THC range (ng/mL) 6 90.4±20.2 45.6–187.8 6 100.0±10.1 62.8–125.3 1.97 6 119.8±10.6 44.5–180.9 2.40 18 63.0±8.6 2.54 6 162.6±18.7 107.4–204.7 Leander (12), 4.84 12 124.2±16.2 44.8–218.0 Ohlsson et al (19) *Data adapted from reference 20 Delta-9-tetrahydrocannabinol flow (%) Reference Undifferentiated stream Sidestream Pyrolyzed Mainstream Butt Fehr and 50–60 40–50 Kalant (17), Truitt (18) Agurell and Huestis (16) Perez-Reyes (20) 20 6–53 31–50 16–69* 10–21 40–50 23–30 20–37 *‘High’ from single-puff smoking machine (20) amounts of cannabichromene. Unexpectedly, for every sample analyzed, 50% of the THC was recovered compared with the original THCA content. THC absorption by inhalation (with a bioavailability of 18% to 50% from cigarettes [16]) is extremely rapid, and is the main reason why this is the route of dosing preferred by many people (23). From experiments with deuteriumlabelled THC given intravenously (5 mg) or smoked in cigarettes (10 mg), heavy smokers (n=14) were found to obtain higher overall bioavailability (23% to 27%) of THC (24) than light (n=13) marijuana smokers (10% to 14%) (25). In the two experiments, there was high intersubject variability (coefficient of variation 40% to 70%) with overlap between groups. A mean bioavailability of 20%, with a range of 10% to 30%, for THC is given by Iversen (23). Standardized cigarettes have been developed by NIDA, and the relationships among cannabis (THC) content, dose administered and resultant plasma levels have been investigated. The inhalation from smoking cannabis, containing 1.64% THC (mean dose 13.0 mg THC), resulted in a mean peak THC plasma level of 77 ng/mL (19). In another experiment, controlled puffing of a 3.55% THC cigarette provided a maximum plasma level of 268.4 ng/mL (20). A comparison between cannabis ‘joint’ potency and resulting plasma THC concentrations from carefully controlled smoking experiments is shown in Table 2. Even in these controlled experiments, there is clearly great variation in the amount absorbed among individuals and a poor relationship between the amount of THC in cigarettes (1% to 4.8%) and peak plasma THC concentrations. It is possibe that individuals control their own levels by limiting inhalation according to effect. It is noted that the total THC level of Health Canada medical marijuana is approximately 10%, although that estimate likely includes THCA. There does not appear to be any new pharmacokinetic information on this dose level. Arguably, the most reliable information on absorption of marijuana is from work by Huestis et al (26), where a strict smoking protocol and an extremely rapid blood sampling technique were applied to six volunteers with cigarettes at two THC dose levels (1.75% and 3.55%). Concentrations of THC were detected in 2 min, just after the first puff, and peak concentrations occurred at 9 min, just before the last puff (which began at 9.8 min). Average peak plasma concentrations of 79±25.2 ng/mL and 152±86.3 ng/mL were obtained for the Pain Res Manage Vol 10 Suppl A Autumn 2005 THC content in cannabis (%) n 1.00 1.32 11.7–137.0 cigarettes containing 1.75% and 3.55% THC, respectively. Despite a rigorous smoking protocol, the variation displayed from the higher dose ranged from approximately 80 ng/mL to 260 ng/mL. Although the reported average maximum concentration occurred at 9 min, just before the final puff, the investigators noted that the time to peak concentration was influenced by the number of puffs, time between puffs, and the volume and length of inhalations; this was clear from other detailed studies (27,28). However, the effectiveness of breathholding with 3.55% THC potency cigarettes appears to be limited. After puffing the cigarette, a 20 s hold did not increase plasma concentrations significantly over a 10 s hold (27). There is little information for THC and other cannabinoids comparing pharmacokinetics in men and women. In a study with tritiated THC administered intravenously and orally to six young men and women, no differences in pharmacokinetics, including disposition and metabolism were noted (28). In another small study (29), three men and three women who were experienced marijuana smokers smoked two 1% THC cigarettes, with a 2 h interval between doses. They were asked to smoke at their usual rate. There was a difference between men and women in smoking rate, with men smoking more rapidly with more puffs (28 puffs versus 11 puffs for the women). There was a tendency for peak concentrations to be lower for the women, but there was no significant difference in the area under the concentrationtime curve (AUC) (28,29). THC plasma levels decreased rapidly after cessation of smoking and, at 2 h after smoking, were below 5 ng/mL; 15 min after reaching the maximum, mean concentrations declined by approximately 50% (26,30). Using modern sensitive analytical techniques, THC can be detected in the plasma for at least one day after a single dose, and for 13 days in chronic users (31). The decline of THC in plasma is multiphasic and, as Harvey (32) notes, estimates of the terminal half-life of THC in humans have increased as analytical methods have become more sensitive. Although there is still no consensus, it is probably safe to say that the terminal half-life of THC averages at least one week, but could be considerably longer. The half-life in plasma does not appear to be different between heavy and light users (33,34). Oral THC Information on oral THC was obtained mainly with dronabinol. THC is almost completely absorbed (90% to 95%) after single oral doses according to the recovery of 14C-labelled dose (35), although these data include both THC and its degradation products. From an oral dose of 20 mg THC in a chocolate cookie, compared with an intravenous infusion of 17A mcgilveray_8902.qxd 9/8/2005 1:27 PM Page 18 McGilveray 5 mg, the systemic availability was only 4% to 12% (19), and is described as being slowly and unreliably absorbed (13). While most subjects had peak plasma THC concentrations between 1 h and 2 h, some of the 11 subjects only peaked at 6 h and many had more than one peak. When tritiated THC was administered in oil enclosed in capsules (total doses of 15 mg in women and 20 mg in men) 10% to 20% of the administered dose reached the systemic circulation. The peak THC concentrations observed ranged from 10 ng/mL to 15 ng/mL, approximately 10% of the levels attained by efficient smoking (28). Only 10% to 20% of synthetic THC (dronabinol) administered in capsules with sesame oil enters the systemic circulation, indicating extensive first-pass metabolism (5). The psychomotor effect or ‘high’ has been observed to occur more quickly by the smoking than by the oral route (13,19); Iversen (36) remarks this as the reason “smoking is the preferred route of cannabis for many people”. As with the administration by smoking, the elimination phase from oral THC in plasma can be described using a twocompartment model with an initial (alpha) half-life of approximately 4 h and a beta half-life of 25 h to 36 h (37). However, as noted previously, the terminal half-life of THC can be much longer with considerable individual variability (31,32). Rectal THC In a pilot study (38), a suppository containing 11.8 mg of the THC hemisuccinate ester (equivalent to 9 mg THC) was administered to three women (two of whom had previously exhibited low plasma THC levels with a 10 mg dose of the oral THC dronabinol [Marinol]) and it provided comparatively high plasma THC concentrations. The AUC for plasma THC was more than 30-fold higher than after oral dosing. In another pilot study (39), in two patients with spasticity, multiple 10 mg to 15 mg doses of oral THC (dronabinol [Marinol]) were compared with rectal THC hemisuccinate suppositories (2.5 mg to 5 mg) over 24 h. After oral doses, peak plasma levels from 2.1 ng/mL to 16.9 ng/mL THC and 74.5 ng/mL to 244.0 ng/mL metabolite were found and, after rectal doses, peak plasma levels from 1.1 ng/mL to 4.1 ng/mL THC and 6.1 ng/mL to 42.0 ng/mL metabolite were measured over 8 h. Corrected for dose, rectal THC was approximately twice as bioavailable as the oral form, and this is attributed both to lower absorption and higher first-pass metabolism from the oral versus rectal route. DISTRIBUTION Distribution of THC begins immediately and rapidly after absorption. The plasma protein binding of THC and its metabolites is approximately 97% (40,41). THC is mainly bound to low density lipoproteins, with up to 10% present in red blood cells (42), while the metabolite, 11-hydroxy-THC, is even more strongly bound, with only 1% found in the free fraction (43). Because of its lipid solubility, THC has a large apparent volume of distribution, approximately 10 L/kg (13). Moreover, animal studies show that it is sequestered to the fat tissues, including the brain (32); however, considerably less than 1% of an administered dose reaches the brain (44).The highest concentrations are found in the heart and adipose tissue, with levels reaching 10- to 1000-fold that of plasma, respectively (18). THC readily crosses the blood-brain barrier and the slight delay in correlating peak plasma concentration to effects is assumed to reflect this distribution (19). In animal studies, while immediate distribution is high in the liver, the spleen and body fat are the major 18A sites of distribution after 72 h and are the long-term THC storage sites (45). There has been concern about the possible consequences of the long persistence of THC in fatty tissues. However, there is no evidence that the THC residues persist in the brain, and release from the fatty storage sites into blood is slow enough that the levels attained are not high enough to cause psychological effects. However, with regular use, THC will accumulate (32). METABOLISM The majority of the metabolism of cannabinoids occurs in the liver, and different metabolites predominate when different routes of administration are used. The complex metabolism of THC involves allylic oxidation, epoxidation, decarboxylation and conjugation (13). Cannabinoids are good substrates for cytochrome P450 (CYP) mixed-function oxidases and, in humans, the major site of hydroxylation is carbon 11, catalyzed by CYP 2CP (32). This is considered to be the enzyme that may influence potential drug interactions; however, the Marinol monograph (37) states, “in studies involving patients with AIDS and/or cancer, (dronabinol) capsules have been coadministered with a variety of medications (eg, cytotoxic agents, antiinfective agents, sedatives or opioid analgesics) without resulting in any clinically significant drug to drug interactions.” The major initial metabolites of THC are 11-hydroxy-THC and 11-nor-9-carboxy-THC. Over 80 other metabolites of THC, most of which are polar and acidic, have been identified and isolated by conducting in vivo experiments in humans or in vitro studies with human tissue (13). 11-hydroxy-THC is rapidly formed by the action of hepatic microsomal oxidases, and plasma levels parallel the duration of observable drug action. 11-hydroxy-THC has been found to have psychotomimetic properties equal to THC (46-48). After smoking 1.75% and 3.55% THC cigarettes, this metabolite appears rapidly and peaks shortly after THC, approximately 15 min after the start of smoking (16). 11-hydroxy-THC also exhibits peak plasma concentrations of approximately 7.5 ng/mL (approximately 5% of parent THC) and the AUC profile of this metabolite averages 20% of the parent. Similar results were obtained with intravenous administration (13). The psychoinactive 11-nor-9-carboxy-THC is the primary acid metabolite of THC excreted in the urine (49), and it is the cannabinoid most often monitored in forensic analysis of body fluids (50). Peak plasma values of this metabolite occur 1.5 h to 2.5 h after smoking and are approximately one-third the concentration of parent THC. There are numerous oxidative products from the side chain and further oxidation of the alcohols yield carboxylic acid products (13). Following oxidation, the phase II metabolites of the free drug or hydroxy-THC appear to be glucuronide conjugates (13) that appear in the urine; however, they are not major or appreciably active. There is limited information on the metabolism of CBD and CBN in humans. As with THC, the 11-hydroxy metabolites appear to be the major phase I products (51). For CBD, hydroxylation was found in all positions of the side chain, with several minor dihydroxylated metabolites being identified (52). For CBD, the amount of polar metabolites formed seems greater than for THC (13). For CBN, as well as the 11-hydroxy metabolite, dihydroxy-CBN, CBN-7-oic acid and more polar metabolites are formed after intravenous administration (53). It is known that polyaromatic hydrocarbons found in tobacco and cannabis smoke induce the action of CYP 1A2. If it can be Pain Res Manage Vol 10 Suppl A Autumn 2005 mcgilveray_8902.qxd 8/30/2005 4:26 PM Page 19 Pharmacokinetics of cannabinoids shown that the metabolism of THC also involves this CYP, then repeated exposure to cannabis could cause the more rapid disappearance of THC via this specific enzyme. However, animal studies (54) suggest that there may be functional interactions between THC and nicotine, which might have addiction implications. Various other CYP enzymes are of interest for potential drug interactions. In human liver microsome preparations, CBD has been shown to inhibit formation of THC metabolites catalyzed by CYP 3A, with less effect on CYP 2C9 (32). However, others suggest that CBD decreases formation of 11-hydroxy-THC by inhibition of CYP 2C9 (55), but it does not appear to present as a clinical interaction (56). After oral doses of THC, parent THC and its active metabolite, 11-hydroxy-THC, are present in approximately equal concentrations in the plasma (54,57). Concentrations of both parent drug and metabolite peak approximately 2 h to 4 h after oral dosing and decline over several days. Clearance averages approximately 0.2 L/kg⋅h, but is highly variable due to the complexity of cannabinoid distribution (37). The larger amount of 11-hydroxy-THC metabolite from first-pass metabolism by this route, which is similar in potency to THC, complicates the interpretation of potential effects. With oral THC dosing, absorption is slow and variable, and peak concentrations of THC may be only 10% of that from an efficiently smoked administration; however, the plasma levels of active 11-hydroxy metabolite are approximately threefold higher than that observed in the plasma from smoking (28,56). EXCRETION Elimination of inhaled THC and its metabolites occurs via the feces (65%) and urine (20%). After five days, 80% to 90% of the total dose is excreted (28,34). Metabolites in the urine (of which there are 20) are mainly acidic, such as 11-nor-9-carboxyTHC. Those in the feces are both acidic and neutral, the most abundant metabolites being 9-carboxy-THC (29%) and 11-hydroxy-THC (21%) (28,32). Similarly, following oral doses, THC and its biotransformation products are excreted in both feces and urine (37). Biliary excretion (complicated with enterohepatic recycling) is the major route of elimination, with approximately one-half of a radiolabelled oral dose being recovered from the feces within 72 h, compared with 10% to 15% recovered from urine (58). Less than 5% of an oral dose is recovered unchanged in the feces (37). Following administration of a single oral dose, low levels of THC metabolites have been detected for more than five weeks in the urine and feces (37,59). In forensic or employment situations when such testing may be applied, it is important for patients (or recreational users) to be aware that traces of marijuana can be detected in urine even weeks after dosing (50). NABILONE As previously stated, dronabinol is identical to THC from the marijuana plant. However, nabilone was developed from structure-action evaluation by industry (7) and has been marketed in Europe and Canada for over 20 years for the management of severe nausea and vomiting associated with cancer chemotherapy (8). It has also been studied for the treatment of chronic pain of various etiologies, with some patients experiencing useful benefits; however, there remains a need for more clinical trials on this aspect (60). Pain Res Manage Vol 10 Suppl A Autumn 2005 Absorption In radiotracer studies with 14C-nabilone administered intravenously and orally, 95.8% was absorbed (61) from oral administration (the disappearance from plasma was rapid due to extensive distribution and rapid metabolism). Additionally, for both the intravenous and the oral routes, the total radioactivity exhibited half-lives of 20.6 h and 35 h, respectively, and the parent nabilone had a plasma elimination half-life of approximately 2 h (62). As with THC, there is a high first-pass effect, but it is well absorbed and the pharmacokinetics appear to be linear (but variable) from oral doses of 1 mg to 4 mg (62). Distribution Information on the protein binding of nabilone is lacking. However, after intravenous tracer administration, the disappearance of total radioactivity, parent nabilone and the alcohol metabolite was biphasic, with the first phase (half-life approximately 10 min) attributed to distribution into tissue and the slower phase to elimination (61,63). Metabolism The metabolism of nabilone has not yet been fully elucidated. The major metabolites of nabilone in plasma are a mixture of isomeric alcohols yielded from the reduction of the ketone group on carbon 9 (61,62). These metabolites are excreted in feces, but not urine. The metabolites in urine are uncharacterized, and are highly polar and acidic, although there is no evidence of sulfate or glucuronide conjugates (62). It is speculated that, like THC, there is hydroxylation of various sites on the dimethylheptyl side chain (61) and one nabilone diol has been recovered from feces with one alcohol on the carbon 9 and the other on the side chain (64). There is virtually no information on drug interactions for this agent. Excretion From both oral and intravenous tracer doses, over 90% of the dose was recovered over seven days, with approximately 67% in feces and 23% in urine (61,62). These values are similar to the feces to urine ratio of excretion found with other cannabinoids (62). PHARMACOKINETIC-PHARMACODYNAMIC RELATIONSHIPS Most studies (65) of plasma concentration and effect relationships for marijuana have been directed at the psychotropic effect (‘high’) and the temporal relationship between this effect and plasma levels, as well as at intoxication; impairment of cognitive or motor function is not yet clear but is of major forensic interest (66). The acute effect on heart rate has also been used for such modelling (30). Dose and plasma concentration versus response for possible therapeutic applications are ill-defined, except for some information obtained for oral dosing with dronabinol for its limited indications (19). Such correlations of THC pharmacokinetics are complicated by the emergence of active metabolites, particularly 11-hydroxy-THC (47,49), which attains higher plasma concentrations after oral than inhalation doses. In one study (30), six volunteers smoked 1% by weight cigarettes with an average weight of 894 mg (total THC dose 8.9 mg), and then smoked a second cigarette after 2 h. Plasma THC concentrations, heart rate and self-reported ‘high’ profiles were documented. Similar psychological ‘highs’ occurred after both cigarettes, but less heart rate acceleration was found for the second cigarette. The heart rate for the first cigarette peaked at an 19A mcgilveray_8902.qxd 9/8/2005 1:27 PM Page 20 McGilveray average of 11 min after the start of smoking and was maintained until approximately 30 min after smoking. Thus, the effect was observed to begin at approximately 5 min after peak plasma concentration (mean 45 ng/mL) was reached – it exhibited a lag time – and it returned to baseline at approximately 30 min, when the plasma concentration was approximately 7 ng/mL. Although the heart rate increase was much smaller with the second cigarette, the lag time was similar. For the psychotropic effect, there was a different pattern with a gradual emergence of effect at 10 min (concentration of 30 ng/mL, post-peak), peaking at 30 min after smoking (concentration of approximately 7 ng/mL) and diminishing rapidly 45 min after smoking (concentration of 4.5 ng/mL). The second dose showed very similar pharmacokinetic and response profiles to the first cigarette. The data were fitted with a lag time model because the effect emerged approximately 20 min after the peak plasma concentration. In another experiment (67), the relationship between THC plasma concentrations and self-reported ‘highs’ with single cigarettes of three different potencies were examined. The cigarettes were 1.3%, 2.0% and 2.5% in THC potency. Because NIDA cigarettes average 900 mg, the total dose available ranged from 11.7 mg to 22.5 mg. The results indicated a proportional dose response, with the intensity and duration greatest for the 2.5% cigarette. As with the experiment above, there was a lag time from the peak plasma concentration until the ‘high’ and, for the highest dose, the feeling commenced at 5 min after smoking, when the plasma concentration was approximately 140 ng/mL. However, with the lowest dose, a similar intensity was noted at 5 min at a concentration of 90 ng/mL (which appears near the peak concentration for this dose). For the low dose, the intensity of the ‘high’ reached 50% of its maximum at 30 min and then gradually declined over 2 h, whereas, for the high dose, the ‘high’ almost plateaued at 20 min for 60 min to 75 min at 70% intensity, before declining. Modelling this data suggests that the steadystate plasma concentration at 50% of the maximum high-effect (Css[50]) would be 25 ng/mL to 29 ng/mL (67). Other reports (16,66) showed similar results using a 3.55% THC cigarette (which can yield an available dose of 32 mg of THC). In this case, the effect was perceptible within 2 min to 3 min and exhibited a plateau that commenced at 9 min and continued for 1.5 h before diminishing over 3 h to 4 h. A simultaneous average plasma concentration profile showed that at 1.5 h, the THC level is approximately 10 ng/mL and the 11-hydroxy-THC level is somewhat less. It was noted that the lack of correspondence between the plasma profile and the subjective ‘high’ response can be fitted with a more complex pharmacodynamic mode. This includes an ‘effect compartment’, which, after a lag time, reaches equilibrium with an effect curve. After equilibrium is reached, the intensity of the effect is proportional to the plasma THC profile. This concentration-effect response demonstrates a counterclockwise hysteresis. This type of modelling (66) supports a 10 ng/mL cutoff as evidence of functional impairment, which agrees with the above Css(50) estimate (67). In addition, the model was used to simulate multiple dosing with a 1% cigarette containing 9 mg THC (68). The duration of the maximal ‘high’ for this dose was estimated at approximately 45 min after dosing and declined to 50% of this peak effect at approximately 100 min after smoking. At this dose, a dosing interval of 1 h gave a ‘continuous high’ and the recovery after the last dose occurred at 150 min. The peak plasma concentration during this dosage was estimated at approximately 70 ng/mL and the Css(50) at approximately 30 ng/mL THC. 20A The data relating concentration and response were limited to the cardiac and subjective ‘high’ responses, and these show dissimilarities in profile. The information obtained from oral dosing with dronabinol was complicated because there is a greater amount of psychoactive 11-hydroxy-THC metabolite formed by this route of administration (49). Thus, the target THC plasma concentrations derived actually depend on the subjective ‘high’ response that may or may not be related to the potential therapeutic applications. However, it is likely that the psychoactivity that elicits this response from the central nervous system is receptor-derived and the concentrations are useful for suggesting doses from smoking. There are no formal pharmacokinetic-pharmacodynamic correlations with nabilone; however, there have only been a limited number of dose-response studies performed using doses between 1 mg and 5 mg (69). The responses examined were heart rate, blood pressure and subjective signs and symptoms, particularly euphoria. There was a decrease in pulse rate with the 1 mg dose and a slight increase of 7 beats/min to 8 beats/min with the 2.5 mg and 5 mg doses. There was no change in blood pressure when the subjects were recumbent but, with the 5 mg dose, blood pressure dropped significantly on standing and this was associated with dizziness (68). Subjective signs and symptoms, including euphoria and relaxation, were also more marked after the 5 mg dose. In experiments examining tolerance to these effects, a 2 mg dose was administered twice daily for seven days, and subjects were challenged with a 5 mg dose on the eighth day and again after a week of washout. After six days on the 2 mg dose, postural hypotension and euphoria were absent and did not recur with an immediate higher dose. However, after the washout, there was a change in blood pressure and subjective responses, although this was not as marked as in naïve subjects. It is important to note that tolerance did not develop to the relaxant and antiemetic effects of nabilone, as has been confirmed by clinical trials (6,7,61). These trials have also found that compared with THC, nabilone produces less tachycardia and less euphoria for a similar antiemetic response (70). CONCLUSIONS The chemistry of cannabinoids is complex (for a more detailed description, see Grotenhermen [1], Mechoulam et al [2] and Mechoulam [70]) and the information on pharmacokinetics is limited, except for the THC component. Absorption from smoked cannabis is rapid and highly variable, with fast effects on heart rate and a quick attainment of the marijuana ‘high’; these effects may be somewhat controlled by the smoker’s uptake. Absorption from oral dosing is also variable; however, it is much slower and the production of active THC metabolites (first-pass metabolism) leads to some prolonged activity. The synthetic derivative nabilone is rapidly well absorbed from the oral route and also appears to have active metabolites, being more than 90% eliminated in seven days. Less is known about the pharmacokinetics of other cannabis components, and the absorption and fate by other routes, such as rectal or dermal. There is very recent information on dermal uptake of ∆-8-THC (71) indicating that a constant plasma concentration can be maintained for 48 h. While there have been concerns about possible drug interactions of marijuana or THC (particularly with other central nervous system drugs), and concerns in the treatment of frail patients on many other treatments (eg, AIDS), there is no substantive literature to indicate that these are clinically significant (72). Pain Res Manage Vol 10 Suppl A Autumn 2005 mcgilveray_8902.qxd 8/30/2005 4:26 PM Page 21 Pharmacokinetics of cannabinoids REFERENCES 1. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42:327-60. 2. Mechoulam R, Devane WA, Glaser R. Cannabinoid geometry and biological activity. In: Nahas GG, Sutin KM, Harvey D, Agurell S, eds. Marihuana and Medicine. Totowa, New Jersey: Humana Press, 1999:65-90. 3. Joy JE, Watson SJ Jr, Benson JA Jr. Marijuana and medicine: Assessing the science base. <www.nap.edu/books/0309071550/html/> (Version current at June 20, 2005). 4. Garrett ER, Hunt CA. Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol. J Pharm Sci 1974;63:1056-64. 5. Marinol® monograph. Compendium of Pharmaceuticals and Specialties (CPS), Canadian Pharmacists Association, Ottawa, 2003:949. 6. Lemberger L. Nabilone, a synthetic cannabinoid of medical utility. In: Nahas GG, Sutin KM, Harvey D, Agurell S, eds. Marihuana and Medicine. Totowa, New Jersey: Humana Press, 1999:561-66. 7. Cesamet® monograph. Compendium of Pharmaceuticals and Specialties (CPS), Canadian Pharmacists Association, Ottawa, 2003:332. 8. Razdan RK. Structure-activity relationships in cannabinoids. Pharmacol Rev 1986;38:75-149. 9. Martin BR, Mechoulam R, Razdan RK. Discovery and characterization of endogenous cannabinoids. Life Sci 1999;65:573-95. 10. Martin BR, Jefferson R, Winckler R, et al. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J Pharmacol Exp Ther 1999;290:1065-79. 11. Brenneisen R. [Psychotropic drugs. II. Determination of cannabinoids in Cannabis sativa L. and in cannabis products with high pressure liquid chromatography (HPLC)]. Pharm Acta Helv 1984;59:247-59. 12. Agurell S, Leander K. Stability, transfer and absorption of cannabinoid constituents of cannabis (hashish) during smoking. Acta Pharm Suec 1971;8:391-402. 13. Agurell S, Halldin M, Lindgren JE, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 1986;38:21-43. 14. Pitts JE, Neal JD, Gough TA. Some features of Cannabis plants grown in the United Kingdom from seeds of known origin. J Pharm Pharmacol 1992;44:947-51. 15. Health Canada. http://www.hc-sc.gc.ca/hecs-sesc/marihuana/ pdf/qc-result-cq_e.pdf> (Version current at August 3, 2005). 16. Huestis M. Pharmacokinetics of THC in inhaled and oral preparations. In: Nahas GG, Sutin KM, Harvey D, Agurell S, eds. Marihuana and Medicine. Totowa, New Jersey: Humana Press, 1999:105-16. 17. Fehr KO, Kalant H. Analysis of cannabis smoke obtained under different combustion conditions. Can J Physiol Pharmacol 1972;50:761-7. 18. Truitt EB Jr. Biological disposition of tetrahydrocannabinols. Pharmacol Rev 1971;23:273-8. 19. Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther 1980;28:409-16. 20. Perez-Reyes M. Marijuana smoking: Factors that influence the bioavailability of tetrahydrocannabinol. NIDA Res Monogr 1990;99:42-62. 21. Davis KH Jr, McDaniel IA Jr, Caldwell LW, Moody P. Some smoking characteristics of marijuana cigarettes. In: Agurell S, Dewey WL, Willette RE, eds. The Cannabinoids: Chemical, Pharmacologic and Therapeutic Aspects. New York: Academic Press, 1984:97-109. 22. Jack A, McKay G. Qualitative and quantitative analysis of the cannabinoid content of combusted plant tissue. Abstract no 36, Canadian Society for Pharmaceutical Sciences, 7th annual symposium, Vancouver, British Columbia, June 9-12, 2004. 23. Iversen LL. The Science of Marijuana. Oxford: Oxford University Press, 2000:37. 24. Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl) 1981;74:208-12. Pain Res Manage Vol 10 Suppl A Autumn 2005 25. Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: Effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther 1992;261:114-22. 26. Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther 1992;52:31-41. 27. Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: Effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther 1995;272:560-9. 28. Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther 1983;34:352-63. 29. Perez-Reyes M, Owens SM, Di Guiseppi S. The clinical pharmacology and dynamics of marihuana cigarette smoking. J Clin Pharmacol 1981;21(Suppl 8-9):201S-7S. 30. Barnett G, Chiang CW, Perez-Reyes M, Owens SM. Kinetic study of smoking marijuana. J Pharmacokinet Biopharm 1982;10:495-506. 31. Johansson E, Agurell S, Hollister LE, Halldin MM. Prolonged apparent half-life of delta 1-tetrahydrocannabinol in plasma of chronic marijuana users. J Pharm Pharmacol 1988;40:374-5. 32. Harvey DJ. Absorption, distribution and biotransformation of the cannabinoids. In: Nahas GG, Sutin KM, Harvey D, Agurell S, eds. Marihuana and Medicine. Totowa, New Jersey: Humana Press, 1999:91-103. 33. Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Single dose kinetics of deuterium labelled delta 1-tetrahydrocannabinol in heavy and light cannabis users. Biomed Mass Spectrom 1982;9:6-10. 34. Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther 1980;215:35-44. 35. Lemberger L, Weiss JL, Watanabe AM, Galanter IM, Wyatt RJ, Cardon PV. Delta-9-tetrahydrocannabinol. Temporal correlation of the psychologic effects and blood levels after various routes of administration. N Engl J Med 1972;286:685-8. 36. Iversen LL. The Science of Marijuana. Oxford: Oxford University Press, 2000:46-7. 37. Unimed Pharmaceuticals Inc. Marinol® US monograph. <http://www.unimed.com/proddisc3.html> (Version current at June 20, 2005). 38. Mattes RD, Shaw LM, Edling-Owens J, Engelman K, Elsohly MA. Bypassing the first-pass effect for the therapeutic use of cannabinoids. Pharmacol Biochem Behav 1993;44:745-7. 39. Brenneisen R, Egli A, Elsohly MA, Henn V, Spiess Y. The effect of orally and rectally administered delta 9-tetrahydrocannabinol on spasticity: A pilot study with 2 patients. Int J Clin Pharmacol Ther 1996;34:446-52. 40. Garrett ER, Hunt CA. Pharmacokinetics of delta-9tetrahydrocannabinol in dogs. J Pharm Sci 1977;66:395-407. 41. Widman M, Agurell S, Ehrnebo M, Jones G. Binding of (+)- and (minus)-delta-1-tetrahydrocannabinols and (minus)-7-hydroxydelta-1-tetrahydrocannabinol to blood cells and plasma proteins in man. J Pharm Pharmacol 1974;26:914-6. 42. Wahlqvist M, Nilsson IM, Sandberg F, Agurell S. Binding of delta-1-tetrahydrocannabinol to human plasma proteins. Biochem Pharmacol 1970;19:2579-84. 43. Widman M, Nilsson IM, Agurell S, Borg H, Granstrand B. Plasma protein binding of 7-hydroxy-1-tetrahydrocannabinol: An active 1-tetrahydrocannabinol metabolite. J Pharm Pharmacol 1973;25:453-7. 44. Nahas G, Leger C, Tocque B, Hoellinger H. The kinetics of cannabinoid distribution and storage with special reference to the brain and testis. J Clin Pharmacol 1981;21(8-9 Suppl):208S-214S. 45. Nahas GG, Frick HC, Lattimer JK, Latour C, Harvey D. Pharmacokinetics of THC in brain and testis, male gametotoxicity and premature apoptosis of spermatozoa. Hum Psychopharmacol 2002;17:103-13. 46. Iversen LL. The Science of Marijuana. Oxford: Oxford University Press, 2000:51. 47. Perez-Reyes M, Timmons MC, Lipton MA, Davis KH, Wall ME. Intravenous injection in man of 9-tetrahydrocannabinol and 11-OH-9-tetrahydrocannabinol. Science 1972;177:633-5. 48. Christensen HD, Freudenthal RI, Gidley JT, et al. Activity of delta8- and delta9-tetrahydrocannabinol and related compounds in the mouse. Science 1971;172:165-7. 21A mcgilveray_8902.qxd 8/30/2005 4:26 PM Page 22 McGilveray 49. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992;16:276-82. 50. Huestis MA, Mitchell JM, Cone EJ. Urinary excretion profiles of 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in humans after single smoked doses of marijuana. J Anal Toxicol 1996;20:441-52. 51. Martin BR, Cone EJ. Chemistry and pharmacology of cannabis. In: Kalant H, Corrigall W, Hall W, Smart R, eds. The Health Effects of Cannabis. Toronto: Centre for Addiction and Mental Health, 1999:21-68. 52. Martin B, Nordqvist M, Agurell S, Lindgren JE, Leander K, Binder M. Identification of monohydroxylated metabolites of cannabidiol formed by rat liver. J Pharm Pharmacol 1976;28:275-9. 53. Martin B, Agurell S, Nordqvist M, Lindgren JE. Dioxygenated metabolites of cannabidiol formed by rat liver. J Pharm Pharmacol 1976;28:603-8. 54. Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol 2002;135:564-78. 55. Bornheim LM, Everhart ET, Li J, Correia MA. Characterization of cannabidiol-mediated cytochrome P450 inactivation. Biochem Pharmacol 1993;45:1323-31. 56. Wall ME, Brine DR, Perez-Reyes M. Metabolism of cannabinoids in man. In: Braude MC, Szara S, eds. Pharmacology of Marihuana. New York: Raven Press, 1976:93-113. 57. Hollister LE, Gillespie H. Interactions in man of delta-9-tetrahydrocannabinol. II. Cannabinol and cannabidiol. Clin Pharmacol Ther 1975;18:80-3. 58. Hawks RL. The constituents of cannabis and the disposition and metabolism of cannabinoids. NIDA Res Monogr 1982;42:125-37. 59. Ellis GM Jr, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther 1985;38:572-8. 60. Notcutt W, Price M, Blossfeldt P, Chapman G. Clinical experience of the synthetic cannabinoid nabilone for chronic pain. In: Nahas GG, Sutin KM, Harvey D, Agurell S, eds. Marijuana and Medicine. Totowa, New Jersey: Humana Press, 1999:561-6. 22A 61. Ward A, Holmes B. Nabilone. A preliminary review of its pharmacological properties and therapeutic use. Drugs 1985;30:127-44. 62. Rubin A, Lemberger L, Warrick P, et al. Physiologic disposition of nabilone, a cannabinol derivative, in man. Clin Pharmacol Ther 1977;22:85-91. 63. Lemberger L, Rubin A, Wolen R, et al. Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev 1982;9(Suppl B):17-23. 64. Sullivan HR, Hanasono GK, Miller WM, Wood PG. Species specificity in the metabolism of nabilone. Relationship between toxicity and metabolic routes. Xenobiotica 1987;17:459-68. 65. Harder S, Rietbrock S. Concentration-effect relationship of delta-9-tetrahydrocannabiol and prediction of psychotropic effects after smoking marijuana. Int J Clin Pharmacol Ther 1997;35:155-9. 66. Cone EJ, Huestis MA. Relating blood concentrations of tetrahydrocannabinol and metabolites to pharmacologic effects and time of marijuana usage. Ther Drug Monit 1993;15:527-32. 67. Chiang CW, Barnett G. Marijuana effect and delta-9-tetrahydrocannabinol plasma level. Clin Pharmacol Ther 1984;36:234-8. 68. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH). J Anal Toxicol 1992;16:283-90. 69. Lemberger L, Rowe H. Clinical pharmacology of nabilone, a cannabinol derivative. Clin Pharmacol Ther 1975;18:720-6. 70. Mechoulam R. Chemistry of cannabis. Handbook Exp Pharmacol 1981;55:119-34. 71. Valiveti S, Hammell DC, Earles DC, Stinchcomb AL. In vitro/in vivo correlation studies for transdermal delta 8-THC development. J Pharm Sci 2004;93:1154-64. 72. Hollister L. Interactions of marihuana and THC: What we don’t but should know. In: Nahas GG, Sutin KM, Harvey D, Agurell S, eds. Marijuana and Medicine. Totowa, New Jersey: Humana Press, 1999:273-8. Pain Res Manage Vol 10 Suppl A Autumn 2005 beaulieu_8903.qxd 8/30/2005 10:55 AM Page 23 REVIEW Toxic effects of cannabis and cannabinoids: Animal data Pierre Beaulieu MD PhD FRCA P Beaulieu. Toxic effects of cannabis and cannabinoids: Animal data. Pain Res Manage 2005;10(Suppl A):23A-26A. Effets toxiques du cannabis et des cannabinoïdes : données d’études sur animal The present article reviews the main toxic effects of cannabis and cannabinoids in animals. Toxic effects can be separated into acute and chronic classifications. Acute toxicity studies show that it is virtually impossible to die from acute administration of marijuana or tetrahydrocannabinol, the main psychoactive component of cannabis. Chronic toxicity involves lesions of airway and lung tissues, as well as problems of neurotoxicity, tolerance and dependence, and dysregulations in the immune and hormonal systems. Animal toxicity data, however, are difficult to extrapolate to humans. Le présent article passe en revue les principaux effets toxiques du cannabis et des cannabinoïdes chez les animaux. On peut classer les effets toxiques en deux catégories : aigus et chroniques. Selon les études de toxicité aiguë, il est presque impossible de mourir d’une surdose de marijuana ou de tétrahydrocannabinol, principal composant psychoactif du cannabis. La toxicité chronique, de son côté, est associée à des lésions des voies aériennes et des poumons, ainsi qu’à la neurotoxicité, tolérance et dépendance, et à la dysrégulation des systèmes immunitaire et hormonal. Il est cependant difficile d’appliquer aux humains les données sur la toxicité animale. Key Words: Animal; Cannabinoids; Cannabis; Toxicity ince its use in ancient times for therapeutic purposes, cannabis has been known to produce deleterious effects. The adverse effects of cannabis in humans are reviewed in the present supplement to Pain Research & Management (1). Acute effects of cannabis and cannabinoids are well established, while some uncertainties exist with regard to its long-term effects (2). However, as is often the case with cannabis, results of various studies can be interpreted differently depending on the author; for example, some authors may concentrate on the existence of toxic effects, while others may insist that these effects are minor (2). Overall, the most appropriate opinion on the use of medical marijuana may come from the United States Institute of Medicine when they stated that “except for the harms associated with smoking, the adverse effects of marijuana use are within the range of effects tolerated with other medications” (3). It has been suggested that there are 426 chemical entities in the marijuana plant, of which more than 60 are cannabinoids (4). Therefore, it is not surprising that some of these substances can exert adverse effects. Hundreds of studies, starting in the 1970s, have been published on the toxicity of cannabinoids in animals, and it is well beyond the scope of the present article to review them all. We will only concentrate on key animal data regarding the acute and chronic toxic effects of cannabis and cannabinoids. One must keep in mind that most of the animal studies performed have used delta-9-tetrahydrocannabinol (THC) injections of 10 mg/kg to 20 mg/kg, whereas, for an average adult of 70 kg smoking a cigarette containing 15 mg of THC, this corresponds to an administration of 40 µg/kg of THC (5). S ACUTE TOXICITY In animals, the administration of high doses of THC, other cannabinoids or endogenous cannabinoids (endocannabinoids) such as anandamide, produces a typical response characterized by hypothermia, hypolocomotion, catalepsia and antinociception. From studies in knockout animals, it has been shown that the cannabinoid receptor CB1 is responsible for these effects (6). The overall acute toxicity of THC is low. The mean lethal dose (that which kills 50% of animals) of oral THC in rats is 800 mg/kg to 1900 mg/kg depending on sex and strain (7). Animal studies have shown a very large separation (by a factor of more than 10,000) between pharmacologically effective and lethal doses (8). Furthermore, no cases of death were reported following maximum THC oral doses of up to 3 g/kg and 9 g/kg in dogs and monkeys, respectively (8). However, monkeys treated acutely with 128 mg/kg or more intravenously, died from respiratory arrest and cardiac failure, whereas all monkeys survived with doses of 92 mg/kg or less (9). Phillips et al (10) investigated the acute toxicity of pure THC in rats and mice (Table 1). Both rats and mice became ataxic 1 min to 2 min after having received an intravenous injection of THC. If stimulated, they became hyperactive for 1 s to 2 s. The righting reflex was lost and dyspnea progressed to death by respiratory depression. Postmortem examination revealed that all organs (except the lungs, which were congested and edematous) were unremarkable. Survivors were free of toxic signs after 24 h to 72 h. In rodents, low doses of cannabinoids decrease locomotor activities, while higher doses stimulate movements and even higher doses lead to catalepsy (11). Similarly, in mice, Adams and Martin (12) describe a ‘popcorn effect’ in animals treated with THC (sedation associated with a jump in response to a stimulus wthat, in turn, triggers another stimulation and the jump of another mouse, etc). Furthermore, cannabinoids cause an increase in gait width (13) and show rotarod Department of Anesthesiology, CHUM – Hôtel-Dieu, Montréal, Québec Correspondence: Dr Pierre Beaulieu, Department of Anesthesiology, CHUM – Hôtel-Dieu, 3840 rue St-Urbain, Montréal, Québec H2W 1T8. Telephone 514-890-8000 ext 14570, fax 514-412-7222, e-mail pierre.beaulieu@umontreal.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 ©2005 Pulsus Group Inc. All rights reserved 23A beaulieu_8903.qxd 8/30/2005 10:55 AM Page 24 Beaulieu TABLE 1 Dose range of delta-9-tetrahydrocannabinol administered by different routes in animals to produce various effects Spontaneous Decreased activity rectal temperature Antinociception Catalepsy Mouse 1.0 mg/kg (iv) 1.4 mg/kg (iv) 1.4 mg/kg (iv) LD50 1.5 mg/kg (iv) Drug Static discrimination ataxia 42.5 mg/kg (iv) Operant suppression Mortality – – – – 0.6 mg/kg (ip) – – – 482 mg/kg (orally) Rat – – – 6.0 mg/kg (ip) 28.6 mg/kg (iv) 800–1900 mg/kg (orally) Dog – – – – – – 0.2 mg/kg (iv) Monkey – – – – – – – No deaths after doses up to 3 g/kg (orally) 0.2 mg/kg (iv) No deaths after doses up to 9 g/kg (orally). Death after doses of 128 mg/kg or more (iv) Data from Forney (18), Thompson et al (9), and Phillips et al (10). ip Intraperitoneally; iv Intravenously; LD50 Lethal dose (that which kills 50% of animals) impairments in mice after direct injection of synthetic cannabinoids into the cerebellum (14). Low doses of THC or other psychotropic cannabinoids produce a combination of sedative and stimulant effects, whereas higher doses are mainly sedative (15). Animal studies have also found that THC and anandamide cause deficits in shortterm memory in spatial learning tasks (16). These effects are reversed by a cannabinoid CB1 antagonist. In addition, cannabinoids and endocannabinoids reduce motor activity, reduce body temperature, decrease reflex responses and muscle tone, impair the ability to carry out complex behaviour and decrease overt aggressive behaviour, especially in primates for the latter. In isolated heart, THC produces a biphasic effect on heart rate with an initial increase followed by a decrease. THC also decreases coronary blood flow and cardiac contractile force (17). In the whole animal (dogs, cats and rats), THC produces a decrease in blood pressure associated with bradycardia, but these effects may vary with other species. Milzoff et al (cited by Forney [18]) have studied the effects of THC on heart rate, respiratory rate and body temperature in anesthetized rats after doses of 0.625 mg/kg to 10 mg/kg. They have reported decreases in all the parameters measured. Finally, in rodents, THC and, to a lesser degree, other cannabinoids, such as nabilone and cannabinol, reduce intestinal motility by a CB1 receptor-mediated mechanism (19). CHRONIC TOXICITY A great number of chronic and potentially toxic effects of cannabis on various systems have been described. Lung toxicity Animals exposed to varying doses of marijuana smoke for 12 to 30 months show extensive damage to the smaller airways as well as acute and chronic pneumonia. However, rats exposed to marijuana smoke for one year failed to demonstrate any anatomical or functional evidence of emphysema (20). exposure to THC or marijuana extracts persistently alters the structure and function of the rat hippocampus, a paleocortical brain region involved with learning and memory processes (21). It is suggested that both age during exposure and duration of exposure may be critical determinants of neurotoxicity. Periods of cannabis or THC exposure shorter than three months have not yet been demonstrated to produce neurotoxic effects in rats (21). Studies of monkeys with up to 12 months of daily exposure have not consistently reported neurotoxicity, although one must keep in mind that it represents less time of exposure for these animals compared with rats (lifespan of approximately 40 years for monkeys compared with two to three years for rats). Studies of the effects of cannabinoids on neurons in vitro have yielded inconsistent results. Indeed, the mixed reports of neurotoxic and neuroprotective effects of cannabinoids are confusing (22) (Figure 1). TOLERANCE AND DEPENDENCE Phillips et al (10) administered THC to rats intraperitoneally for 30 days at five dose levels ranging from 0 mg/kg to 30 mg/kg. Animals displayed signs of increasing ataxia, lacrimation, diarrhea and depression. There was no evidence of developing tolerance, although Carlini (23) has reported that rats can develop tolerance to the behavioural effects of THC when low doses are administered over a short period of time. Tolerance to the biological effects of THC has been demonstrated in cultured cells and animal species. Using the CB1 receptor antagonist SR141716A, a withdrawal syndrome can be produced in rats, mice and dogs that have been maintained on THC (24). The syndrome includes scratching, licking, arched back and ptosis. However, there is no animal model of cannabis dependence because animals do not typically selfadminister cannabis in the same way as they do with opioids, cocaine or alcohol (24). IMMUNE SYSTEM Neurotoxicity Although the presumed neurotoxic effects of marijuana enter into the legalization argument, surprisingly few experimental studies of marijuana toxicity have been published, at least until recently. Several laboratories have reported that chronic 24A Cell and animal experiments have shown that THC exerts complex effects on humoral and cellular immunity (25). Cannabinoids and endocannabinoids can be considered immunomodulators that have an influence on almost every component of the immune response machinery. Generally, Pain Res Manage Vol 10 Suppl A Autumn 2005 beaulieu_8903.qxd 8/30/2005 10:55 AM Page 25 Toxic effects of cannabinoids in animals endocannabinoids exert a negative action on the onset of a variety of parameters of the immune response, although their role in normal immune homeostasis and the development of immune system disorder is still far from being resolved (25). Guinea pigs and mice have been used extensively as experimental models for documenting the effects of cannabinoids (THC doses in the range of 0.2 mg/kg to 100 mg/kg) on host resistance in the intact animal. It was established that THC has the potential to compromise host resistance to both viruses and bacteria (26). Furthermore, Mishkin and Cabral (27) have demonstrated that the decreased antiviral responsiveness is paralleled by decreased cellular and humoral immunity, suggesting that THC targets specific elements of the immune system involved in antivirus responses. In vitro studies have also demonstrated that cannabinoids alter the functional activities of a variety of immune cell types (28). However, at present, there are no definitive data that demonstrate that these in vitro cellular effects are operative in humans (26). CANCER The antineoplastic activities of THC and its analogue were first observed in the early 1970s (29). Based on the immunosuppressive effects of cannabis, animal studies were originally performed to investigate the possibility that marijuana smoking, or long-term THC treatment, might favour tumour growth. These studies, however, initially produced contradictory results. The data of one study suggested that the growth of a lung carcinoma was enhanced due to CB2 receptor-mediated immune suppression (30). However, in a two-year administration of high oral doses (50 mg/kg) of THC to rats and mice, Chan et al (31) showed that THC treatment tended to increase survival (70% in the treated animals compared with 45% in the untreated controls) and lower the incidence of primary tumours with no marked histopathological alterations in the brain or other organs. Indeed, it is now a fact that cannabinoids inhibit tumour growth in laboratory animals by modulating key cell signalling pathways, thereby inducing direct growth arrest and the death of tumour cells, as well as by inhibiting tumour angiogenesis and metastasis (32). Thus, cannabinoids are selective antitumour compounds that kill tumour cells without affecting their nontransformed counterparts. Therefore, cannabinoids are potential anticancer agents, which appear to be well tolerated in animal studies and which do not produce the generalized toxic effects in normal tissues that limit most conventional agents used in chemotherapy (33). HORMONAL SYSTEM AND FERTILITY Cannabis and THC act on the hypothalamic-pituitary adrenal axis. In animal studies, a multitude of endocrine processes are influenced by these drugs, including adrenocorticotropic hormone, thyroid-stimulating hormone and growth hormone. Indeed, the administration of cannabinoids decreases plasma growth hormone levels, reduces serum thyroid-stimulating hormone levels (by 90% within 60 min of treatment in rats) and stimulates the release of adrenocorticotropic hormone and glucocorticoids (34). Thus, the regulation of blood glucose levels may be affected. THC has been reported to account for the majority of the reproductive hazards of marijuana use. Animal studies in males largely confirm the ability of cannabinoids to suppress Pain Res Manage Vol 10 Suppl A Autumn 2005 Figure 1) Dual effect of cannabinoids on neurons. Cannabinoids may lead to opposite effects on neuron survival/death. Stimulation of cannabinoid receptors located on brain neurons (CB1) provides protection against excitotoxicity (through inhibition of glutamate release and activation of intracellular signalling cascades) or augmentation of excitotoxicity (by decreasing gamma aminobutyric acid [GABA] release from GABAergic inhibitory interneurons). Finally, cannabinoids may exert their neuroprotective effects via a third mechanism which does not involve CB1 receptors. CNS Central nervous system; NMDA N-methylD-aspartate; THC Delta-9-tetrahydrocannabinol. Reproduced with permission from Mechoulam and Lichtman (22) spermatogenesis, to induce aberrations in sperm morphology (35), to reduce the weight of reproductive organs and to decrease the plasma concentration of hormones such as testosterone (an acute dose of THC produces a 65% reduction in plasma testosterone levels by 60 min in the rhesus monkey) (36). These studies suggest that THC inhibits luteinizing hormone and follicle-stimulating hormone secretion, consequently decreasing testosterone production and altering spermatogenesis (34). High THC doses cause a modest increase in abnormally formed sperm (5.3% in mice treated with 10 mg/kg of THC per day for five days compared with 1.5% in controls) (35). In females, THC prolongs the estrous cycle and decreases the proestrous surge of luteinizing hormone inhibiting ovulation (37). Acute cannabinoid exposure inhibits basal prolactin release in monkeys and rats, and blocks the prolactin surge that occurs on the day of proestrus or in response to suckling (34). In addition, exposure to natural cannabis extracts during pregnancy has been correlated with embryotoxicity and specific teratological malformations in rats, hamsters and rabbits (37). Finally, anandamide has been shown to impair pregnancy and embryonic development in mice. However, anandamide has been suggested to have a dual role, with low anandamide levels being associated with implantation and high levels with uterine changes during gestation (37). EXTRAPOLATION OF ANIMAL DATA TO HUMANS Animal studies are important in determining the overall toxicity of compounds such as cannabis and cannabinoids. They allow the use of various animal species, the choice of the route of administration and doses, as well as the duration of the treatment to obtain acute or chronic conditions. Furthermore, it is possible for animal studies to control for confounding factors, and also to allow direct pathological studies of all the organs. 25A beaulieu_8903.qxd 8/30/2005 10:55 AM Page 26 Beaulieu When it comes to extrapolation of the data to humans, the picture is more complex. Three main approaches based on body weight, body surface area and pharmacokinetic data have been used to extrapolate animal data to humans (2). However, none of these approaches is ideal, and sometimes quite puzzling results are obtained. For example, the lethal dose of THC in nonhuman primates turned out to be five- to 10-fold higher than that found in rats and dogs (6). CONCLUSIONS More data are needed on the adverse effects of cannabinoids in animals, especially on controversial issues such as their effects on the brain and immune system. However, one should be very careful when interpreting the results and appying them to humans. The best approach to human toxicology rests on the study of human data. REFERENCES 1. Ware M, Tawfik VL. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manage 2005;10(Suppl A):31A-37A. 2. Grotenhermen F. Review of unwanted actions of cannabis and THC. In: Grotenhermen F, Russo E, eds. Cannabis and Cannabinoids. New York: The Haworth Integrative Healing Press, 2002:233-47. 3. Joy JE, Watson SJ, Benson JA, eds. Marijuana and Medicine: Assessing the Science Base. Washington, DC: Institute of Medicine, National Academy Press, 1999. 4. Dewey WL. Cannabinoid pharmacology. Pharmacol Rev 1986;38:151-78. 5. Collective authorship. Cannabis: Quels effets sur le comportement et la santé? Inserm Ed. 2001. 6. Ledent C, Valverde O, Cossu G, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999;283:401-4. 7. Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol 1973;25:363-72. 8. House of Lords Select Committee on Science and Technology. Cannabis: The scientific and medical evidence. London: The Stationary Office. 1998. 9. Thompson GR, Fleischman RW, Rosenkrantz H, Braude MC. Oral and intravenous toxicity of delta-9-tetrahydrocannabinol in rhesus monkeys. Toxicol Appl Pharmacol 1974;27:648-65. 10. Phillips RN, Turk RF, Forney RB. Acute toxicity of delta-9tetrahydrocannabinol in rats and mice. Proc Soc Exp Biol Med 1971;136:260-3. 11. Sanudo-Pena MC, Romero J, Seale GE, et al. Activational role of cannabinoids on movement. Eur J Pharmacol 2000;391:269-74. 12. Adams IB, Martin BR. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 1996;91:1585-614. 13. Patel S, Hillard CJ. Cannabinoid CB1 receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther 2001;297:629-37. 14. DeSanty KP, Dar MS. Cannabinoid-induced motor incoordination through the cerebellar CB(1) receptor in mice. Pharmacol Biochem Behav 2001;69:251-9. 15. Leweke FM. Acute effects of cannabis and the cannabinoids. In: Grotenhermen F, Russo E, eds. Cannabis and Cannabinoids. New York: The Haworth Integrative Healing Press, 2002:249-56. 16. Iversen L. Cannabis and the brain. Brain 2003;126:1252-70. 26A 17. Trouve R, Nahas G. Cardiovascular effects of marihuana and cannabinoids. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, eds. Marihuana and Medicine. Totowa, New Jeresey: Humana Press, 1999:291-304. 18. Forney RB. Toxicology of marihuana. Pharmacol Rev 1971;23:279-84. 19. Krowicki ZK, Moerschbaecher JM, Winsauer PJ, Digavalli SV, Hornby PJ. Delta9-tetrahydrocannabinol inhibits gastric motility in the rat through cannabinoid CB1 receptors. Eur J Pharmacol 1999;371:187-96. 20. Tashkin DP. Marihuana and the lung. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, eds. Marihuana and Medicine. Totowa, New Jersey: Humana Press, 1999:279-87. 21. Scallet AC. Neurotoxicity of cannabis and THC: A review of chronic exposure studies in animals. Pharmacol Biochem Behav 1991;40:671-6. 22. Mechoulam R, Lichtman AH. Neuroscience. Stout guards of the central nervous system. Science 2003;302:65-7. 23. Carlini EA. Tolerance to chronic administration of Cannabis sativa (marihuana) in rats. Pharmacology 1968;1:135-42. 24. Swift W, Hall W. Cannabis and dependence. In: Grotenhermen F, Russo E, eds. Cannabis and Cannabinoids. New York: The Haworth Integrative Healing Press, 2002;257-68. 25. Parolaro D, Massi P, Rubino T, Monti E. Endocannaboids in the immune system and cancer. Prostaglandins Leukot Essent Fatty Acids 2002;66:319-32. 26. Cabral GA. Immune system. In: Grotenhermen F, Russo E, eds. Cannabis and Cannabinoids. New York: The Haworth Integrative Healing Press, 2002;279-87. 27. Mishkin EM, Cabral G. Delta-9-tetrahydrocannabinol decreases host resistance to herpes simplex virus type 2 vaginal infection in the B6C3F1 mouse. J Gen Virol 1985;66:2539-49. 28. McCoy KL, Gainey D, Cabral GA. delta 9-Tetrahydrocannabinol modulates antigen processing by macrophages. J Pharmacol Exp Ther 1995;273:1216-23. 29. Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA. Antineoplastic activity of cannabinoids. J Natl Cancer Inst 1975;55:597-602. 30. Zhu LX, Sharma S, Stolina M, et al. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol 2000;165:373-80. 31. Chan PC, Sills RC, Braun AG, Haseman JK, Bucher JR. Toxicity and carcinogenicity of delta 9-tetrahydrocannabinol in Fischer rats and B6C3F1 mice. Fundam Appl Toxicol 1996;30:109-17. 32. Guzman M. Cannabinoids: Potential anticancer agents. Nat Rev Cancer 2003;3:745-55. 33. Bifulco M, Di Marzo V. Targeting the endocannabinoid system in cancer therapy: A call for further research. Nat Med 2002;8:547-50. 34. Murphy LL. Hormonal system and reproduction. In: Grotenhermen F, Russo E, eds. Cannabis and Cannabinoids. New York: The Haworth Integrative Healing Press, 2002:289-97. 35. Zimmerman AM, Zimmerman S, Raj AY. Effects of cannabinoids on spermatogenesis in mice. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, eds. Marihuana and Medicine. Totowa, New Jersey: Humana Press, 1999:347-57. 36. Smith CG, Moore CE, Besch NF, Besch PK. The effects of marihuana (Delta-9-THC) on the secretion of sex hormones in the mature male rhesus monkey. Clin Chem 1976;22:1184. 37. Maccarrone M, Falciglia K, Di Rienzo, Finazzi-Agro A. Endocannaboids, hormone-cytokine networks and human fertility. Prostaglandins Leukot Essent Fatty Acids 2002;66:309-17. 38. Martin BR, Compton DR, Thomas BF, et al. Behavioral, biochemical and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 1991;40:471-8. Pain Res Manage Vol 10 Suppl A Autumn 2005 ware_8904.qxd 8/30/2005 11:00 AM Page 27 REVIEW Cannabinoids for the treatment of pain: An update on recent clinical trials Mark Ware MBBS MRCP MSc1, Pierre Beaulieu MD PhD2 M Ware, P Beaulieu. Cannabinoids for the treatment of pain: An update on recent clinical trials. Pain Res Manage 2005;10(Suppl A):27A-30A. Over the past five years, there has been a considerable increase in clinical research on cannabinoid use for a range of pain syndromes. Cannabinoid products are becoming available for research and clinical use, and pharmaceutical industry interest in the potential for cannabinoids in therapeutics is also gaining momentum. The present article summarizes recent clinical trial data in the field of pain management and suggests that the potential for cannabinoid therapy for chronic pain states is encouraging. Clinicians working in pain management should be aware of the options becoming available from the cannabinoid class of medications. Les cannabinoïdes pour le traitement de la douleur : Le point sur les essais cliniques récents Au cours des cinq dernières années, on a effectué beaucoup de recherches cliniques sur l’utilisation des cannabinoïdes dans divers syndromes douloureux. Les dérivés des cannabinoïdes deviennent plus accessibles pour la recherche, leur utilisation clinique se répand et l’industrie pharmaceutique manifeste un intérêt croissant pour leur potentiel thérapeutique. Le présent article fait le point sur les essais cliniques récents dans le domaine du traitement de la douleur et confirme le potentiel des cannabinoïdes dans le traitement de la douleur chronique. Les médecins qui travaillent dans le domaine du contrôle de la douleur doivent être au courant des options qu’offrent les cannabinoïdes en tant que classe pharmacologique. Key Words: Cannbinoid; Cannabis; Clinical trial; Pain; Review he use of cannabinoids for the treatment of acute and chronic pain has a long and well-documented history. As reviewed elsewhere in this supplement, the mechanisms of cannabinoid action, which have recently been identified, provide evidence for a pain pathway mediated by cannabinoids (1). It is now recognized that 10% to 15% of patients with chronic pain use herbal cannabis as part of their treatment (2) and that this may result in reductions in opioid requirements (3-5). The use of cannabis or cannabinoids in the treatment of acute pain has not been as widely reported. Recent advances in cannabinoid pharmacology have resulted in increasing attention on the therapeutic potential of cannabinoids, and a number of preparations have been or are being developed and investigated in randomized controlled trials. The difficulties with conducting clinical trials on pain include the fact that pain is a subjective experience, and pain patients comprise a heterogenous group consisting of many different syndromes with a variety of physical, psychological and social problems. In addition to this, cannabinoids are associated with considerable social stigma, and cannabis for medical purposes has become a major politicolegal issue for many Western governments. Historically, clinical data for the efficacy of cannabinoids in pain relief have been equivocal; a qualitative systematic review published in 2001 (6) concluded “cannabinoids are no more effective than codeine in controlling pain and have depressant effects on the central nervous system that limit their use. Their widespread introduction into clinical practice for pain management is therefore undesirable”. The study was criticized for T reaching conclusions that were not supported by the existing literature (7). Reports of the use of cannabis for pain continue to suggest a role for cannabinoids in pain management; nabilone has been used with reported benefit for chronic pain in clinical practice (8), and case reports (9) and case series (10) suggest that cannabinoids deserve further enquiry. In the past two years, a number of clinical trials have been reported investigating the efficacy of number of therapeutic cannabinoid compounds. The purpose of the present paper is to summarize these recent reports, to consolidate our current understanding of the effects of cannabinoids in pain management and to provide a stimulus for further research efforts. METHODS A review of publications relating to the use of cannabinoids in the treatment of pain in humans was conducted. The search was based on the authors’ libraries of material and communications with other investigators. In addition, MEDLINE was searched for clinical trials of cannabinoids for acute and chronic pain; these were summarized and categorized according to the compound used, including naturally occurring and synthetic cannabinoids. RESULTS Acute pain Since 1990, only three clinical studies of cannabinoids have been performed in acute pain, two in human volunteers (11,12) and one on postoperative pain patients (13). 1McGill University; 2Université de Montréal, Montreal, Quebec Correspondence: Dr Mark Ware, E19.145 Montreal General Hospital, 1650 Cedar Avenue, Montreal, Quebec H3G 1A4. Telephone 514-934-8222 ext 4386, fax 514-934-8096, e-mail mark.ware@muhc.mcgill.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 ©2005 Pulsus Group Inc. All rights reserved 27A ware_8904.qxd 8/30/2005 11:00 AM Page 28 Ware and Beaulieu In 2000, Greenwald and Stitzer (11) reported a study of the antinociceptive properties of smoked cannabis in recreational drug users. The volunteers were healthy regular marijuana users. Of the 13 participants enrolled, only five male volunteers completed the entire study, which consisted of three test sessions, during which they smoked cigarettes containing 0% (placebo) and 3.55% delta-9-tetrahydrocannabinol (THC) (active). Cannabis smoking was divided in four bouts, during which participants inhaled nine puffs (zero, three, six or nine active puffs separated by 40 min each). Patients were also given naltrexone (an opioid antagonist) – 0 mg, 50 mg or 200 mg orally – 1 h before the first smoking session, to assess whether endogenous opioids influence cannabis effects in humans. Test sessions consisted mainly of antinociceptive measures using finger withdrawal from radiant heat stimulation, a procedure in humans that is comparable with a tail-flick assay. Overall, cannabis produced significant dose-dependent antinociception that was not antagonized by naltrexone. The effect was weak and only significantly present at the highest dose. Despite these positive results, the authors argued that because all participants were relatively frequent cannabis users, a greater tolerance to the antinociceptive effects of cannabis smoking may have developed. The analgesic effect of oral THC, morphine or their combination was reported in healthy subjects under experimental pain conditions (12). This was a randomized, placebo-controlled, double-blind, crossover study involving 12 healthy cannabis-naïve volunteers (six female and six male). Each subject orally received either 20 mg THC (dronabinol), 30 mg morphine, a mixture of 20 mg THC and 30 mg morphine, or placebo as a single dose. The between-session washout phases were at least seven days. Pain tests consisting of pressure pain tolerance, heat pain (thermode), ice-cold immersion, and single and repeated transcutaneous electrical stimulation were performed every hour up to 8 h post-drug administration. Furthermore, side effects and vital functions were monitored, as well as plasma concentrations of THC and its metabolites. In the heat test, neither morphine nor THC produced any analgesic effect. In the cold test, morphine alone and the combination morphine/THC were analgesic, but not THC alone, whereas in the pressure test, only morphine alone was analgesic. Finally, in the electrical stimulation test, morphine increased the pain detection threshold during single mode stimulation, while morphine alone and in combination with THC was analgesic during the repeated mode of stimulation. Drug administration was usually associated with mild side effects, with most patients feeling sleepy and confused after THC or THC/morphine administration. Interestingly, side effects of THC (in particular, euphoria, hallucinations and confusion) were lowered in the presence of morphine, and in the reverse, THC decreased nausea and vomiting associated with morphine when the two were given together. Taken together, these results illustrate that THC provides poor pain control in this battery of acute pain tests, which are characteristic of superficial pain (heat test and electrical stimulation) and more deep pain (pressure and cold test). However, opioids and non-steroidal anti-inflammatory drugs are known to provide no analgesia in the heat and cold tests, respectively. Furthermore, none of the experimental pain tests produced inflammation or tissue damage. Therefore, as the authors mentioned in their discussion, it is not possible to rule out the fact that THC would have an analgesic effect after induction of 28A inflammation, or tissue or nerve damage. Finally, the plasma levels of THC in this study were very low; therefore, THC given with a better bioavailability could increase its analgesic effect and should be tested further. A randomized clinical study (13) looking at postoperative pain showed a lack of efficacy of oral THC in women undergoing elective total abdominal hysterectomy. After surgery, all patients used a patient-controlled analgesia (PCA) device with morphine for 24 h. After this time, the PCA was discontinued, and any patient requesting further analgesia was randomly assigned to receive either 5 mg THC capsules or placebo. The primary outcome measure was the sum of the pain intensity differences over a 6 h period, while the second outcome measure was time to request for rescue analgesia in the form of oral codeine (30 mg). Twenty patients were recruited in each group. No differences in mean sum of the pain intensity differences scores were found between the THC and placebo groups. The only side effect reported to be different between the groups was an increased awareness of surroundings in the THC group. The lack of efficacy of THC should be put into context of a study with a small number of patients using a small dose of THC as a single dose at day 2 after surgery. This study needs to be repeated using larger doses of THC (see below) or a combination of cannabinoids for a longer period of time before cannabinoids can be excluded from being effective in postoperative pain management. Chronic pain Ajulemic acid: Karst et al (14) conducted a small randomized controlled trial of 1’,1’-dimethylheptyl-delta-8-THC-11-oic acid in 21 patients with chronic neuropathic pain. In this crossover study, 20 mg of 1’,1’-dimethylheptyl-delta-8-THC11-oic acid twice daily for four days followed by 40 mg twice daily for three days was associated with reduced pain measured 3 h after intake of the study drug compared with placebo. Eight hours after intake of the drug, the pain scale differences between groups were not significant. Adverse effects were transient dry mouth and tiredness, with no serious adverse event reported. Cannabis extracts (sublingual): Berman et al (15) conducted a randomized controlled trial of two cannabis-based medicinal extracts for neuropathic pain resulting from brachial plexus avulsion. In this crossover trial, 48 patients received THC, a mixture of THC/cannabidiol (CBD) or placebo in a sublingual spray over a six-week period. Both THC and THC/CBD improved sleep quality, pain and quality of life. Wade et al (16) conducted a series of double-blind, randomized, placebo-controlled, single-patient, crossover trials with two-week treatment periods in patients with intractable neurogenic symptoms. Patients had a range of disorders, including multiple sclerosis, spinal cord injury, brachial plexus avulsion and amputation. The patients were administered whole-plant extracts of THC, CBD, a combination of the two or a matched placebo by sublingual spray at doses determined by titration against symptom relief or unwanted effects within the range of 2.5 mg/24 h to 120 mg/24 h. Pain relief from THC and CBD was superior to placebo, while impaired bladder control, muscle spasms and spasticity were improved in some patients. Three patients had transient hypotension and intoxication with rapid initial dosing of THC. Notcutt et al (17) conducted a series of ‘n-of-1’ studies using a sublingual cannabis extract in 34 patients with chronic Pain Res Manage Vol 10 Suppl A Autumn 2005 ware_8904.qxd 8/30/2005 11:00 AM Page 29 Cannabinoids for the treatment of pain: Update pain. Three cannabis-based medicine extracts (THC, CBD and a 1:1 mixture of both) were given over a 12-week period. After an initial open-label period, the cannabis-based medicine extracts were used in a randomized, double-blind, placebocontrolled, crossover trial. The extracts that contained THC proved most effective in symptom control. Regimens for the use of the sublingual spray emerged and a wide range of dosing requirements was observed. Side effects were common and were generally acceptable and similar to other psychoactive agents that are used for chronic pain. As the present paper went to press, Health Canada granted conditional approval for the use of a buccal cannabis extract for chronic neuropathic pain associated with multiple sclerosis. Some of the data to support this have been cited above; other data are currently only available in abstract form or are awaiting publication. Prescribing information has been added to the recommendations section of this issue. Cannabis extracts (oral): Zajicek et al (18) reported the results of a large-scale, multicentre, randomized, controlled trial of oral cannabinoids in the treatment of muscle spasticity associated with multiple sclerosis. Six hundred thirty participants were treated at 33 United Kingdom centres with oral cannabis extract (n=211), THC (n=206) or placebo (n=213). The trial duration was 15 weeks. No treatment effect of cannabinoids was observed on the primary outcome of muscle spasticity measured by the Ashworth scale. There was evidence of a treatment effect on patient-reported spasticity and pain. Side effects were principally expected. Svendsen et al (19) reported the results of a small, randomized controlled trial of oral THC (dronabinol) versus placebo in 24 patients with central pain associated with multiple sclerosis. Treatment duration was three weeks with up to 10 mg of THC. Median pain intensity during the last week of treatment was significantly reduced compared with placebo (visual analogue scale 4.0 versus 5.0, P=0.02). Dizziness was the most frequent adverse event associated with THC, especially during the first week of treatment (19). Ongoing studies Two studies with different designs are underway in the same setting of postoperative pain and may shed light into the role of cannabinoids in postoperative pain. The first study, funded by the Medical Research Council (United Kingdom), is a multicentre study called CANPOP (Trial of Cannabis for Acute Post-Operative Pain). Preliminary results of a dose-finding study were presented in September 2003 at a European congress (European Federation of IASP Chapters) in Prague, Czech Republic. Patients undergoing major orthopedic surgery or hysterectomy were recruited and received a capsule from one of the four following groups: cannabis extracts containing mainly THC and CBD (Cannador, Society for Oncological and Immunological Research, Germany), a synthetic delta-9THC (dronabinol), a standard analgesic (acetaminophen) or a placebo. The primary objective of the study was to evaluate pain scores using a visual analogue scale for 6 h following the end of surgery. The dose-finding study showed that a dose of 10 mg of THC (Cannador) was effective (30 patients completed the study and 15 required rescue analgesia), whereas a dose of 5 mg was not (the first 11 patients requested rescue analgesia, so the dose was stopped). At the 10 mg dose, 29 patients were without nausea and one patient had moderate nausea for 2 h. Based on these results, a full-scale study has begun using THC Pain Res Manage Vol 10 Suppl A Autumn 2005 10 mg, and definitive results should be available in a few months. Another study, funded by the Canadian Institute of Health Research, has recently started and is testing the role of a synthetic analogue of THC, nabilone, in postoperative pain, nausea and vomiting (Pierre Beaulieu, Université de Montréal – Centre hospitalier de l’Université de Montréal, Québec). Patients undergoing major (orthopedic, gynecological, plastic or urological) surgery receive capsules of either 1 mg or 2 mg of nabilone, ketoprofen or placebo every 8 h for 24 h while simultaneously receiving morphine PCA. The primary objectives are total quantity of morphine used in 24 h and pain scores at rest and on movement. Finally, the incidence of nausea and vomiting, tolerance of the study medications and quality of sleep are also recorded. This prospective, randomized, doubleblind study will recruit a total of 160 patients. For chronic pain, one pilot, randomized, placebo-controlled study of smoked cannabis is underway at the McGill University Health Centre, Montreal, Quebec. Thirty-two subjects are to be randomly assigned to receive four different cannabis preparations ranging in THC content from 0% to 9.5%. Subjects will be allocated to receive all four preparations in a crossover design trial. In each cycle, they will use 25 mg three times daily for five days, on an outpatient basis, with nine-day washout periods between cycles. The primary outcome is average pain intensity measured during the smoking phase. The study is expected to be completed in late 2005. An additional study is underway in patients with HIVassociated neuropathic pain in San Francisco, California, USA. Preliminary findings of an open-label phase suggest that patients using smoked cannabis obtain some pain relief, but the data have not yet been published. DISCUSSION The present review was not conducted systematically and only refers to studies available through the MEDLINE database. Important trial results or other data that may exist elsewhere may have been missed. However, the publications cited in the present paper are not uniformly positive, and the possibility of publication bias, while always a concern with controversial drugs like cannabis, is assumed to be minimal because the therapeutic potential is of great interest to physicians and regulators, and the direction of any potential bias is not obviously apparent. These reports suggest that there is some therapeutic potential for cannabinoids in acute and chronic pain, but that significant hurdles exist in achieving clinically relevant outcomes with minimal side effects. Much more work is needed to evaluate appropriate outcomes, to develop alternative means of administration and to develop dosing strategies. Further work is needed using new and existing cannabinoids to continue to explore the therapeutic potential of cannabinoids in chronic pain. As with morphine and nonsteroidal anti-inflammatory drugs, cannabinoids may not be very effective in experimental acute pain. Their role in postoperative pain may be more attractive in the presence of tissue injury and inflammation. Cannabinoids may act as adjuvants to opioids to control pain, and also nausea and vomiting (20), which are not trivial symptoms in the postoperative period. From the current evidence, when given in combination, opioids and cannabinoids may reduce each other’s side effects and produce an adequate combination in the treatment of acute pain. 29A ware_8904.qxd 8/30/2005 11:00 AM Page 30 Ware and Beaulieu For chronic pain, the evidence is mounting that cannabinoids may constitute a new class of agents to add to the pharmaceutical toolbox in the management of chronic pain. The effects of cannabinoids on pain, including peripheral and central neuropathic pain states, and spasticity are measurable and meaningful to patients, and suggest that with appropriate prescribing and monitoring, additional benefit to some patients may be expected with cannabinoid therapy. Short-term side effects are well described, and often are the main drawbacks to patient compliance with therapy. More specific information is required on the long-term side effects of cannabinoid therapy, including drugdrug interactions, tolerance, cognitive impairment and risks of addiction, but these issues are not unique to cannabinoids and indeed point to the need for physicians to exercise diligence in following patients on psychoactive medications. SUMMARY The past two years have seen the publication of several important clinical trials that point to the potential role for cannabinoids in pain management, and ongoing work promises to further explore and define these effects. Cannabinoids are worthy of consideration for refractory pain conditions, but it is important to fully inform patients who may embark on this line of therapy of the side effect profile, and to monitor doses used and adverse events carefully. Not surprisingly, the short-term risks are easier to evaluate than the long-term ones. The former do not appear to be excessive. Definite long-term risks exist, particularly for respiratory ailments, cognitive functions and psychosis. While long-term use may be quite sound, it requires active attention to an evolving topic. FINANCIAL SUPPORT: MW is supported by the fonds de la recherche en santé (FRSQ) (Boursier-clinicien junior 1) and holds grants from the Canadian Institutes of Health Research (CIHR). MW has received honoraria from Valeant and AstraZeneca, has sat on advisory boards for Bayer and Valeant, has acted as a consultant to Cannasat, and has conducted research with grants from GW Pharmaceuticals and Valeant. PB is supported by the FRSQ (Boursier-clinicien junior 1), the Research Center of the Centre hospitalier de l’Université de Montréal and holds a grant from the CIHR. REFERENCES 1. Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature 1998;395:381-3. 2. Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain 2003;102:211-6. 3. Holdcroft A, Smith M, Jacklin A, et al. Pain relief with oral cannabinoids in familial Mediterranean fever. Anaesthesia 1997;52:483-6. 4. Chatterjee A, Almahrezi A, Ware M, Fitzcharles MA. A dramatic response to inhaled cannabis in a woman with central thalamic pain and dystonia. J Pain Symptom Manage 2002;24:4-6. 5. Lynch ME, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manage 2003;25:496-8. 6. Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 2001;323:13-6. 7. Iversen L. Cannabinoids in pain management. Few well controlled trials of cannabis exist for systemic review. BMJ 2001;323:1250. 8. Price MAP, Notcutt WG. Cannabis and cannabinoids in pain relief. In: Brown DT, ed. Cannabis: The Genus Cannabis, 1st edn. Amsterdam: Harwood Academic Publishers, 1998:223-46. 9. Hays H. Marijuana for the management of proximal mytonic myopathy. J Pain Symptom Manage 2001;21:267-9. 10. Ware MA, Gamsa A, Persson J, Fitzcharles MA. Cannabis for chronic pain: Case series and implications for clinicians. Pain Res Manage 2002;7:95-9. 11. Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend 2000;59:261-75. 12. Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, 30A 13. 14. 15. 16. 17. 18. 19. 20. Zbinden A, Brenneisen R. The analgesic effect of oral delta-9tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 2003;105:79-88. Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9tetrahydrocannabinol in postoperative pain. Pain 2003;106:169-72. Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: A randomized controlled trial. JAMA 2003;290:1757-62. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: Results of a randomised controlled trial. Pain 2004 Dec;112:299-306. Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 2003;17:21-9. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: Results from 34 ‘N of 1’ studies. Anaesthesia 2004;59:440-52. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomised placebo-controlled trial. Lancet 2003;362:1517-26. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;329:253. Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy-induced nausea and vomiting: Quantitative systematic review. BMJ 2001;323:16-21. Pain Res Manage Vol 10 Suppl A Autumn 2005 ware_8905.qxd 8/30/2005 11:01 AM Page 31 REVIEW Safety issues concerning the medical use of cannabis and cannabinoids Mark A Ware MBBS MRCP MSc1,2, Vivianne L Tawfik BSc2 MA Ware, VL Tawfik. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manage 2005;10(Suppl A):31A-37A. Safety issues are a major barrier to the use of cannabis and cannabinoid medications for clinical purposes. Information on the safety of herbal cannabis may be derived from studies of recreational cannabis use, but cannabis exposure and effects may differ widely between medical and recreational cannabis users. Standardized, quality-controlled cannabinoid products are available in Canada, and safety profiles of approved medications are available through the Canadian formulary. In the present article, the evidence behind major safety issues related to cannabis use is summarized, with the aim of promoting informed dialogue between physicians and patients in whom cannabinoid therapy is being considered. Caution is advised in interpreting these data, because clinical experience with cannabinoid use is in the early stages. There is a need for long-term safety monitoring of patients using cannabinoids for a wide variety of conditions, to further guide therapeutic decisions and public policy. Key Words: Adverse events; Cannabis/cannabinoid; Safety; Therapy hysicians’ concerns about the use of cannabis for medical purposes, particularly in its widely used and unregulated herbal form, are often focused on safety issues. Because herbal cannabis has been used recreationally for many years and has been extensively studied, information on the safety concerns may be obtained by extrapolating results from epidemiological studies. Safety information about medicinal cannabinoid use may also be obtained from preparations of single cannabinoid compounds, which have been approved by regulatory agencies and have been prescribed for more than 20 years. The use of cannabis by patients with diseases such as HIV/AIDS, epilepsy, chronic noncancer pain, glaucoma and multiple sclerosis gives rise to potential safety concerns that are not addressed in observational research on recreational users. Examples of such concerns are potential drug-drug interactions, alterations in the immune functions of immunocompromised patients, and the risk of developing dependency disorders when cannabis is used in a medical context. The present paper is an overview of safety issues regarding medicinal cannabis use. The aim is to promote a meaningful and informed dialogue between patients and health care providers regarding cannabis use. P Innocuité du cannabis et des cannabinoïdes utilisés à des fins médicales Les problèmes d’innocuité représentent un obstacle de taille à l’utilisation du cannabis et des médicaments dérivés des cannabinoïdes à des fins cliniques. Les données d’innocuité relatives à l’utilisation de la plante peuvent en effet provenir d’études sur l’utilisation du cannabis à des fins récréatives. Or, l’exposition au cannabis et ses effets peuvent différer considérablement selon que les utilisateurs le consomment à des fins médicales ou récréatives. Au Canada, on trouve des produits dérivés des cannabinoïdes standardisés et soumis à un contrôle de la qualité et les profils d’innocuité des médicaments approuvés peuvent être consultés par l’entremise du Formulaire canadien. Dans le présent article, l’auteur offre un résumé des principaux enjeux liés à l’innocuité du cannabis dans le but de favoriser un dialogue éclairé entre médecins et patients chez qui on envisage un traitement par cannabinoïdes. La prudence s’impose lorsque l’on interprète les données de la recherche clinique, puisque l’expérience pratique avec les cannabinoïdes en est à ses débuts. Il faudra exercer une surveillance à long terme de l’innocuité des cannabinoïdes chez les patients atteints de divers problèmes de santé pour mieux orienter les décisions thérapeutiques et les politiques en matière de réglementation. METHODS Search strategy The published literature on MEDLINE from 1966 to December 2004 was searched using the medical subject headings “marijuana smoking” and “adverse effects”, and with the limitations of human studies, studies published in English and studies available with abstracts. Papers on the effects of prenatal cannabis exposure on offspring were not reviewed in detail. Abstracts were reviewed by the lead author (MAW), and relevant papers were obtained and reviewed. The quality of the studies was not formally evaluated. Further safety information was obtained from safety summaries previously prepared by both authors in preparation for clinical trials, and further sources were identified from antecedent references. Where multiple studies were found to report on the same safety concerns, the most recent or representative reports were reviewed. Data on the adverse events of cannabinoid drugs that are available on the Canadian market were taken from the 2004 Compendium of Pharmaceuticals and Specialties (1). Data on investigational cannabinoid drugs in development were not considered. Major safety issues were categorized and explored in more detail. 1Montreal General Hospital; 2McGill University, Montreal, Quebec Correspondence: Dr Mark Ware, E19.145 Montreal General Hospital, 1650 Cedar Avenue, Montreal, Quebec H3G 1A4. Telephone 514-934-8222 ext 4386, fax 514-934-8096, e-mail mark.ware@muhc.mcgill.ca Pain Res Manage Vol 10 Suppl A Autumn 2005 ©2005 Pulsus Group Inc. All rights reserved 31A ware_8905.qxd 8/30/2005 11:01 AM Page 32 Ware and Tawfik TABLE 1 Incidence of adverse events of regulated cannabinoids: Probable causal relationships with incidences of greater than 1% Adverse event Dronabinol (%) Nabilone (%) ‘High’ 8–24 38.8 Somnolence 3–10 66 Dizziness 3–10 58.8 Dry mouth 21.6 Euphoria 3–10 Paranoid reaction 3–10 Nausea 3–10 Abdominal pain 3–10 Thinking abnormalities/confusion 3–10 Ataxia 4.0 >1 12.8 Asthenia >1 7.6 Amnesia >1 Anxiety/nervousness >1 Depersonalization >1 Vomiting >1 Palpitations >1 Tachycardia >1 Vasodilation/facial flush >1 Blurred vision 12.8 Sensation disturbance 12.4 Anorexia 7.6 Headache 7.2 Orthostatic hypertension 5.2 Hallucinations 2.0 Data from reference 1 RESULTS One hundred fifty-seven papers were identified from the literature search for adverse effects, of which 79 were felt to be of relevance. The product monographs for nabilone and dronabinol were obtained. Cannabinoid-based drugs A summary of the adverse event profiles obtained from the 2004 Compendium of Pharmaceuticals and Specialties (1) for dronabinol and nabilone are provided in Table 1. The most commonly reported adverse events are a ‘high’, drowsiness, dizziness and dry mouth. For further information, refer to the product monographs of these drugs (2,3). Herbal cannabis Herbal cannabis is most often smoked. Survey data suggest that patients with chronic pain smoke between one and four puffs from a cannabis joint two to three times a day (4) (although larger doses are also known to be used by some patients). The exposures reported in recreational epidemiological and experimental studies range widely, from single exposures to over 20 years of daily heavy cannabis use. The major safety concerns may be divided into those about the quality of the product and those about the administration of the drug itself. Drug administration effects are further divided into effects related to the delivery system and effects directly related to the cannabinoid compounds. 32A Quality concerns Adverse events due to the use of contaminated cannabis were reported only in cannabis smokers. Contamination with Aspergillus has given rise to concerns of lung infections in immunocompromised patients (5-7). Contamination with paraquat (a potent pesticide) has not been associated with adverse effects (8). Contamination with formaldehyde has been reported to impair memory (9) and may be life threatening (10). Cannabis soaked in embalming fluid has been reported to cause phencyclidine-like responses (11). The sharing of cannabis with contaminated smoking paraphernalia has been associated with small outbreaks of tuberculosis (12) and meningococcal disease (13,14). Safety concerns related to cannabis smoking: Respiratory function Cannabis smoking poses a potential health risk. Cannabis smoke has been shown to have qualitatively the same constitution as tobacco smoke but with quantitatively higher concentrations of polyaromatic hydrocarbons, which are known carcinogens (15). Cannabis smoke, like tobacco smoke, contains carbon monoxide, which preferentially binds hemoglobin at the expense of oxygen binding. A higher prevalence of chronic bronchitis symptoms, such as cough, phlegm and wheeze, has also been noted in cannabis smokers (16-19). All symptoms were most evident in heavy, chronic users, defined as those who had smoked more than three joints per day for 25 years or more. There is a published report (20) of four cases of emphysema in adults with a history of cannabis smoking. However, observational surveys of heavy, chronic cannabis use have failed to find any lung damage in long-term smokers (21). Pneumomediastinum and pneumothorax have been reported following the prolonged Valsalva manoeuvre that may accompany cannabis smoking (possibly through rupturing emphysematous bullae) (22-25). Several case studies of young patients with carcinoma of the upper respiratory tract have been published (26). There is concern that heavy cannabis smoking is a causative factor of this type of cancer, which is rare in adults under the age of 60 years, even in those who smoke tobacco and drink alcohol (27,28). One case-control study (29) reported an increased risk of upper respiratory tract cancer due to cannabis smoking; however, two recent case-control studies (30,31) have failed to find any increased risk of oral squamous cell cancers due to cannabis smoking. No association between cannabis smoking and tobacco smoking-related cancers was found in a large retrospective cohort study (32). The effects of exposure to cannabis smoke in low doses in patients using cannabis therapeutically have not been determined. The doses used by patients for symptom relief may be low, and risks increase with heavy, chronic use of cannabis. Acute effects Mood effects: Acute reactions, such as nausea, anxiety, paranoia and disorientation often occur in new cannabis users but are uncommon in regular cannabis users (33). Many patients were considered to be asymptomatic after abstinence from cannabis for four months (34). For patients seeking symptom relief, the psychological high associated with cannabis smoke inhalation may be another unwanted effect, but this moodaltering effect may be an important part of the overall therapeutic response. Euphoria, altered time perception and Pain Res Manage Vol 10 Suppl A Autumn 2005 ware_8905.qxd 9/8/2005 1:28 PM Page 33 Safety issues with the medical use of cannabis/cannabinoids relaxation are acute reactions that disappear within 3 h to 4 h and are considered part of the high (35). Acute toxicity: Unlike opioids, cannabis does not cause central respiratory depression (36). Acute hyperthermia has been reported following cannabis use and jogging on a warm day (37). Overdosing is extremely rare and is usually accompanied by the use of other drugs, such as alcohol. A lethal tetrahydrocannabinol (THC) dose has not been reported. From a purely pharmacological perspective, cannabinoids appear to be very safe. For a more detailed review of toxicity, please see the article by Beaulieu (pages 23A-26A). Doctors at the HaightAshbury clinic in the San Francisco Bay area who work primarily with drug addicts have stated that it is virtually impossible to die of a cannabis overdose (33). Anxiety and panic: Acute anxiety and panic are recognized as possible complications of cannabis use, usually in the new user (35,38). Patients usually respond to reassurance. Cannabis use in a relaxed and supportive atmosphere may help reduce anxiety. In addition, patients appreciate being made aware of likely psychoactive effects on first doses, and gradual titration to an effective dose may promote tolerance to adverse psychoactive effects. Some subjects may find the anxiety unpleasant enough to stop using cannabis. Feelings of paranoia have been observed among recreational users with bipolar and panic disorders (39). The illegal status of the drug may be an important confounder in this regard. Cardiac and other vascular effects: The cardiovascular effects of cannabis were recently reviewed (40). An increased relative risk of nonfatal myocardial infarction in the first hour following cannabis smoking has been described (41). Myocardial infarction following cannabis use and Viagra (Pfizer Canada Inc) consumption has been reported (42). The increase in heart rate following cannabis use may play a role by increasing myocardial oxygen demand, but other factors, such as plaque rupture and arrhythmias, may also have an effect (43-45). Cannabis smoking-induced tachycardia may be problematic for patients with comorbid ischemic heart disease or arrhythmias. It has been found that inhalation of cannabis smoke reduces the amount of exercise required to cause an attack of angina by 50% (46). Cannabis-induced tachycardia is reduced by clonidine, an alpha-2 agonist, which suggests that THC may play a role in sympathetic nervous system stimulation (47), although a reduction in parasympathetic tone has also been suggested (48). Cannabis is known to cause postural hypotension immediately after smoking (49). It also causes peripheral vasodilation, resulting in characteristic conjunctival reddening. It is plausible that the increased heart rate and drop in blood pressure may be secondary to a drop in peripheral vascular resistance. In addition, antiretroviral therapy (ie, highly active antiretroviral therapy) has been shown to cause lipid abnormalities in patients with HIV/AIDS. These lipid abnormalities may result in an increased risk for ischemic heart disease, which could be exacerbated by cannabis (50). Transient ischemic attacks (51) and cerebrovascular stroke, usually following acute cannabis use, have also been reported in several case reports (52-56). Renal infarction following cannabis smoking has also been reported (57). Cognitive function: Impaired performance has been observed using the circular lights test after subjects smoked two cannabis cigarettes containing 2.8% THC (58). A slight slowing of reaction was found by using digit symbol substitution and automated Pain Res Manage Vol 10 Suppl A Autumn 2005 tracking tests after three doses of 0%, 1.3% and 2.7% THC. Inhalation of cannabis smoke had no effect on performance in the divided attention task (59). Performance measures have shown no dose-related effects on reaction times, but a doseresponse effect on accuracy has been observed (60). Acute cannabis exposure has been associated with a hangover or residual effect on psychomotor performance (61,62). Effects on driving: The literature concerning the risks of cannabis and driving is controversial, and no studies have been published on the effects of medicinal cannabis use on driving. The results are often influenced by the confounding effects of alcohol. Cannabis is known to cause mild euphoria, altered time perception and decreased motor coordination, which affect driving skills. Studies (63) have found that perceptual motor speed and accuracy are impaired after smoking a cannabis joint. However, it has been suggested that, unlike users of alcohol, cannabis users are aware of their level of intoxication and compensate for the effects by becoming very cautious, resulting in a decrease in the speed and the frequency of overtaking, as well as an increase in the following distance (64). It is recognized that cannabis may have significant effects on driving ability, with exaggerated effects in the presence of alcohol (65). Seizures: Data on the risk of epileptic seizures following cannabis use were nonconclusive (66). Long-term effects Dependency: The risk of cannabis dependency is an important consideration when contemplating its medical use. In the present supplement, this is discussed further by Gourlay (pages 38A43A). Although dependence on cannabis has been described, it is difficult to quantify the extent of this risk. Cannabis has a lower rate of conditional dependence (the risk of developing dependence among those who have used the drug) than alcohol, cocaine, heroin or tobacco, although the rate increases with the amount used (67). Substance abuse rarely begins with therapeutic use alone, as the experience with opioid analgesics has shown (68). Withdrawal symptoms such as cravings, irritability, anxiety, depression, reduced appetite and poor sleep after withdrawal from oral THC and smoked cannabis have been described (69-71), but the symptoms are limited to heavy, chronic users and are relatively short lived (72). The abuse potential of nabilone (73) and dronabinol (74) have been examined and there is no published evidence that these drugs are prone to abuse or diversion (however, see the article by Gourlay). Long-term monitoring data on addictive behaviours are needed. Cognitive function: The effects of long-term cannabis use on cognitive function remain controversial (75,76). In chronic users (10 to 15 years), cognitive impairments, such as deficits in memory of word lists, compared with nonusers are observed, but these resolve after 30 days of abstinence and may be related to acute effects (77). A meta-analysis (78) of the residual neurocognitive effects of cannabis use reported decreases in the performance of memory tasks. A recent study (79) suggested that patients with advanced HIV/AIDS may be at risk for aggravated memory impairment due to cannabis use. The long-term effects of cannabis use on neurocognitive function may be due to a direct effect of cannabis use or may be due to confounding effects (80); further research is required to draw conclusions. Drug interactions: THC and other cannabinoids are metabolized by enzymes that are also responsible for the metabolism of 33A ware_8905.qxd 8/30/2005 11:01 AM Page 34 Ware and Tawfik commonly prescribed medicines. This may potentially result in important drug-drug interactions. At least two cytochrome (CY) P450 enzyme systems, CYP2C and CYP3A, have been shown to be involved in the metabolism of cannabinoids (81). Recently, it was found that although delta-9-THC and antiretroviral drugs are metabolized by CYP3A, administration of THC (smoked or orally) does not significantly reduce plasma concentrations of antiretroviral drugs in patients with HIV/AIDS (82). Potential interactions with tricyclic antidepressants have been reported (83-85), but a conclusive link has not been established. Immunity: It is apparent that delta-9-THC has immunomodulating effects, but the related health risks are not well defined. The dose required to obtain such effects is greater than that required for psychoactive or therapeutic effects (86). In a randomized, double-blind study of the effects of smoked cannabis (3.95% THC, 1 g three times daily) and oral THC (2.5 mg three times daily) administered over 21 days in patients with HIV/AIDS, neither the smoked nor the oral THC had a significant effect on CD4 cell counts or viral loads compared with placebo (82). Nausea and vomiting: A recent case series (87) described a cyclical vomiting syndrome associated with chronic, heavy cannabis use (‘cannabinoid hyperemesis’), which was linked to an abnormal washing behaviour. Psychological effects: While cannabis use is associated with depression (88) and anxiety (89), a causative link has not been established. A recent systematic review (90) did not find a strong association between chronic cannabis use in young people and psychosocial harm. Long-term cognitive effects: The presence of long-term cognitive effects following chronic, heavy use has been shown (75,78), particularly in the domains of memory and learning, and there is debate over whether these effects are reversible (91,92). Under medical use conditions, the relevance of these effects has been questioned (78). Psychosis and schizophrenia: An association between cannabis use and an increased risk of psychosis and schizophrenia has been reported (93). In a study by Zammit et al (93), cannabis use was found to be a risk factor for developing schizophrenia (in a dose-dependent manner). Cannabis has also been shown to be associated with a schizotypal personality disorder (94), but the direction of this association is unclear. Cannabis has been shown to be a risk factor in the development of psychotic symptoms in young people, particularly among those with a predisposition for psychosis (95). Recent modelling studies (96) have suggested that daily cannabis use is causally associated with the development of psychosis. ‘Cannabis psychosis’ (97) has been shown to be clinically distinct from acute schizophrenia, with a shorter duration and high rates of remission (98); however, one report (99) has questioned the existence of cannabis psychosis disorder. A recent retrospective study (100) and a review (101) have confirmed the association between cannabis use and precipitation of schizophrenia in predisposed people and in people without a history of schizophrenia. Effects on pregnancy: The effects of cannabis on the reproductive system in humans are uncertain because the published evidence is limited and inconsistent (35). Results from human epidemiological studies are difficult to interpret because cannabis users are more likely than nonusers to smoke tobacco, drink alcohol and use other illicit drugs during pregnancy. 34A Cannabis use during pregnancy has been correlated with low birth weight (102,103), prematurity (104) and intrauterine growth retardation (105), although contradictory findings have also been reported (106). Frequent maternal cannabis use may be a weak risk factor for sudden infant death syndrome (107). Risk of death: In one large retrospective cohort study of patients with HIV/AIDS (108), current cannabis use was not associated with an increased risk of non-AIDS death in men (RR=1.72, 95% CI 0.89 to 1.39); however, it was associated with an increased risk of AIDS-related death (RR=1.90, 95% CI 1.33 to 2.73) when compared with nonusers and experimental users of cannabis. For women, current cannabis use was not associated with total mortality (RR=1.09, 95% CI 0.80 to 1.49) (107). It is not clear whether the use of cannabis was causally related to AIDS-related mortality or whether cannabis smoking was used to treat worsening symptoms and was a confounder in this analysis. Vascular effects: Peripheral arteritis, which is analogous to Buerger’s disease in tobacco smokers, has been reported in several case reports (109,110). DISCUSSION The use of cannabis in any form poses potential health risks that are well described (although some remain controversial). Cannabis smoking clearly poses unique risks, both from the smoke and from potential contamination. The use of standardized and quality-controlled cannabis preparations with accurate monitoring and follow-up may identify and reduce these risks. The use of pharmaceutical cannabis preparations has risks that are well documented on product labels, but further research is required on the long-term effects of these products. The safety of cannabinoids in children, the elderly and patients with comorbid disorders (eg, diabetes, hypertension, ischemic heart disease, renal and hepatic impairment, and diseases that damage the immune system), as well as the effects of cannabinoid use on concurrent psychiatric illness (eg, depression, anxiety, psychosis and drug abuse) are all subjects for further research. Some broad clinical recommendations based on existing safety information may be put forward, but these must be continuously revised as new data are published. Patients with a history of a psychotic disorder such as schizophrenia should not use cannabis. The use of cannabis during pregnancy should be avoided. Patients with uncontrolled hypertension and active ischemic heart disease should avoid cannabis. Patients using cannabis therapeutically should not drive or operate heavy machinery while experiencing the psychoactive effects of cannabis (consistent with advice concerning the therapeutic use of other psychoactive agents such as benzodiazepines and opioids). Patients with comorbid depression and other psychiatric disorders should be carefully monitored. Cannabinoids should be administered initially at low doses and titrated slowly to balance the positive and negative acute effects. Patients should be advised of the nature and likelihood of acute effects, and close monitoring is advised during the initial dose titration. Most of our current knowledge about the risks of herbal cannabis is derived from studies of recreational users, and these risks may or may not be relevant in a medical use paradigm. The doses used may be different, the psychoactive effects at therapeutic doses may have a different impact, and the total lifetime exposure may be different. A considerable cumulative dose response to cannabinoids has been observed in many Pain Res Manage Vol 10 Suppl A Autumn 2005 ware_8905.qxd 8/30/2005 11:01 AM Page 35 Safety issues with the medical use of cannabis/cannabinoids areas and, therefore, some risks apply only to those who use cannabis over a long period of time. CONCLUSIONS Cannabis is used by many patients with a wide range of chronic disorders. Canadian physicians are being asked to support patient applications for authorizations to cultivate and possess cannabis for medical purposes. Physicians need to be able to provide a concise summary of known or suspected risks to their patients. It is hoped that this review will be a useful tool in this regard. SUGGESTED FURTHER READING • Zimmer L, Morgan JP. Marijuana Myths, Marijuana Facts: A Review of the Scientific Evidence. New York: Lindesmith Centre, 1997. • Joy JE, Watson SJ, Benson JA, eds. Marijuana and Medicine: Assessing the Science Base. Institute of Medicine report. Washington: National Academy Press, 1999. • Iversen LL. The Science of Marijuana. Oxford: Oxford University Press, 2000. • Castle D, Murray R, eds. Marijuana and Madness. Cambridge: Cambridge University Press, 2004. • Grinspoon L, Bakalar JB. Marijuana: The Forbidden Medicine. New Haven: Yale University Press, 1997. SUPPORT: Dr Mark Ware is supported by the fonds de la recherche en santé (Boursier-clinicien junior 1) and holds grants from the Canadian Institutes of Health Research. He has received honoraria from Valeant and AstraZeneca, has sat on advisory boards for Bayer and Valeant, has acted as a consultant to Cannasat, and has conducted research with grants from GW Pharmaceuticals and Valeant. REFERENCES 1. Compendium of Pharmaceuticals and Specialties. Ottawa: Canadian Pharmacists Association, 2004. 2. Product monograph: Marinol. <www.solvaypharma.ca/en/ products/HCP/Marinol.asp> (Version current at June 29, 2005). 3. Cesamet product monograph. In: CPS: Compendium of Pharmaceuticals and Specialties. Ottawa: Canadian Pharmacists Association, 2004:409-10. 4. Ware MA, Gamsa A, Persson J, Fitzcharles MA. Cannabis for chronic pain: Case series and implications for clinicians. Pain Res Manage 2002;7:95-9. 5. Hamadeh R, Ardehali A, Locksley RM, York MK. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest 1988;94:432-3. 6. Marks WH, Florence L, Lieberman J, et al. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation 1996;61:1771-4. 7. Szyper-Kravitz M, Lang R, Manor Y, Lahav M. Early invasive pulmonary aspergillosis in a leukemia patient linked to aspergillus contaminated marijuana smoking. Leuk Lymphoma 2001;42:1433-7. 8. Landrigan PJ, Powell KE, James LM, Taylor PR. Paraquat and marijuana: Epidemiologic risk assessment. Am J Public Health 1983;73:784-8. 9. Hawkins KA, Schwartz-Thompson J, Kahane AI. Abuse of formaldehyde-laced marijuana may cause dysmnesia. J Neuropsychiatry Clin Neurosci 1994;6:67. 10. Nelson LS, Holland JA, Ravikumar PR. Dangerous form of marijuana. Ann Emerg Med 1999;34:115-6. 11. Holland JA, Nelson L, Ravikumar PR, Elwood WN. Embalming fluid-soaked marijuana: New high or new guise for PCP? J Psychoactive Drugs 1998;30:215-9. 12. Munckhof WJ, Konstantinos A, Wamsley M, Mortlock M, Gilpin C. A cluster of tuberculosis associated with use of a marijuana water pipe. Int J Tuberc Lung Dis 2003;7:860-5. Pain Res Manage Vol 10 Suppl A Autumn 2005 13. Finn R, Groves C, Coe M, Pass M, Harrison LH. Cluster of serogroup C meningococcal disease associated with attendance at a party. South Med J 2001;94:1192-4. 14. Krause G, Blackmore C, Wiersma S, et al. Marijuana use and social networks in a community outbreak of meningococcal disease. South Med J 2001;94:482-5. 15. Hoffmann D, Brunnemann KD, Gori GB, Wynder EL. On the carcinogenicity of marijuana smoke. Recent Adv Phytochem 1975;9:63-81. 16. Bloom JW, Kaltenborn WT, Paoletti P, Camilli A, Lebowitz MD. Respiratory effects of non-tobacco cigarettes. Br Med J (Clin Res Ed) 1987;295:1516-8. 17. Tashkin DP, Coulson AH, Clark VA, et al. Respiratory symptoms and lung function in habitual heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and nonsmokers. Am Rev Respir Dis 1987;135:209-16. 18. Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst 1998;90:1198-205. 19. Sherrill DL, Krzyzanowski M, Bloom JW, Lebowitz MD. Respiratory effects of non-tobacco cigarettes: a longitudinal study in general population. Int J Epidemiol 1991;20:132-7. 20. Johnson MK, Smith RP, Morrison D, Laszlo G, White RJ. Large lung bullae in marijuana smokers. Thorax 2000;55:340-2. 21. Cruickshank EK. Physical assessment of 30 chronic cannabis users and 30 matched controls. Ann N Y Acad Sci 1976;282:162-7. 22. Birrer RB, Calderon J. Pneumothorax, pneumomediastinum, and pneumopericardium following Valsalva’s maneuver during marijuana smoking. NY State J Med 1984;84:619-20. 23. Okereke UN, Weber BE, Israel RH. Spontaneous pneumomediastinum in an 18-year-old black Sudanese high school student. J Natl Med Assoc 1999;91:357-9. 24. Hazouard E, Koninck JC, Attucci S, Fauchier-Rolland F, Brunereau L, Diot P. Pneumorachis and pneumomediastinum caused by repeated Muller’s maneuvers: Complications of marijuana smoking. Ann Emerg Med 2001;38:694-7. 25. Feldman AL, Sullivan JT, Passero MA, Lewis DC. Pneumothorax in polysubstance-abusing marijuana and tobacco smokers: Three cases. J Subst Abuse 1993;5:183-6. 26. Endicott JN, Skipper P, Hernandez L. Marijuana and head and neck cancer. Adv Exp Med Biol 1993;335:107-13. 27. Taylor FM III. Marijuana as a potential respiratory tract carcinogen: A retrospective analysis of a community hospital population. South Med J 1988;81:1213-6. 28. Donald PJ. Marijuana smoking – possible cause of head and neck carcinoma in young patients. Otolaryngol Head Neck Surg 1986;94:517-21. 29. Zhang ZF, Morgenstern H, Spitz MR, et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev 1999;8:1071-8. 30. Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S. An analysis of risk factors for oral cancer in young people: A case-control study. Oral Oncol 2004;40:304-13. 31. Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res 2004;64:4049-54. 32. Sidney S, Quesenberry CP Jr, Friedman GD, Tekawa IS. Marijuana use and cancer incidence (California, United States). Cancer Causes Control 1997;8:722-8. 33. Smith DE, Mehl C. An analysis of marijuana toxicity. Clin Toxicol 1970;3:101-15. 34. Kolansky H, Moore WT. Toxic effects of chronic marihuana use. JAMA 1972;222:35-41. 35. Hall W, Solowij N. Adverse effects of cannabis. Lancet 1998;352:1611-6. 36. Vachon L, FitzGerald MX, Solliday NH, Gould IA, Gaensler EA. Single-dose effects of marihuana smoke. Bronchial dynamics and respiratory-center sensitivity in normal subjects. N Engl J Med 1973;288:985-9. 37. Walter FG, Bey TA, Ruschke DS, Benowitz NL. Marijuana and hyperthermia. J Toxicol Clin Toxicol 1996;34:217-21. 38. Strohle A, Muller M, Rupprecht R. Marijuana precipitation of panic disorder with agoraphobia. Acta Psychiatr Scand 1998;98:254-5. 39. Seibyl JP, Krystal JH, Charney DS. Marijuana (cannabis) use is anecdotally said to precipitate anxiety symptoms in patients with 35A ware_8905.qxd 8/30/2005 11:01 AM Page 36 Ware and Tawfik 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. panic disorder. Is there any research evidence to support this? Also, can marijuana use precipitate or expose paranoia in patients with an underlying bipolar disorder? J Clin Psychopharmacol 1990;10:78. Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol 2002;42(Suppl 11):64S-70S. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805-9. McLeod AL, McKenna CJ, Northridge DB. Myocardial infarction following the combined recreational use of Viagra and cannabis. Clin Cardiol 2002;25:133-4. Kosior DA, Filipiak KJ, Stolarz P, Opolski G. Paroxysmal atrial fibrillation following marijuana intoxication: A two-case report of possible association. Int J Cardiol 2001;78:183-4. Rezkalla SH, Sharma P, Kloner RA. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann Emerg Med 2003;42:365-9. Bachs L, Morland H. Acute cardiovascular fatalities following cannabis use. Forensic Sci Int 2001;124:200-3. Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med 1974;291:65-7. Cone EJ, Welch P, Lange WR. Clonidine partially blocks the physiologic effects but not the subjective effects produced by smoking marijuana in male human subjects. Pharmacol Biochem Behav 1988;29:649-52. Newlin DB, Pretorius MB, Wong CJ, Dax EM. Acute marijuana smoking reduces vagal tone. NIDA Res Monogr 1991;105:565-6. Merritt JC, Cook CE, Davis KH. Orthostatic hypotension after delta 9-tetrahydrocannabinol marihuana inhalation. Ophthalmic Res 1982;14:124-8. Purnell JQ, Zambon A, Knopp RH, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS 2000;14:51-7. Mouzak A, Agathos P, Kerezoudi E, Mantas A, Vourdeli-Yiannakoura E. Transient ischemic attack in heavy cannabis smokers-how ‘safe’ is it? Eur Neurol 2000;44:42-4. Cooles P, Michaud R. Stroke after heavy cannabis smoking. Postgrad Med J 1987;63:511. Zachariah SB. Stroke after heavy marijuana smoking. Stroke 1991;22:406-9. White D, Martin D, Geller T, Pittman T. Stroke associated with marijuana abuse. Pediatr Neurosurg 2000;32:92-4. Finsterer J, Christian P, Wolfgang K. Occipital stroke shortly after cannabis consumption. Clin Neurol Neurosurg 2004;106:305-8. Geller T, Loftis L, Brink DS. Cerebellar infarction in adolescent males associated with acute marijuana use. Pediatrics 2004;113:e365-70. Lambrecht GL, Malbrain ML, Coremans P, Verbist L, Verhaegen H. Acute renal infarction and heavy marijuana smoking. Nephron 1995;70:494-6. Cone EJ, Johnson RE, Moore JD, Roache JD. Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol Biochem Behav 1986;24:1749-54. Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav 1989;34:173-9. Chait LD, Corwin RL, Johanson CE. A cumulative dosing procedure for administering marijuana smoke to humans. Pharmacol Biochem Behav 1988;29:553-7. Chait LD, Fischman MW, Schuster CR. ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Depend 1985;15:229-38. Chait LD. Subjective and behavioral effects of marijuana the morning after smoking. Psychopharmacology (Berl) 1990;100:328-33. Kurzthaler I, Hummer M, Miller C, et al. Effect of cannabis use on cognitive functions and driving ability. J Clin Psychiatry 1999;60:395-9. Gieringer DH. Marijuana, driving, and accident safety. J Psychoactive Drugs 1988;20:93-101. O’Kane CJ, Tutt DC, Bauer LA. Cannabis and driving: A new perspective. Emerg Med (Fremantle) 2002;14:296-303. Gordon E, Devinsky O. Alcohol and marijuana: Effects on epilepsy and use by patients with epilepsy. Epilepsia 2001;42:1266-72. Budney AJ, Moore BA. Development and consequences of cannabis dependence. J Clin Pharmacol 2002;42(Suppl 11):28S-33S. Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet 1996;347:143-7. 36A 69. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141:395-404. 70. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999;141:385-94. 71. Kouri EM, Pope HG Jr. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol 2000;8:483-92. 72. Smith NT. A review of the published literature into cannabis withdrawal symptoms in human users. Addiction 2002;97:621-32. 73. Lemberger L, Rubin A, Wolen R, et al. Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev 1982;9(Suppl B):17-23. 74. Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol). J Psychoactive Drugs 1998;30:187-96. 75. Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002;287:1123-31. 76. Pope HG Jr. Cannabis, cognition, and residual confounding. JAMA 2002;287:1172-4. 77. Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 2001;58:909-15. 78. Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J Int Neuropsychol Soc 2003;9:679-89. 79. Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci 2004;16:330-5. 80. Pope HG Jr, Yurgelun-Todd D. Residual cognitive effects of longterm cannabis use. In: Castle DJ, Murray R, eds. Marijuana and Madness. Cambridge: Cambridge University Press, 2003:198-210. 81. Yamamoto I, Watanabe K, Narimatsu S, Yoshimura H. Recent advances in the metabolism of cannabinoids. Int J Biochem Cell Biol 1995;27:741-6. 82. Abrams DI, Hilton JF, Leiser RJ, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: A randomized, placebo-controlled clinical trial. Ann Intern Med 2003;139:258-66. 83. Hillard JR, Vieweg WV. Marked sinus tachycardia resulting from the synergistic effects of marijuana and nortriptyline. Am J Psychiatry 1983;140:626-7. 84. Wilens TE, Biederman J, Spencer TJ. Case study: Adverse effects of smoking marijuana while receiving tricyclic antidepressants. J Am Acad Child Adolesc Psychiatry 1997;36:45-8. 85. Mannion V. Case report: Adverse effects of taking tricyclic antidepressants and smoking marijuana. Can Fam Physician 1999;45:2683-4. 86. Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol 1998;83:102-15. 87. Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: Cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004;53:1566-70. 88. Green BE, Ritter C. Marijuana use and depression. J Health Soc Behav 2000;41:40-9. 89. Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: Cohort study. BMJ 2002;325:1195-8. 90. Macleod J, Oakes R, Copello A, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: A systematic review of longitudinal, general population studies. Lancet 2004;363:1579-88. 91. Harrison GP Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol 2002;42(Suppl 11):41S-7S. 92. Solowij N. Do cognitive impairments recover following cessation of cannabis use? Life Sci 1995;56:2119-26. 93. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: Historical cohort study. BMJ 2002;325:1199. 94. Williams JH, Wellman NA, Rawlins JN. Cannabis use correlates with schizotypy in healthy people. Addiction 1996;91:869-77. 95. Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ 2005;330:11. 96. Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction 2005;100:354-66. Pain Res Manage Vol 10 Suppl A Autumn 2005 ware_8905.qxd 8/30/2005 11:01 AM Page 37 Safety issues with the medical use of cannabis/cannabinoids 97. Bartolucci G, Fryer L, Perris C, Shagass C. Marijuana psychosis: A case report. Can Psychiatr Assoc J 1969;14:77-9. 98. Basu D, Malhotra A, Bhagat A, Varma VK. Cannabis psychosis and acute schizophrenia. A case-control study from India. Eur Addict Res 1999;5:71-3. 99. McGuire PK, Jones P, Harvey I, et al. Cannabis and acute psychosis. Schizophr Res 1994;13:161-7. 100. van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: A longitudinal population-based study. Am J Epidemiol 2002;156:319-27. 101. Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: A review. Addiction 2004;99:425-30. 102. Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG 2002;109:21-7. 103. Zuckerman B, Frank DA, Hingson R, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med 1989;320:762-8. 104. Fried PA, Watkinson B, Willan A. Marijuana use during pregnancy and Pain Res Manage Vol 10 Suppl A Autumn 2005 decreased length of gestation. Am J Obstet Gynecol 1984;150:23-7. 105. Gibson GT, Baghurst PA, Colley DP. Maternal alcohol, tobacco and cannabis consumption and the outcome of pregnancy. Aust NZ J Obstet Gynaecol 1983;23:15-9. 106. Witter FR, Niebyl JR. Marijuana use in pregnancy and pregnancy outcome. Am J Perinatol 1990;7:36-8. 107. Scragg RK, Mitchell EA, Ford RP, Thompson JM, Taylor BJ, Stewart AW. Maternal cannabis use in the sudden death syndrome. Acta Paediatr 2001;90:57-60. 108. Sidney S, Beck JE, Tekawa IS, Quesenberry CP, Friedman GD. Marijuana use and mortality. Am J Public Health 1997;87:585-90. 109. Disdier P, Granel B, Serratrice J, et al. Cannabis arteritis revisited – ten new case reports. Angiology 2001;52:1-5. 110. Ducasse E, Chevalier J, Dasnoy D, Speziale F, Fiorani P, Puppinck P. Popliteal artery entrapment associated with cannabis arteritis. Eur J Vasc Endovasc Surg 2004;27:327-32. 37A gourlay_8906.qxd 8/30/2005 10:58 AM Page 38 REVIEW Addiction and pain medicine Douglas Gourlay MD FRCPC FASAM D Gourlay. Addiction and pain medicine. Pain Res Manage 2005;10(Suppl A):38A-43A. The adequate cotreatment of chronic pain and addiction disorders is a complex and challenging problem for health care professionals. There is great potential for cannabinoids in the treatment of pain; however, the increasing prevalence of recreational cannabis use has led to a considerable increase in the number of people seeking treatment for cannabis use disorders. Evidence that cannabis abuse liability is higher than previously thought suggests that individuals with a history of substance abuse may be at an increased risk after taking cannabinoids, even for medicinal purposes. Smoked cannabis is significantly more reinforcing than other cannabinoid administration methods. In addition, it is clear that the smoked route of cannabis delivery is associated with a number of adverse health consequences. Thus, there is a need for pharmaceutical-grade products of known purity and concentration using delivery systems optimized for safety. Another factor that needs to be considered when assessing the practicality of prescribing medicinal cannabinoids is the difficulty in differentiating illicit from prescribed cannabinoids in urine drug testing. Overall, a thorough assessment of the risk/benefit profile of cannabinoids as they relate to a patient’s substance abuse history is suggested. La dépendance et les analgésiques Le cotraitement pertinent de la douleur chronique et des troubles de dépendance est un problème complexe et ambitieux pour les professionnels de la santé. Les cannabinoïdes ont un grand potentiel dans le traitement de la douleur, mais la prévalence croissante d’utilisation récréative du cannabis s’associe à une augmentation considérable du nombre de personnes qui cherchent à se faire traiter pour des troubles reliés à l’utilisation du cannabis. Des données démontrant que les dommages reliés à l’usage de cannabis sont plus élevés qu’on le pensait auparavant laissent supposer que les personnes ayant des antécédents d’abus de drogues seraient plus vulnérables après avoir pris des cannabinoïdes, même pour des besoins médicinaux. Le cannabis fumé est un agent considérablement plus renforçant que les cannabinoïdes administrés autrement. En outre, il est clair que la voie d’administration du cannabis fumé entraîne diverses conséquences nocives pour la santé. Ainsi, il est nécessaire de mettre au point des produits de qualité pharmaceutique dont la pureté et la concentration sont connues, au moyen de systèmes de perfusion à l’innocuité optimisée. Un autre facteur dont il faut tenir compte lorsqu’on évalue la possibilité de prescrire des cannabinoïdes médicinaux demeure la difficulté de distinguer les cannabinoïdes prescrits des cannabinoïdes illicites dans les tests de dopage. Dans l’ensemble, une analyse approfondie du profil risque-avantage des cannabinoïdes par rapport aux antécédents d’abus de drogues du patient est suggérée. Key Words: Addiction; Cannabis; Risk management; Substance abuse; Urine drug testing epending on the prevailing attitudes of the time, certain pharmacotherapies have been perceived as either villains or heros. At the crux of this debate has been the risk of substance misuse and addiction in the management of chronic pain. As cannabis enters the field of pain medicine, it must also be examined in this light. D THE PAIN AND ADDICTION CONTINUUM The notion that pain and addiction can coexist is a relatively recent concept. Previously, the literature suggested that the prevalence of addictive disorders in the pain population was so uncommon that it did not merit investigation (1,2). This belief still persists today (albeit much less so) despite the fact that the prevalence of addictive disorders within the general population is typically quoted as 10% (3). The reasons for this are complex. A dichotomous approach to pain and addiction is simple, even if it is potentially incorrect. The only requirement for this approach is to establish a ‘legitimate’ pain diagnosis and, thus, obviate the need to enquire into issues related to substance misuse and addiction. In some circles, asking questions related to drug and alcohol use is seen as tantamount to dismissing the patients’ complaints of pain. In no other area of medicine would such a conclusion be drawn. It is becoming clear that pain and addiction can, and do, coexist (4-7). In understanding the relationship between pain and addiction, part of the problem seems to be nomenclature. Pain specialists and addiction practitioners alike have used common terms that, depending on the individual’s point of view and training, have come to mean entirely different things. To this end, in 1999, the American Pain Society, the American Academy of Pain Medicine and the American Society of Addiction Medicine formed a group called the Liaison Committee for Pain and Addiction. The first task for this group was the creation of a set of definitions for addiction, dependence and tolerance that could be embraced by all three organizations. These definitions were published in 2003 (8). Addiction “Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviours that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm and craving.” (8) Unlike the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (9) definition for addiction, Centre for Addiction and Mental Health, Toronto, Ontario Correspondence: Dr Douglas Gourlay, Addiction Medicine Clinic, Centre for Addiction and Mental Health, 33 Russell Street, Toronto, Ontario M5S 2S1. Telephone 416-535-8501 ext 6754, fax 416-595-6821, e-mail doug-gourlay@camh.net 38A ©2005 Pulsus Group Inc. All rights reserved Pain Res Manage Vol 10 Suppl A Autumn 2005 gourlay_8906.qxd 8/30/2005 10:58 AM Page 39 Addiction and pain medicine physical dependency (withdrawal) and tolerance are not considered in making the diagnosis. The usefulness of the DSM-IV definition is severely reduced when the identified drug of misuse is also a drug which could ultimately be an appropriate part of the patients’ pharmacological management. Although validated tools to assess risk in the context of pain and addiction are being developed (10), at the present time there are no tools that can differentiate between appropriate and problematic use (either misuse or addiction) of a medication where the identified drug of choice may also play a useful role in the management of chronic pain. This is currently the case with opioids used in chronic noncancer pain management (11) and will undoubtedly present a diagnostic challenge when cannabis becomes more widely used to treat chronic pain. Physical dependence “Physical dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.” (8) The phenomenon of physical dependence is an expected, neuroadaptive change that occurs in response to chronic exposure to certain drugs. It is not, in itself, suggestive of anything beyond this. Tolerance “Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.” (8) In the case of tolerance, it is important to remember that it is neither good nor bad; it simply is. In some cases, such as the cognitive blunting effects of opioids, tolerance occurs relatively quickly and is considered positive. On the other hand, tolerance to the constipating effects of opioids virtually never occurs. What is particularly important about these definitions is that the boards of representative organizations from both the pain management and addiction medicine communities have endorsed them. While these definitions represent a compromise, they are an important step forward in reducing the risk of the continued use of imprecise terms in both the pain management and addiction medicine literature. Although the true prevalence of addiction within the chronic pain management population is unknown, the enormous problem that chronic pain represents in the substance abuse treatment population is beginning to be appreciated (12,13). A study by Rosenblum et al (14) found that 37% of patients surveyed within methadone maintenance treatment programs suffered from chronic severe pain. They also concluded that self-medication with psychoactive drugs for pain was especially problematic for substance abusers enrolled in drug-free (abstinence-based) treatment programs. THE DISEASE OF ADDICTION Drug abuse and addiction represent challenges to society at several levels. While it is tempting to focus on the impact of Pain Res Manage Vol 10 Suppl A Autumn 2005 addiction on individualistic health, there is also an adverse impact on public health. In 1998, the Substance Abuse and Mental Health Services Administration estimated that 13.6 million Americans aged 12 years and older had used an illicit drug in the previous month (15). The harm done by this use occurs not only at the level of the brain, but is also seen in increased rates of HIV transmission, hepatitis B and C, and tuberculosis. The societal cost of associated criminality and social dysfunction is difficult to accurately ascertain but has been estimated at US$109 billion annually (16). There are many different factors that lead to drug use including genetic, physiological and even social factors (17). In addition, there are both risk and protective factors that modify the expression of a substance use disorder. Risk factors include weak family structure, peer group pressures and growing up in high-crime neighbourhoods. On the other hand, strong family bonds and academic achievement may be protective (18). By stepping beyond the question of why some people choose to use drugs recreationally and by focusing only on those who do, it is realized that in the at-risk individual, the chronic exposure to certain psychotropic substances causes changes in brain structure and function. Addiction is not only a ‘disease of the brain’ (19) but also a disease with both behavioural and social expression. Treatment of addiction requires changes to be made both within the patient and in the surrounding environment. PREVALENCE OF DRUG USE AND DEPENDENCE There is considerable variation in the prevalence of drug use depending on the drug being considered and the population under study. Alcohol, tobacco and caffeine are the most commonly used psychotropics (20). Drugs like heroin and cocaine are much less commonly used. In general, the use of cannabis has remained relatively constant over recent years; however, it is on the rise among young adults in certain ethnic and racial groups (21). In fact, cannabis has been shown to be the most widely used illicit substance in communities around the world (22-24). In the United States, according to the 2000 United States National Household Survey on Drug Abuse (22), 2.8 million Americans met the criteria in the previous year for either cannabis dependence (1.6 million) or abuse (1.2 million). Furthermore, this survey reported that 25% (713,000) of those with cannabis use disorder actually seek treatment. That is more than those seeking treatment for cocaine, hallucinogens, stimulants, pain relievers, inhalants, tranquilizers, heroin or sedatives (25). In the United States, it is estimated that of those who have ever used cannabis, 10% will develop dependence (26). Of those who try cannabis more than once, approximately one-third will become regular users even though most will have stopped using it by their late 20s or 30s (27). In fact, the relative potential for dependence on cannabis, expressed in terms of conditional dependence (the risk of developing dependence among those who have ever used that substance) (26), is approximately 9%, which is lower than other substances such as alcohol (15%), cocaine (17%), heroin (23%) or tobacco (32%) (26,28). In Canada, the number of Canadians aged 15 years and older who admit to using cannabis nearly doubled between 1989 and 2002, with the highest rate among teenagers (29). In fact, 6.5% of Canadians reported using cannabis in 1989, 7.4% in 39A gourlay_8906.qxd 8/30/2005 10:58 AM Page 40 Gourlay 1994 and 12.2% in 2002. Of those who reported use in the previous year, approximately 47% had used cannabis less than once per month. However, 20% reported regular use, with onehalf of these using it weekly and the other one-half using it daily (29). In the Ontario Student Drug Use Survey of grades 7, 9 and 11 students, 21.8% had used cannabis in the past year in 1977, 9.9% in 1991 and 27.8% in 2003 (30). A recent study (31) in Nova Scotia examining cannabis use in patients suffering from chronic pain showed that 36% had previously used cannabis, 15% had tried it for pain relief and 10% were currently using it for the treatment of their chronic pain. CANNABIS USE Cannabis as a drug with abuse liability For some time, cannabinoids were considered unimportant in brain reward systems, despite the fact that they are clearly euphorigenic in humans and do have abuse liability (32,33). More recent work over the past 15 years has shown cannabinoids to interact with the brain reward systems in a similar fashion to other drugs with addiction liability (34,35). Drugs with addiction liability often share certain qualities, which can be demonstrated in the animal laboratory setting. One of these is the involvement of the endogenous opioid system even for nonopioid addictive drugs. In this case, the rewarding effect of cannabinoids can be attenuated by opioid antagonists, which is characteristic of drugs with addiction liability (32). More recently, Justinova et al (36) demonstrated this attenuating effect of the opioid antagonist naltrexone on the reinforcing effects of delta-9-tetrahydrocannabinol (THC) in squirrel monkeys. Finally, in previously dependent but now abstinent animals, drugs with addiction liability are often able to reinstate drugseeking and self-administration behaviour in response to a ‘priming’ dose of the drug. Although there is a paucity of data in this area, there is evidence that the intravenous priming of former heroin-dependent rats with cannabinoid agonists can lead to reinstatement of drug-seeking behaviour (32,37). Recently, it has been demonstrated with cannabis-naïve squirrel monkeys that THC can act as an effective reinforcer of drug-taking behaviour in monkeys with no history of exposure to other drugs. This suggests that self-administration of THC by monkeys may provide a reliable animal model of human cannabis abuse (38). Thus, it would be reasonable to conclude that cannabinoids share many of the qualities demonstrated in other drugs, which are clearly seen as having a high addiction liability (33). This becomes important when assessing risk in the use of this class of drug for the management of pain, especially in those individuals with a history or an increased risk of a substance use disorder. Cannabis dependence, tolerance and withdrawal The existence of a definable withdrawal syndrome with cannabis has long been questioned. Before the publishing of the DSM-IV, cannabis dependence did not exist as a specific diagnostic category (34). More recently, research has begun to characterize this phenomenon more clearly. Tolerance to cannabis can develop after periods of daily use as short as one to three weeks. The resultant withdrawal symptoms that occur on abrupt discontinuation of cannabis typically begin after 24 h, peak at 72 h and are usually over within seven to 10 days (39). The symptoms are nonspecific and include irritability, anxiety, physical tension, and decreases in mood and appetite. In some cases, there is 40A restlessness, tremors, sweating, insomnia and even vivid dreams. The time frame is highly variable and appears to be similar to that seen with other drugs of abuse (40). Cannabis use disorders Recent studies in the United States have shown that more people are self-identifying themselves as having cannabis use disorders (as indicated by a tremendous increase in admissions to publicly funded treatment programs). The demand for treatment from health professionals, however, remains low relative to the apparent problem. In 1999, the United States Treatment Episode Data Set (41) recorded more than 220,000 admissions for cannabis use disorder treatment, which represents 14% of all admissions to these facilities – a doubling in rate compared with 1993 (42). By 2000, cannabis admissions accounted for 61% of all adolescent admissions. These trends are also observed in Australia, where they have seen a doubling in the national rates of cannabis treatment-seeking individuals from 2000/2001 to 2001/2002, with an overall rate of 21% and a specific rate of 45% in those younger than 20 years of age (43). There has also been a concomitant increase in cannabisrelated emergency room visits in the United States. Adjusting for population changes, this represents a 139% increase in reported visits for the period of 1995 to 2002 (44). In a recent article (21) examining the prevalence of cannabis use disorders in the United States duing 1991/1992 and 2001/2002, the authors indicate that despite an overall stable prevalence of cannabis use, more adults in the United States had a cannabis use disorder in 2001/2002 compared with 1991/1992. While these rates did not increase for young, white men and women, their rates of use remained high (34.4% for 2001/2002). In contrast, the rates of cannabis abuse or dependence increased by 18% from 30.2% in 1991/1992 to 35.6% in 2001/2002 across the general population surveyed (21). Two important factors were identified that might help to explain the increase in cannabis use disorders. The first is the significant increase in the potency of cannabis from police seizures over the time period of 1992 to 2002 (45). In the absence of any systematic increase in frequency or quantity of cannabis used over the time frame studied, the increasing rates of cannabis use disorders may reflect an increased potency of the cannabis used (21). Although research clearly indicates that concentrations of seized cannabis have been rising, the actual percentage increase has been difficult to assess (45,46). The cannabinoid concentration of cannabis seized in Canada does appear to have been increasing over the years. Although the concentrations quoted vary widely, depending on the sample and method of analysis, the average cannabinoid concentration of cannabis seized in the 1980s was less than 1%, while the average over the period of 1996 to 2000 was 6.7% (47). The use of selective breeding and hydroponic cultivation has dramatically increased the potency of today’s cannabis (48). Information from ‘street seizures’ in Canada from April 2000 to April 2002 indicates a mean THC content of 10% with a range of 3% to 30% (49). According to the World Health Organization, the average joint contains 0.5 g to 1.0 g of cannabis plant matter (50). This makes the estimation of ‘average dose’ exceedingly difficult. The second possible factor to explain increased cannabis use disorders relates to the increase in use observed in the younger age groups. Because an early onset of drug use has been consistently associated with an increased risk of developing a Pain Res Manage Vol 10 Suppl A Autumn 2005 gourlay_8906.qxd 8/30/2005 10:58 AM Page 41 Addiction and pain medicine substance use disorder (51-53), the increase in duration of use associated with this younger using population may help to account for the increased prevalence in rates of cannabis abuse and dependence (21). Methods of cannabis use Cannabis is used in a wide variety of ways. Most commonly, it is smoked either alone as a cannabis cigarette or mixed with tobacco (27). It can also be refined into a smokable resinous mass (hashish) or further refined into a potent oil (‘hash oil’ or ‘honey oil’) that is either smoked with tobacco-containing products or vaporized with a constant-temperature device (54). In some cultures, cannabis is taken orally as either an ingredient in foods (55) (eg, ‘hash brownies’) or as a tea (‘ganja tea’). The mode of delivery directly affects the speed of onset and duration of action of the desired (and sometimes undesired) effects of this drug and, therefore, affects the abuse liability. The speed of drug delivery also affects the reinforcing nature of a drug and, hence, its abuse liability. The inhaled route is a fast and effective method of delivering precise quantities of drug at the instant the individual desires it (56). With inhalation (ie, smoked), the drug is delivered into the cerebral vascular circulation very rapidly. Coupled with the fact that the user can titrate the quantity of drug delivered by the degree of force used during inhalation and the time that the breath is held, the inhaled route is a fast and very reinforcing means of delivering substances to the human brain (57). Smoking as a delivery system It is important to separate the drug from the delivery system in the assessment of risk. As presented in other articles in this symposium, there is significant potential for cannabinoids in the treatment of pain. In addition, it is clear that the smoked route of delivery is potentially associated with a number of adverse health consequences (58). Thus, it is a priority to minimize the risks of smoking. Appreciating this point, both natural plant extract (59,60) and synthetic oral cannabinoids have been developed (61). SYNTHETIC CANNABINOIDS At the present time, two synthetic cannabinoids are available for medical use in Canada. The first is a true synthetic THC, dronabinol (Marinol, Solvay Pharma Inc, Canada) (62), and the other is a synthetic cannabinoid called nabilone (Cesamet, Valeant Canada limitée/Limited) (63). Marinol Marinol was first approved for use in the United States in 1985 and it is currently available for two specific indications: the treatment of anorexia associated with weight loss in patients with AIDS, and the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments (62). Marinol is a synthetic THC dissolved in sesame seed oil and, when taken orally, the drug is unpredictably absorbed from the gastrointestinal tract. In contrast to the inhaled route of administration, there is very little opportunity for the patient to titrate the dose to effect. Adverse side effects are common (64). In the past, it has been reported that the abuse liability of Marinol is low (65); the authors support this by stating that the drug lacks the desirable qualities of rapid onset and titratability Pain Res Manage Vol 10 Suppl A Autumn 2005 commonly seen in drugs of abuse (65). At the present time, Marinol is available in 2.5 mg, 5 mg and 10 mg capsules. Cesamet Cesamet is a synthetic cannabinoid that has been developed for use as an antiemetic agent, notably for chemotherapy-induced nausea. It is available as a pulvule (powder-containing capsule) in 0.5 mg and 1 mg strengths. This form of delivery does not appear to lend itself to volatilization or combustion and, thereby, limits misuse via the pulmonary route of administration. Nabilone has been studied in comparison with the reinforcing properties of oral THC and smoked cannabis in subjects with previous cannabis experience. These data indicate that smoked cannabis was significantly more reinforcing than all other cannabis compounds studied, regardless of drug use history (66). DRUG MONITORING Whenever a drug or member of a class of drugs is legitimately present in a test sample, as is the case with any prescribed medication, it becomes very difficult to monitor misuse of that drug or class of drug. An example of this would be the problems associated with monitoring an abstinent heroin user who has been prescribed codeine-containing analgesics for the management of pain. In this case, morphine that results from the metabolism of heroin (diacetyl morphine) will be impossible to distinguish from the morphine that would be expected to be present due to the metabolism of the prescribed codeine (67,68). From a monitoring perspective, it is relatively difficult to distinguish smoked cannabis from orally administered or smoked Marinol. Both routes of administration produce immunoassay-positive screen results for THC and, because they are identical molecules, they cannot even be distinguished by gas chromatography/mass spectroscopy. Naturally occurring THC-containing products are a mix of cannabinoids. One specific cannabinoid that can be identified by sophisticated testing, delta-9-tetrahydrocannabivarin (THCV), has been proposed as a way to specifically identify the naturally occurring product from the synthetic agent (69,70). In 2001, ElSohly et al (69) reported results from a cleverly designed study using a small number of cannabis users as test subjects. The study used a three-session, within-subject, crossover design. Each subject randomly received on separate sessions either an oral dose of Marinol (15 mg), a smoked dose of pure THC (16.98 mg dronabinol) in a placebo cannabis cigarette or an equivalent smoked dose of cannabis (2.11% THC and 0.12 mg THCV/800 mg cigarette = 16.98 mg THC and 0.96 mg THCV). They found that use of cannabis with or without Marinol could be distinguished from the use of Marinol alone (69). Previously, they had found that the confirmatory analytes, THC carboxylic acid and THCV carboxylic acid, found in urine drug specimens supported these findings (70). Cesamet, however, does not trigger a positive immunoassay screen or a confirmatory gas chromatography/mass spectroscopy test for THC (71). In this context, a positive screen for THC in a patient being prescribed Cesamet is consistent only with the use of a THC-containing product such as cannabis or Marinol. PRACTICAL CONSIDERATIONS IN DRUG TESTING Urine drug testing can and should play an important role in chronic pain management. There are, however, some limitations 41A gourlay_8906.qxd 8/30/2005 10:58 AM Page 42 Gourlay to its use. Apart from therapeutic drug level monitoring, the application of drug toxicology in clinical medicine has been largely limited to the emergency room setting. Its use is well known within forensic settings, such as the Department of Transportation testing, and in workplace testing. In this context, testing is often adversarial and certainly often not in the patient’s best clinical interests. Drug testing may, however, be patient-centred if used properly (68,72). Motivating behavioural change, encouraging maintenance of healthy changes already made, advocacy with third parties and early identification of undiagnosed substance use disorders, where they exist, make the rational use of urine drug testing a welcome addition to clinical medicine. No medical test should be used to limit treatment or deny appropriate care. Urine drug testing should be no exception. Testing techniques can be divided into initial ‘screening’ and the more definitive ‘confirmatory’ tests. Screening tests are commonly immunoassay-based and usually identify classes of drugs rather than specific agents. Screen tests are typically very sensitive because they often react to all members of the test class rather than to simply one specific drug. However, there are exceptions to this, as in the case of methadone. In contrast, confirmatory testing is often less sensitive but able to accurately identify specific drugs. For example, a patient taking codeine would be expected to have an initial positive screen for ‘opiates’, but only on confirmatory testing do the specific agents responsible for this positive screen (codeine and its active metabolite morphine) become identified (67,68). It is important to remember that neither screening nor confirmatory testing can detect all substances that may, in fact, be present in a sample. A number of factors, including both concentration effects (dilute urine) and limitations in the testing methodology can account for the failure to detect drugs that may be present in a sample, including prescription drugs that the patient is known to be taking (67,68). A detailed description of the patient-centred approach to urine drug testing is beyond the scope of this paper; however, useful recommendations for the use of drug testing in the primary care setting can be found on the Internet at <http://www.alaskaafp.org/udt.pdf> (67), and in a report by Heit and Gourlay (68). CONCLUSIONS The abuse liability and reinforcing nature of cannabis are well established. In trying to assess the role of cannabinoids in the management of chronic pain, one must consider the risk/benefit profile of this class of drugs and the population in which the agent will be used. Similarly, when determining the most effective route of administration, the patient’s history must be examined. If future research suggests that the smoked route of administration of cannabinoids is the ‘gold standard’ in pain management, it will be important to know the test populations’ previous experience with cannabinoids. If this is the perspective of cannabis-naïve subjects, inhaled cannabis may indeed be the preferred route of administration. On the other hand, if this preference seems to be largely associated with those subjects who have extensive histories with smoked cannabis, this gold standard may become severely tarnished. This should not diminish the potential role for cannabinoids in medicine, but rather emphasize the need for pharmaceutical grade products of known purity and concentration using delivery systems optimized for safety, and for further research on the safety of smoked cannabis when used for therapeutic purposes. In particular, in this case, the risks of the delivery system must be separated from the risks of the drug. Given that pain and addictive disorders coexist, and that cannabinoids appear to have similar abuse liability to other drugs that activate the brain reward systems, these drugs must be approached with an appropriate level of caution. As is often the case, risk lies not solely with the drug, but with the drug used in the context of an at-risk individual. Taking into account the prevalence of recreational cannabis use in modern society, coupled with the increased potency of the available street product, cannabis use disorder should be seen as a progressive entity. Failure to rationally approach this issue will put both the patient and the practitioner at an unacceptable and potentially reducible risk. Those who have previously met the DSM-IV criteria for a substance use disorder with any licit or illicit substance will likely be at an increased risk when using cannabinoids, even for medical purposes. Whether this elevated risk will rise to the level of an absolute contraindication remains to be seen. REFERENCES 1. Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med 1980;302:123. 2. Perry S, Heidrich G. Management of pain during debridement: A survey of US burn units. Pain 1982;13:267-80. 3. Savage SR. Long-term opioid therapy: Assessment of consequences and risks. J Pain Symptom Manage 1996;11:274-86. 4. Black RG. The chronic pain syndrome. Surg Clin North Am 1975;55:999-1011. 5. Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain 1992;8:77-85. 6. Halpern L. Substitution-detoxification and its role in the management of chronic benign pain. J Clin Psychiatry 1982;43:10-4. 7. Savage SR. Assessment for addiction in pain-treatment settings. Clin J Pain 2002;18(Suppl 4):S28-38. 8. Savage SR, Joranson DE, Covington EC, Schnoll SH, Heit HA, Gilson AM. Definitions related to the medical use of opioids: Evolution towards universal agreement. J Pain Symptom Manage 2003;26:655-67. 9. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington: American Psychiatric Association, 1994. 10. Passik SD, Kirsh KL, Whitcomb L, et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy. Clin Ther 2004;26:552-61. 11. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain 42A 12. 13. 14. 15. 16. 17. 18. medicine: A rational approach to the treatment of chronic pain. Pain Med 2005;6:107-12. Jones EM, Knutson D, Haines D. Common problems in patients recovering from chemical dependency. Am Fam Physician 2003;68:1971-8. Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: Implications for concurrent treatment. Drug Alcohol Depend 2004;73:23-31. Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 2003;289:2370-8. Substance Abuse and Mental Health Services Administration. National Household Survey on Drug Abuse: Main Findings 1998. <http://www.oas.samhsa.gov/NHSDA/98MF.pdf> (Version current at August 7, 2005). Leshner AI. The disease of addiction. Lippincotts Prim Care Pract 2000;4:249-53. Galea S, Nandi A, Vlahov D. The social epidemiology of substance use. Epidemiol Rev 2004;26:36-52. National Institute of Health. Risk and protective factors in drug abuse prevention. <http://www.drugabuse.gov/ NIDA_Notes/ NNVol16N6/Risk.html> (Version current at August 7, 2005) . Pain Res Manage Vol 10 Suppl A Autumn 2005 gourlay_8906.qxd 8/30/2005 10:58 AM Page 43 Addiction and pain medicine 19. Leshner AI. Addiction is a brain disease, and it matters. Science 1997;278:45-7. 20. Room R. Smoking and drinking as complementary behaviours. Biomed Pharmacother 2004;58:111-5. 21. Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA 2004;291:2114-21. 22. Summary of Findings from the 2000 National Household Survey on Drug Abuse. NHSDA Series H-13, DHHS Publication No (SMA) 01-3549. <http://www.oas.samhsa.gov/NHSDA/2kNHSDA/ 2kNHSDA.htm> (Version current at August 7, 2005). 23. Australian Institute of Health and Welfare. 2001 National Drug Strategy Household Survey: First results. <http://www.aihw.gov.au/ publications/phe/ndshs01/ndshs01-020717.pdf> (Version current at June 15, 2005). 24. European Monitoring Centre for Drugs and Drug Addiction. Annual report on the state of the drugs problem in European Union and Norway. <http://ar2002.emcdda.eu.int/en/home-en.html> (Version current at June 15, 2005). 25. Clark HW, MacNeill Horton A, Dennis M, Babor TF. Moving from research to practice just in time: The treatment of cannabis use disorders comes of age. Addiction 2002;97(Suppl 1):1-3. 26. McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: A review of the literature. J Subst Abuse Treat 2003;24:369-76. 27. Gruber AJ, Pope HG Jr. Marijuana use among adolescents. Pediatr Clin North Am 2002;49:389-413. 28. Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substansces and inhalants: Basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 1994;2:244-68. 29. Tjepkema M. Use of cannabis and other illicit drugs. Health Rep 2004;15:41-7. 30. Adlaf EM, Paglia A. Drug use among Ontario students: Detailed OSDUS findings, 1977-2003. <http://www.camh.net/pdf/OSDUS03drugdetail-final.pdf> (Version current at June 15, 2005). 31. Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain 2003;102:211-6. 32. Gardner EL. Addictive potential of cannabinoids: The underlying neurobiology. Chem Phys Lipids 2002;121:267-90. 33. Tanda G, Goldberg SR. Cannabinoids: Reward, dependence, and underlying neurochemical mechanisms – a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115-34. 34. Smith NT. A review of the published literature into cannabis withdrawal symptoms in human users. Addiction 2002;97:621-32. 35. Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiatry 2001;178:101-6. 36. Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186-94. 37. Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci 2003;17:1723-6. 38. Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135-40. 39. Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol 2003;112:393-402. 40. Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: Similarities and dissimilarities. Addiction 1994;89:1461-70. 41. Office of Applied Studies. 1994-1999 Treatment Episode Data Set (TEDS). <http://www.dasis.samhsa.gov/teds99/ack.htm> (Version current at August 7, 2005). 42. The DASIS report: Marijuana treatment admissions increase: 1993-1999. <http://oas.samhsa.gov/2k2/MJtx/MJtx.pdf> (Version current at June 15, 2005). 43. Australian Institute of Health and Welfare. Alcohol and other drug treatment services in Australia: Findings from the national minimum data set 2001-02. <http://www.aihw.gov.au/publications/aus/bulletin17/ bulletin17-040909.pdf> (Version current at June 15, 2005). 44. Copeland J. Developments in the treatment of cannabis use disorder. Curr Opin Psychiatry 2004;17:161-8. Pain Res Manage Vol 10 Suppl A Autumn 2005 45. ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF III. Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980-1997. J Forensic Sci 2000;45:24-30. 46. Hall W, Swift W. The THC content of cannabis in Australia: Evidence and implications. Aust N Z J Public Health 2000;24:503-8. 47. Royal Canadian Mounted Police. Marijuana cultivation in Canada: Evolution and current trends. <http://www.rcmp-grc.gc.ca/ crimint/cultivation_e.htm> (Version current at June 15, 2005). 48. Iverson L. The Science of Marijuana. Oxford: Oxford University Press, 2000. 49. Health Canada. Information for Health Care Professionals (Revised) – Marihuana (Marijuana, Cannabis). <http://www.hc-sc.gc.ca/dhp-mps/ marihuana/how-comment/medpract/infoprof/information_rev_e.html> (Version current at August 7, 2005). 50. World Health Organization 1997. Cannabis: A health perspective and research agenda. <http://whqlibdoc.who.int/hq/1997/ WHO_MSA_PSA_97.4.pdf> (Version current at June 15, 2005). 51. Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction 2000;95:1621-30. 52. Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend 1995;40:9-15. 53. Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 1998;10:163-73. 54. Gieringer D, St Laurent J, Goodrich S. Cannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compounds. J Cannabis Therapy 2004;4:7-27. 55. Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J. Marijuanalaced brownies: Behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol 1988;12:169-75. 56. Warner EA. Cocaine abuse. Ann Intern Med 1993;119:226-35. 57. Cone EJ. Recent discoveries in pharmacokinetics of drugs of abuse. Toxicol Lett 1998;102-103:97-101. 58. Ware MA, Tawfik VL. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manage 2005;10(Suppl A):31A-37A 59. Cannabis-based medicines – GW pharmaceuticals: High CBD, high THC, medicinal cannabis – GW pharmaceuticals, THC:CBD. Drugs R D 2003;4:306-9. 60. Smith PF. GW-1000. GW Pharmaceuticals. Curr Opin Investig Drugs 2004;5:748-54. 61. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42:327-60. 62. Unimed Pharmaceuticals, Inc. Marinol. <http://www.marinol.com/ pdfs/MARINOLPI.pdf> (Version current at June 15, 2005). 63. Ward A, Holmes B. Nabilone. A preliminary review of its pharmacological properties and therapeutic use. Drugs 1985;30:127-44. 64. Kinzbrunner BM. Review: Cannabinoids and codeine have similar effects on pain relief, but cannabinoids commonly cause adverse psychotropic effects. ACP J Club 2002;136:18. 65. Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol). J Psychoactive Drugs 1998;30:187-96. 66. Mendelson JH, Mello NK. Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana, and nabilone: Influence of previous marijuana use. Psychopharmacology (Berl) 1984;83:351-6. 67. Urine drug testing in primary care: Dispelling the myths & designing strategies. Pharmacom Grp, 2002. <www.alaskaafp.org/udt.pdf> (Version current at July 14, 2005). 68. Heit HA, Gourlay DL. Urine drug testing in pain medicine. J Pain Symptom Manage 2004;27:260-7. 69. ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. Delta9-tetrahydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: Results of a clinical study. J Anal Toxicol 2001;25:565-71. 70. ElSohly MA, Feng S, Murphy TP, et al. Delta 9-tetrahydrocannabivarin (delta 9-THC V) as a marker for the ingestion of cannabis versus Marinol. J Anal Toxicol 1999;23:222-4. 71. Fraser AD, Meatherall R. Lack of interference by nabilone in the EMIT d.a.u. cannabinoid assay, Abbott TDx cannabinoid assay, and a sensitive TLC assay for delta 9-THC-carboxylic acid. J Anal Toxicol 1989;13:240. 72. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: A rational approach to the treatment of chronic pain. Pain Med 2005;6:107-12. 43A clark_8899.qxd 8/30/2005 10:57 AM Page 44 REVIEW Guidelines for the use of cannabinoid compounds in chronic pain AJ Clark MD FRCPC1, ME Lynch MD FRCPC2, M Ware MBBS MRCP MSc3, P Beaulieu MD PhD FRCA4, IJ McGilveray PhD5, D Gourlay MD FRCPC FASAM6 AJ Clark, ME Lynch, M Ware, P Beaulieu, IJ McGilveray, D Gourlay. Guidelines for the use of cannabinoid compounds in chronic pain. Pain Res Manage 2005;10(Suppl A):44A-46A. OBJECTIVE: To provide clinicians with guidelines for the use of cannabinoid compounds in the treatment of chronic pain. METHODS: Publications indexed from 1990 to 2005 in the National Library of Medicine Index Medicus were searched through PubMed. A consensus concerning these guidelines was achieved by the authors through review and discussion. RESULTS: There are few clinical trials, case reports or case series concerning the use of cannabinoid compounds in the treatment of chronic pain. There are no randomized clinical trials examining the use of herbal cannabis in the treatment of chronic pain. CONCLUSIONS: A practical approach to the treatment of chronic pain with cannabinoid compounds is presented. Specific suggestions about the off-label dosing of nabilone (Cesamet, Valeant Canada limitée/Limited) and dronabinol (Marinol, Solvay Pharma Inc, Canada) in the treatment of chronic pain are provided. Key Words: Cannabinoid; Chronic pain; Dronabinol; Guideline; Nabilone; Treatment n Canada, there are presently two synthetic cannabinoids and one cannabis-based medicine available by prescription. The synthetic agents include nabilone (Cesamet, Valeant Canada limitée/Limited) and the synthetic delta-9-tetrahydrocannabinol dronabinol (Marinol, Solvay Pharma Inc, Canada), both of which are available in oral preparations. The cannabis-based medicine (Sativex, GW Pharma Ltd, United Kingdom) is available as a buccal spray and consists of an extract containing delta-9-tetrahydrocannabinol and cannabidiol. Sativex has only recently been approved by Health Canada (as of April 2005) under a conditional notice of compliance. The approved indication for nabilone and dronabinol is for antiemesis in chemotherapy-induced nausea and vomiting, while the indication listed for the buccal extract is adjunctive treatment for the symptomatic relief of neuropathic pain in multiple sclerosis. Any of these agents prescribed for pain would be considered an ‘off-label’ use. In addition, since July 30, 2001, Canadians have had the opportunity to apply for a license to possess herbal cannabis under the Medical Marijuana Access Regulations. Patients may also apply for a license to grow marijuana or may purchase marijuana supplied by Health Canada by completing appropriate forms. I Lignes directrices à l’égard de l’utilisation des substances cannabinoïdes en cas de douleur chronique OBJECTIF : Fournir aux cliniciens des lignes directrices à l’égard de l’utilisation des substances cannabinoïdes dans le traitement de la douleur chronique. MÉTHODOLOGIE : Une recherche a été effectuée dans les publications indexées entre 1990 et 2005 dans la National Library of Medicine Index Medicus par l’entremise de PubMed. Les auteurs sont parvenus à un consensus à l’égard des présentes lignes directrices par suite d’une analyse et de discussions. RÉSULTATS : Peu d’essais cliniques, de rapports de cas ou de séries de cas portent sur le recours aux substances cannabinoïdes dans le traitement de la douleur chronique. Aucun essai clinique aléatoire ne traite de l’usage du cannabis végétal dans le traitement de la douleur chronique. CONCLUSIONS : Une démarche pratique envers le traitement de la douleur chronique à l’aide de substances cannabinoïdes est présentée. Des suggestions précises sont fournies au sujet du dosage dans une indication non autorisée du nabilone (Cesamet, Valeant Canada limitée/Limited) et du dronabinol (Marinol, Solvay Pharma Inc., Canada) dans le traitement de la douleur chronique. The cannabinoid research field is growing rapidly and there are research programs around the world working on the development of cannabinoids for clinical applications. This includes extracts of naturally occurring agents and the development of novel synthetic cannabinoid agonists and agents that may allow for the modulation of the endogenous cannabinoid system. As reviewed in the present supplement by Ware and Beaulieu (1) and previously by Campbell et al (2), there are few clinical trials regarding synthetic cannabinoids and there are no randomized clinical trials regarding herbal cannabis in the treatment of chronic pain. In the past three years, several reports have been published about the use of smoked herbal cannabis for the treatment of chronic pain related to various causes including HIV, multiple sclerosis, neuropathic pain and general chronic pain (3-8). These reports have identified that 15% to 36% of patients with pain have used, and 10% to 14% continue to use, herbal cannabis for symptom relief. Moreover, these reports have provided information about how herbal cannabis is used, including ‘doses’, route of administration and side effects that were experienced with use. However, this information remains inadequate for the development of guidelines for the use of herbal cannabis. This is also the case with 1University of Calgary, Calgary, Alberta; 2Dalhousie University, Halifax, Nova Scotia; 3McGill University; 4Université de Montréal, Montréal, Québec; 5University of Ottawa, Ottawa; 6Centre for Addiction and Mental Health, Toronto, Ontario Correspondence: Dr AJ Clark, Chronic Pain Centre, Calgary Health Region, #160, 2210 – 2nd Street SW, Calgary, Alberta T2S 3C3. Telephone 403-943-9900, fax 403-209-2955, e-mail john.clark@calgaryhealthregion.ca 44A ©2005 Pulsus Group Inc. All rights reserved Pain Res Manage Vol 10 Suppl A Autumn 2005 clark_8899.qxd 9/8/2005 1:23 PM Page 45 Guidelines: Cannabinoid compounds in chronic pain the buccal cannabis-based extract and, thus, these guidelines will focus on the two oral cannabinoids currently available by prescription in Canada. For information regarding herbal cannabis under the Medical Marijuana Access Regulations, clinicians are referred to the Health Canada Web site (http://www.hc-sc.gc.ca/hecs-sesc/ocma/). In general, contraindications to the use of cannabinoids include pregnancy, uncontrolled hypertension, active ischemic heart disease, arrhythmias and schizophrenia. A history of psychosis is a relative contraindication, and because cannabinoids can cause anxiety, they should be used with caution in patients with a past or current anxiety or panic disorder. Moreover, cannabinoids can cause sedation and cognitive effects. Thus, the usual cautions given for opioids, benzodiazepines or any potentially sedating agent are applicable. Patients should be cautioned not to drive or to operate heavy machinery, etc, if experiencing any side effects that would impair their performance in these activities. Patients with comorbid depression and other psychiatric disorders should be carefully monitored. Cannabinoids should be administered initially at low doses and titrated slowly to balance positive and negative acute effects. Patients should be advised of the likelihood and nature of acute effects, and close monitoring is advised during initial dose titration. EVALUATING A PATIENT FOR A TRIAL OF A CANNABINOID Prudent medical practice incorporates a comprehensive evaluation of a patient when new therapies are considered. The following suggestions are similar to those endorsed by the Canadian Pain Society in its consensus statement and guidelines for the use of opioid analgesics in the treatment of noncancer pain (9): • • • Full assessment, establish diagnosis of pain Assess psychosocial issues and risk of addiction Determine history of previous use of illicit substances or misuse of prescription drugs • Develop a treatment plan assuring that pharmacotherapy takes place within an overall active participatory approach • Ensure that traditional approaches to chronic pain management have been tried or considered Pain is inadequately treated • • • • Consider cautions and ensure no contraindications to use of cannabinoid Discuss potential adverse effects Consider discussing urine testing Review and sign treatment agreement • Cannabinoid-naïve: Start an oral cannabinoid available by prescription* *NCesamet® (synthetic analogue of THC, nabilone) or NMarinol® (synthetic THC, dronabinol) Previous use of cannabinoids: • Obtain a full history of previous cannabinoid use • Specific cannabinoid, dose, route of administration • Symptoms treated and outcome • Adverse effects • Encourage oral route of administration and initiate trial of oral cannabinoid available by prescription* If oral nabilone or dronabinol trial fails or is not financially feasible, consider cannabis: • Discuss the fact that there are not yet clear guidelines regarding efficacy, • doses and toxicity • Raise awareness of oral and vaporized routes of administration • Refer patient to Health Canada Web site and documents regarding cannabis product • Follow the usual clinical guidelines to start low and titrate dose slowly Figure 1) Algorithm for the treatment of chronic pain with cannabinoids • perform a history and examination; • assess the level of pain; • assess psychological contributors and risk of addiction or substance abuse; • document any history or current use of illicit or nonprescribed drugs, including cannabis and synthetic cannabinoids; • determine the effect of the previous use of cannabinoids on pain; • consider urine drug screening to assess the current use of prescribed and nonprescribed medications; • set goals of treatment with a cannabinoid – consider reduction of pain, increased functional abilities, improved sleep quality, increased quality of life and a reduction in the use of other medications; • develop a treatment plan incorporating these goals; • discuss possible side effects that may be experienced with use (eg, central nervous system, cardiovascular and respiratory); • discuss the risks of addiction; • develop a follow-up schedule to periodically review the patient (the five As – Analgesia, Activities, Adverse events, Abuse behaviours and Adequate documentation); • determine whether the goals of treatment are being achieved and the appropriateness of response; and Pain Res Manage Vol 10 Suppl A Autumn 2005 • consider developing a treatment agreement with the patient, particularly if he/she is at risk. Documentation is essential to demonstrate the evaluation of the patient, the rationale for the use of a cannabinoid in the context of the overall management plan and the periodic review of the patient’s status. An algorithm for the treatment of chronic pain is provided in Figure 1. Common side effects associated with the use of the cannabinoids are detailed in Table 1. Risk of addiction to and abuse of Cesamet, Marinol and Sativex Marinol is available suspended in sesame oil in capsule form in 2.5 mg, 5 mg and 10 mg strengths. Cesamet is available as a pulvule (powder-containing capsule) in 0.5 mg and 1 mg strengths. Sativex is available as a buccal spray. As Gourlay (10) notes in the present supplement, it has been previously reported that the abuse liability of Marinol is low because the drug lacks the desirable qualities of rapid onset and titratability commonly seen in drugs of abuse. The same is true for Cesamet due to its compounding as a pulvule; it too does not appear to lead to misuse or abuse. Comparisons of the reinforcing properties of oral tetrahydrocannabinol, smoked cannabis and nabilone in subjects with previous cannabis experience indicate that smoked cannabis was significantly more reinforcing than all other cannabis compounds studied, regardless of 45A clark_8899.qxd 9/8/2005 1:29 PM Page 46 Clark et al TABLE 1 Common and important side effects of cannabinoids Central nervous system Euphoria Anxiety Panic Paranoia When other therapies have failed to provide adequate pain relief or have caused unacceptable side effects, it is reasonable to consider a trial of an oral cannabinoid: • initiate Marinol at 2.5 mg orally at night time; and • increase dose by 2.5 mg every two to three days to a maximum dose of 10 mg twice daily orally. Psychosis Sedation Dizziness Somnolence Depression Ataxia Possible visual/hearing disturbances Asthenia Possible cognitive effects Cardiovascular Tachycardia Postural hypotension Palpitations Vasodilation (flushing, red eyes) Increased risk of myocardial infarction within 1 h of use Respiratory (if smoked) Bronchitis/chronic obstructive pulmonary disease Lung infection Increased risk for upper airway cancer Other Dry mouth Headache Abdominal pain/bloating drug-use history (10). We do not currently have adequate information concerning Sativex and abuse liability. Guidelines for the use of Cesamet Cesamet has been available in Canada since 1981 and is indicated for use as an antiemetic and to treat anorexia associated with AIDS. There have been no adequate randomized clinical trials on the use of Cesamet in chronic pain, although there are two clinical reports (11,12) showing a modest benefit in some patients. When other therapies have failed to provide adequate pain relief or have caused unacceptable side effects, it is reasonable to consider a trial of an oral cannabinoid: • initiate Cesamet at 0.5 mg orally at night time; and • increase dose by 0.5 mg every two to three days to a maximum dose of 2 mg orally twice daily. Guidelines for the use of Marinol Marinol is indicated for use as an antiemetic. There is one randomized clinical trial (13) of Marinol (with a dose up to 10 mg) showing a modest effect in the treatment of central pain in patients with multiple sclerosis. An ‘N-of-1’ trial in noncancer pain subjects has demonstrated improvement in pain similar to that with codeine at doses of 10 mg or higher (14). 46A Sativex Sativex is only approved for use as an adjunctive treatment for the symptomatic relief of neuropathic pain in multiple sclerosis. The product monograph for Sativex recommends a “gradual increase in dose as needed and tolerated until satisfactory pain relief is achieved” (15). There is insufficient published information on the dosing of Sativex to make recommendations about its use at this time. REFERENCES 1. Ware M, Beaulieu P. Cannabinoids for the treatment of pain: An update on recent clinical trials. Pain Res Manage 2005;10(Suppl A):27A-30A. 2. Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 2001;323:13-6. 3. Lynch ME, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manage 2003;25:496-8. 4. Ware M, Gamsa A, Persson J, Fitzcharles M. Cannabis for chronic pain: Case series and implications for clinicians. Pain Res Manag 2002;7:95-9. 5. Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain 2003;102:211-6. 6. Page SA, Verhoef MJ, Stebbins RA, Metz LM, Levy JC. Cannabis use as described by people with multiple sclerosis. Can J Neurol Sci 2003;30:201-5. 7. Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004;62:2098-100. 8. Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr 2004;35:38-45. 9. Jovey RD, Ennis J, Gardner-Nix J, et al; Canadian Pain Society. Use of opioid analgesics for the treatment of chronic noncancer pain – consensus statement and guidelines from the Canadian Pain Society, 2002. Pain Res Manage 2003;8(Suppl A):3A-28A. 10. Gourlay D. Addiction and pain medicine. Pain Res Manag 2005;10(Suppl A):38A-43A. 11. Notcutt WG, Price M, Chapman G. Clinical experience with nabilone for chronic pain. Pharm Sci 1997;3:551-5. 12. Martyn CN, Illis LS, Thom J. Nabilone in the treatment of multiple sclerosis. Lancet 1995;345:579. 13. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;329:253. 14. Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 2003;105:79-88. 15. Sativex product monograph. GW Pharmaceuticals. Salisbury: United Kingdom, 2005. Pain Res Manage Vol 10 Suppl A Autumn 2005 Instructions to Authors_pain.qxd 8/30/2005 11:24 AM Page 1 Pain Research & Management PAIN RESEARCH & MANAGEMENT will consider for publication original papers on any scientific aspect of pain, namely basic science and animal studies, and clinical topics, including epidemiological, social, psychological and psychiatric aspects, as well as reviews and case reports. Management topics, the economic aspects of pain and historical studies are also welcome. Papers may deal with specific Canadian aspects of pain, but there is no restriction in this respect and international submissions will be readily received. The Journal is pleased to accept articles in either English or French. Manuscripts are received with the understanding that they are submitted solely to Pain Research & Management, and that none of the material contained in the manuscript has been published previously or is under consideration for publication elsewhere, with the exception of abstracts. INSTRUCTIONS TO AUTHORS Hardcopy manuscripts can be sent (in triplicate, including three copies of all illustrations) accompanied by an electronic version saved on floppy disk or CD-ROM to: Dr Harold Merskey, Editor-in-Chief, Pain Research & Management, 71 Logan Avenue, London, Ontario N5Y 2P9 Authors are encouraged to contact the editor by telephone at 519-679-1045, by fax at 519-679-6849 or by e-mail at merskey@sympatico.ca IMPORTANT To ensure that the final, published version matches the electronic file, make sure that you use only the following fonts: Arial, Courier, Symbol and Times. The use of nonstandard fonts may lead to missing symbols. The font size should be no smaller than 7 point and no larger that 14 point. There is no charge for black and white figures. However, authors will be charged for colour figures at the rate of $300 for the first figure and $100 for each subsequent figure. SUBMISSIONS 1)Save text and graphics on floppy disk or CD-ROM. 2)Label all submissions with your name and filenames. Please also include details of the platform (PC or Mac) used. 3)Ensure that electronic and hardcopy versions of your manuscript are identical. In cases of a discrepancy, the hardcopy version will be used as the definitive version. TEXT Text files must be saved as .doc files. TABLES: Type double-spaced, on a separate page from the rest of the text with the table number above the table and explanatory notes below. Table numbers should appear in Arabic numerals and should correspond to the order of the tables in the text. If abbreviations are used, an alphabetical listing must be included in the footnote. Written permission from the publisher to reproduce any previously published tables must be included. FIGURE LEGENDS: Type double-spaced, separate from the rest of the text, with figure numbers corresponding to the order in which figures are presented in the text. Identify all abbreviations appearing on figures in alphabetical order at the end of each legend. Enough information should be given to allow interpretation of the figure without reference to the text. Written permission must be obtained from the publisher to reproduce any previously published figures. FIGURES 1)All figures have to be submitted in their original formats. The lettering, decimals, lines and other details on the figures should be sufficiently large to withstand reduction and reproduction. 2)Graphs must be created using Microsoft Word (.doc), Microsoft Power Point (.ppt), Microsoft Excel (.xls), Corel Draw (.cdr), or Adobe Illustrator (.ai or .eps). 3)Any photographs or graphics imported into your figure, must also be submitted separately. Photographs and graphics may be submitted as unmounted glossy proofs (3 sets) or scanned at a resolution of no less than 360 dpi and saved as a .tiff file. Place crop marks on photomicrographs to show the essential field and designate special features with arrows (which must contrast with the background). The first author’s last name, the figure number and the location of the ‘top’ must be indicated on the back of each proof. 4)Photographs and graphics will not be returned. Instructions to Authors_pain.qxd 8/30/2005 11:24 AM Page 2 MANUSCRIPTS All manuscripts must be accompanied by a covering letter detailing what is being submitted and indicating the author to whom we should address correspondence and page proofs in the case of multiple authors (please include a contact address, telephone/fax numbers and e-mail address). Author must sign a publishing agreement supplied by Pulsus Group Inc upon acceptance for publication. Abbreviations must be defined at first mention in the text. All measurements should be in SI units. Appropriate headings and subheadings should be provided in the methods, results and discussion sections. References, figures and tables should be cited in the text with numbers assigned according to the order of mention in the text. Arrange the manuscript as follows: title page, summary, structured abstract and key words, introduction, methods, results, discussion, acknowledgements, references, figure legends, tables and figures. Do not import figures into the text file. Number the pages consecutively, beginning with the title page as 1. The last name of the first author should be typed at the top of each page. TITLE PAGE: In addition to the main title, a short running title of 45 characters or less and the authors’ names (including full, first or middle names) along with their qualifications and affiliations, should appear on the title page. Include the name of the institution from which the work originated. ABSTRACT: On a separate page, type a structured abstract of no more than 250 words for major articles (including review articles). Abstracts for case reports need not be structured, but are limited to 150 words. The abstract should be substantive rather than purely descriptive. Abbreviate only standard units of measurement. KEY WORDS: At the end of the abstract, include a list of 3 to 6 key words for indexing purposes. ACKNOWLEDGEMENTS: Brief acknowledgements may appear at the end of the text, before the references. REFERENCES: Identify references in the text by Arabic numerals in parentheses on the line. Type the reference list doublespaced, separate from the text, with each reference numbered consecutively in the order in that it is mentioned in the text. References cited in figures and tables, but not in the text, should also be numbered following the text references. Personal communications, manuscripts in preparation and other unpublished data should not be cited in the reference list but may be mentioned in the text in parentheses. Identify abstracts with the abbreviation ‘Abst’ and letters to the editor by ‘Lett’ in parentheses; abstracts should not be cited if they are more than 2 years old. The style of references is that of Index Medicus. Journal references should contain inclusive page numbers; book references, specific page numbers; and Web site references, the date of last update, if available, and date of access (references to other types of electronic documents should include the format of the document). Abbreviations of journals should conform to those used in Index Medicus, National Library of Medicine. URLs should be included for all references publicly accessible via the Internet. The style and punctuation of references are as follows: Periodicals List all authors if 6 or less; otherwise list first 3 and add ‘et al’. Do not use periods after authors’ initials. 1. Kohl P, Day K, Noble D, et al. Cellular mechanisms of cardiac mechano-electric feedback in a mathematical model. Can J Cardiol 1998;14:111-9. Books 2. Svensson LG, Crawford ES. Cardiovascular and Vascular Disease of the Aorta. Toronto: WB Saunders Company, 1997:184-5. Chapter in book 3. Trehan S, Anderson JL. Thrombolytic therapy. In: Yusuf S, Cairns JA, eds. Evidence Based Cardiology. London: BMJ Books, 1998:419-44. Web sites 4. National Library of Medicine. Images from the History of Medicine. <http://www.ihm.nlm.nih.gov/> (Version current at January 5, 1999). POLICY ISSUES All statements and opinions are the responsibility of the authors. With submission of the manuscript, a letter of transmittal must indicate that all authors have participated in the research, and have reviewed and agree with the content of the article. The publisher reserves copyright on all published material, which then may not be reproduced without the written permission of the publisher. CONFLICT OF INTEREST: All authors must disclose any commercial associations or other arrangements (eg, financial compensation received, patient-licensing arrangements, potential to profit, consultancy, stock ownership, etc) that may pose a conflict of interest in connection with the article. This information will be made available to the editor and reviewers, and may be included as a footnote at the editor’s discretion. ETHICS OF HUMAN AND ANIMAL EXPERIMENTS: If human subjects are involved, the text must indicate that all gave informed consent and that the protocol was approved by the institutional review committee. If experimental animals are used, provide a statement in the text to indicate that all procedures followed were in accordance with institutional policies. PROOFS: Authors should keep a copy of their original manuscripts as page proofs will be sent to them without the manuscript. In order to avoid delays in publication, authors are required to return proofs within 48 hours by fax or e-mail. REPRINTS: Single reprints may be obtained from the author. Reprints in quantity must be purchased from Pulsus Group Inc. Reprints may not be purchased without the author’s permission. Potential authors who have further questions about these issues should contact the editorial office at 519-679-1045. Pain10(SA)_Cov_spread.qxd 8/30/2005 4:22 PM Page 1 Volume 10 Supplement A • Autumn 2005 Pain Research &Management Cannabinoids for Pain Management Guest Editor: Dr Alexander J Clark Publication of this supplement sponsored by an unrestricted educational grant from Valeant The Journal of the Canadian Pain Society Journal de la société canadienne pour le traitement de la douleur Pulsus Group Inc, 2902 South Sheridan Way, Oakville, Ontario, Canada L6J 7L6 Telephone 905-829-4770, fax 905-829-4799, pulsus@pulsus.com, www.pulsus.com PM 40062595 p u l s u s . c o m p u l s u s . c o m