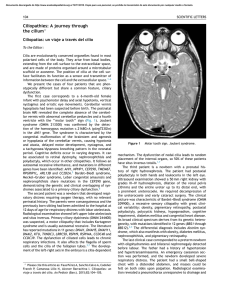
CHAPTER Plasticity as a therapeutic target for improving cognition and behavior in Down syndrome 9 Carmen Martı́nez Cuea, Mara Dierssenb,* a Department of Physiology and Pharmacology, School of Medicine, University of Cantabria, Santander, Spain b Cellular and Systems Neurobiology, Systems Biology Program, Centre for Genomic Regulation (CRG), Barcelona Institute of Science and Technology, Universitat Pompeu Fabra (UPF), Barcelona, Spain *Corresponding author: Tel.: 933160140, e-mail address: maradierssen@gmail.com Abstract Early intervention and environmental optimization have been central to management of Down syndrome (DS) and much of current treatment is still focused in strategies that involve early education plans. This approach has provided significant improvements for Down syndrome but it is not providing a full success. The discovery of an increasing number of genes and molecular pathways linked to intellectual disability and involving a range of synaptic and plasticity-related mechanisms has open new treatment opportunities that focus on targeted treatments boosting neural plasticity. We here discuss some of these approaches, focusing on the effects of environmental enrichment and on the discovery of pharmacological therapies showing beneficial effects even in some clinical trials in adult individuals with Down syndrome. Targeting plasticity impairments in DS is thus a promising strategy to promote cellular mechanisms involved in learning and memory within key cognitive brain region and could lead to improved connectivity. Keywords Neuronal plasticity, Environmental enrichments, Epigenetics, EGCG, Environ-mimetic drugs 1 Introduction For decades, efforts to promote cognitive therapies in DS have been regarded with skepticism for finding any true therapeutic benefit, and with reluctance to invest the time and resources into low-gain clinical trials. The most important reason is that the Progress in Brain Research, Volume 251, ISSN 0079-6123, https://doi.org/10.1016/bs.pbr.2019.11.001 © 2020 Elsevier B.V. All rights reserved. 269 270 CHAPTER 9 Plasticity as a therapeutic target putative mechanism(s) of intellectual impairment specific to trisomy 21 have never been seen as targetable because of the ballast of the developmental effects on the brain. This is also the case for many neurodevelopmental disorders such as autism, epilepsy and other forms of learning disabilities all of which share some form of synaptic dysfunction, with a myriad of variations in synaptic structure and function leading to similar learning and memory deficits. These forms of synaptic dysfunction include variations in synaptic proteins, such as changes in receptors for glutamate, GABA or other neurotransmitters, and impaired function at various sites and mechanisms of the synapse. They most probably lead to an inability to adequately respond to the environmental requirements, called activity-dependent plasticity. The consequences can be found in changes in the physical structure of the synapse, as reflected by abnormalities in spine morphology and density, that adversely affect the information processing efficiency and storage capacity of neural networks (BenavidesPiccione et al., 2004; Dierssen and Ramakers, 2006; Morè et al., 2019). The neurocognitive phenotype of DS stems from deficits in “late-developing” neural systems, with greater gray matter reductions in the frontal lobe, hippocampus and cerebellum (Lott and Dierssen, 2010; Menghini et al., 2011), areas that have major roles in the DS-specific mnesic alterations (Krasuski et al., 2002; Teipel et al., 2004). Those are regions characterized by a rich neuronal connectivity, based predominantly on excitatory connections on the dendritic spines of pyramidal neurons and show the highest plasticity in the brain. In this chapter we focus on some successful strategies increasing neurogenesis, and promoting dendritic rewiring via pro-cognitive treatments, which could provide a unique opportunity to ameliorate neuronal morphology, wiring, and cognitive function. 2 Neurogenesis enhancers During the entire life span of many animals, including mice, cell proliferation takes place in the subventricular (SVZ) zone and in the subgranular zone (SGZ) of the dentate gyrus (DG). Neurogenesis is severely compromised in DS from early developmental stages. In DS fetuses, a reduced number of dividing cells are found in the DG, lateral ventricle (Contestabile et al., 2007; Guidi et al., 2008), external granular layer (EGL) of the cerebellum and in the ventricular zone (VZ; Guidi et al., 2011) (see chapter “Translational validity and implications of pharmacotherapies in preclinical models of Down syndrome” by Martinez Cue). Reduced proliferation of neural precursor cells is also found in mouse models of DS (i.e., Ts65Dn, Ts1Cje and Ts2Cje) during embryonic and perinatal stages in the neocortex, in the subventricular zone (SVZ) and cerebellar EGL (Chakrabarti et al., 2007; Hewitt et al., 2010; Ishihara et al., 2010; Laffaire et al., 2009; Moldrich et al., 2009) and from birth to adulthood in the hippocampal DG (Bianchi et al., 2010a; Contestabile et al., 2007; Lorenzi and Reeves, 2006; Trazzi et al., 2011). These alterations in neurogenesis depend on impaired neuronal precursor proliferation, slowing of the cell cycle and altered 2 Neurogenesis enhancers differentiation (Bahn et al., 2002; Esposito et al., 2008; Hewitt et al., 2010; Laffaire et al., 2009; Moldrich et al., 2009). However, more recent studies conclude that the balance across different process is what defines the final neuronal number. For example, although adult Ts65Dn mice have a lower number of proliferating cells it is compensated by reduced cell death, thus leading to a final number of mature cells similar to controls (Pons-Espinal et al., 2013). Therefore, reduction of adult neurogenesis by itself would not be responsible for the neuronal hypocellularity in the hippocampus (López-Hidalgo et al., 2016) in this model. Even so, if defects in adult neurogenesis in DS were partially responsible for their cognitive alterations, therapies targeted to rescue neurogenesis could be a good approach to treat intellectual disability in DS individuals. There is a wide body of literature that reports different pharmacotherapies that rescue neurogenesis and cognitive deficits in murine models of DS when administered in pre or in postnatal stages. Most of them do not target DS-specific mechanisms, but are explored due to their general neurogenic properties. One example is fluoxetine, a selective serotonin reuptake inhibitor antidepressant that has been shown to increase neurogenesis in mouse (Malberg et al., 2000). In Ts65Dn mice, chronic treatment during prenatal (Guidi et al., 2014), early postnatal (Bianchi et al., 2010a; Stagni et al., 2015) and adult stages (Clark et al., 2006) restored neurogenesis and cognition. Similarly, lithium, a drug prescribed for bipolar depression also rescued neurogenesis in Ts65Dn mice DG at 6 months of age (Contestabile et al., 2013) and in the SVZ at 12-months of age (Bianchi et al., 2010b). Because both fluoxetine and lithium are approved for other indications in humans they could be easier to translate into clinical trials. In fact, a clinical trial is ongoing (Stella Maris Foundation; EudraCT Number: 2011-001556-11), though no results are yet reported. However, possible side effects in neonates upon prenatal treatment with antidepressants cannot be ruled out. Fluoxetine has been associated with risk of specific cardiovascular malformations (Reefhuis et al., 2015) although not all studies find a substantial teratogenic effect of SSRI (Furu et al., 2015). However, the potential risk of preterm birth (Hayes et al., 2012) and pulmonary hypertension in the neonate (Chambers et al., 2006; Olivier et al., 2013) may occur, and in utero exposure to serotonin reuptake inhibitors may result in a neonatal withdrawal syndrome (MosesKolko et al., 2005; Sanz et al., 2005). In any case, there are no published data on DS babies born from mothers taking fluoxetine or other antidepressants and a pilot feasibility trial of perinatal fluoxetine treatment proposed at the Southwestern Medical Center of the University of Texas in 2014 (Byerly, M., Carlin, M., HorsagerBoehrer, R., 2014. A pilot feasibility trial of prenatal and early postnatal fluoxetine treatment for intellectual impairments of Down syndrome) has not yet reported beneficial effects. Increasing evidence indicates that adult hippocampal neurogenesis is implicated in the establishment of long-term potentiation (LTP) and has a role in hippocampaldependent learning and memory (Malberg et al., 2000; Shors et al., 2002). A study employing a CNTF- or BDNF-based prenatal to early postnatal pharmacotherapy in the Ts65Dn mouse model showed beneficial effect both on neurodevelopment and on cognition in adult life (Kazim et al., 2017). In the same direction, a flavone 271 272 CHAPTER 9 Plasticity as a therapeutic target derivative, 7,8-dihydroxyflavone (7,8-DHF), a small molecule that crosses the blood–brain barrier and binds with high affinity and specificity to the TrkB receptor, subcutaneously injected with 7,8-DHF in P3-P15 Ts65Dn pups exhibited a large increase in the number of neural precursor cells in the DG and restoration of granule cell number, density of dendritic spines and levels of the presynaptic protein synaptophysin, indicating that the recovery of the hippocampal anatomy translated into a functional rescue (Stagni et al., 2017). Another example is pharmacological blockade of cannabinoid receptor CB1R, which restores memory deficits, hippocampal synaptic plasticity and adult neurogenesis in the subgranular zone of the DG (Navarro-Romero et al., 2019). Other drugs that have been demonstrated to restore neurogenesis in prenatal and/ or early postnatal treatment studies and improve cognitive deficits in Ts65Dn or other DS mouse models are, among others: the natural flavonoid luteolin (Zhou et al., 2019), the flavonoid agonist of the TRKB receptor for BDNF 7,8dihydroxyflavone (7,8-DHF) (Stagni et al., 2017), a BDNF-mimetic compound (Parrini et al., 2017), corn oil (Giacomini et al., 2018), maternal choline supplementation (Velazquez et al., 2013), APP gamma-secretase inhibitors (Giacomini et al., 2015), melatonin (Corrales et al., 2014), the long-acting β2 agonist formoterol (Dang et al., 2014) and the GABAA α5 receptor negative allosteric modulator RO4938581 (Martı́nez-Cue et al., 2013). Some of these are natural compounds like melatonin or corn oil, the antioxidant α-tocopherol (vitamin E) (Shichiri et al., 2011), the dietary supplement choline (Ash et al., 2014; Kelley et al., 2016; Moon et al., 2010) that could be used safely in humans and thus, have potential for their use in clinics. However, other drugs that have been proven to increase neurogenesis and cognition in animal models of DS could not be used in humans because of their intolerable side effects. One example is the synthetic activator of Sonic hedgehog (Shh) pathway (Das et al., 2013) SAG 1.1. Treatment to Ts65Dn mice with SAG 1.1, increased mitosis, restored cerebellar granule cell precursor populations (Roper et al., 2006) and rescued proliferation in the SVZ and DG (Trazzi et al., 2011). In addition, administration of SAG 1.1 to newborn Ts65Dn mice restored cognition in these mice when they became adults (Das et al., 2013). However, because SAG 1.1 is a very potent mitogenic, it could be carcinogenic, thus, precluding its use in the human population. Finally other agents, such as active peptide fragments of activity-dependent neuroprotective protein (ADNP) and activity-dependent neurotrophic factor (ADNF), two neuroprotective peptides, have also been assayed in DS mouse models. ADNP and ADNF are released by glial cells in the brain and are regulated by vasoactive intestinal peptide and they prevented developmental delay in the Ts65Dn mouse model of DS (Vink et al., 2009). However, it is not knows whether they increase or restore neurogenesis. A number of trisomic HSA21 genes have been proposed to play a role in these phenotypes, including Dyrk1A (Guimera et al., 1999; H€ammerle et al., 2003; Kentrup et al., 1996; Yabut et al., 2010; Yang et al., 2001), Olig1 and Olig2 (Chakrabarti et al., 2010; Lu et al., 2002; Takebayashi et al., 2000; Zhou and Anderson, 2002) and App (Trazzi et al., 2011). In principle, it could be argued that their dosage normalization could also restore neurogenesis. In fact, the inhibitors of the activity of 3 Dendritic remodelers in Down syndrome DYRK1A, ALGERNON, (Nakano-Kobayashi et al., 2017) and epigallocatechin gallate (EGCG) (Stagni et al., 2016) promote neurogenesis in trisomic mice. One important caveat regarding the consideration of neurogenesis as a putative therapeutic target for DS adults is the controversial results about the actual existence of adult neurogenesis in humans. Early studies reporting adult neurogenesis in the human DG (Eriksson et al., 1998; Knoth et al., 2010; Roy et al., 2000; Spalding et al., 2013) have been questioned by a recent report that could not detect neurogenesis in the human DG after the second year of life (Sorrells et al., 2018). Conversely, two studies have demonstrated neurogenesis in the hippocampus of healthy humans up to the eighth (Boldrini et al., 2018) and ninth (Moreno-Jimenez et al., 2019) decade of life. Moreno-Jimenez et al. also reported that the number of newborn neurons progressively declined as AD advanced. In summary, although there are numerous promising therapeutic strategies that increase the cognitive abilities of TS mice through enhancing neurogenesis, only continuous cognitive and physical stimulation are currently used in clinics, and have been proved to be effective in the DS population. It remains to be demonstrated whether drugs that enhance neurogenesis and cognition in mouse models of DS could be useful in the DS population. 3 Dendritic remodelers in Down syndrome: The case of epigallocatechin-3-gallate In DS, the assumption is that deficits in activity-dependent plasticity lead to abnormal dendritic arborization that in turn would impact on a neuron’s function and network connectivity and potentially contribute to learning and memory deficits (Dierssen, 2012). Similar alterations have been linked to other neurodevelopmental disorders, but are also a dominant feature of neurodegenerative diseases, suggesting possible converging mechanisms. In the field of intellectual disability, the correction of the dendritic phenotype has been used as readout of treatment efficacy and, in fact, dendritic spine remodeling is usually associated to cognitive improvements in mouse models. As such, many of the drugs mentioned above to increase neurogenesis, also affect dendritic spine density. In this chapter we will deserve more attention to the flavonoid epigallocatechin-3-gallate (EGCG), because it is one of the few molecules that has demonstrated positive, though sub-clinic, pro-cognitive effects in a phase II clinical trial in adults with DS (De la Torre et al., 2016). Green tea extracts contain a number of catechins, including, EGCG, epigallocatechin (EGC), epicatechin-gallate (ECG), and epicatechin (EC). Profiling catechins in human plasma and urine after tea consumption showed that absorption, excretion, and metabolism of catechins affect the bioavailability and potency, leading to differential bioactivities (Fung et al., 2013; Lee et al., 1995). EGCG is the most abundant and potent catechin extracted from green tea. It crosses both the blood–brain barrier (Lin et al., 2007) and the placental barrier in gestating rats (Chu et al., 2007). EGCG has a plethora of different effects, and has thus been investigated in studies from various research areas, including cancer 273 274 CHAPTER 9 Plasticity as a therapeutic target (Scarpa and Ninfali, 2015) and neurodegenerative disorders. Based upon its chemical structure, EGCG is classified as an antioxidant, which would counteract increased oxidative stress resulting from the over activity of CuZnSOD1 in DS. It also inhibits the transformation of nitrate and peroxynitrite into nitric oxide and the microglia-mediated inflammatory response, thus decreasing neuronal damage (Zhou et al., 2018). Other direct actions of EGCG are independent from antioxidative mechanisms. EGCG interacts with proteins and phospholipids in the plasma membrane and regulates signal transduction pathways, transcription factors, DNA methylation, mitochondrial function and phosphorylation and autophagy (Shi et al., 2018), to exert many of its beneficial biological actions. In vitro EGCG affects a wide array of neuronal signal transduction pathways involved in plasticity, including JAK/STAT, MAPK, PI3K/AKT, WNT and NOTCH (Singh et al., 2011) (Fig. 1). FIG. 1 Mechanisms of action of EGCG. Among others, EGCG targets include cell cycle proteins, protein kinases, transcription factor, antiapoptotic proteins, growth factors and apoptotic proteins. Adapted from Singh, B.N., Shankar, S., Srivastava, R.K., 2011. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 82, 1807–1821. 3 Dendritic remodelers in Down syndrome A recent paper suggested that EGCG could be beneficial through its capability to inhibit metalloproteinase 9 (MMP-9). In DS, the metabolic pathway of nerve growth factor (NGF) is altered due to deregulation of proteolytic enzymes such as MMP-9 and the plasminogen activation system, so that EGCG would protect NGF from degradation acting on MMP-9, but also theaflavin inactivates PAI-1, which inhibits tPA via PI3K/AKT, and MAPK (Wyganowska-Świa˛tkowska et al., 2018). EGCG also interferes with epigenetic mechanisms at various levels, affecting the chromatin opening state. It inhibits both DNA methyltransferases (Fang et al., 2003) and class I histone deacetylases (HDAC 1,2,3,8) (Saldanha et al., 2014; Thakur et al., 2012), it reduces the level of H327me3 and H2AK119 ubiquitination by reducing polycomb protein levels (Choudhury et al., 2011), and affects miRNAs expression (Milenkovic et al., 2012). As an example, EGCG increases methylation of alphasynuclein (SNCA) promoter proximal CpG sites and expression in Ts65Dn mice (Ramakrishna et al., 2016). The property of EGCG of modulating epigenetic changes makes it an ideal candidate for the treatment of DS, as its widespread epigenetic effect might re-establish the lost epigenetic balance (De Toma et al., 2016). DS individuals develop early in life Alzheimer’s disease neuropathology, in part due to APP overexpression. In the hippocampus of Alzheimer’s disease (AD) models, EGCG treatment normalizes the expression of synaptic proteins (Guo et al., 2017; Xicota et al., 2017). Strikingly, EGCG is also effective in other neurodegenerative disorders, such as Parkinson’s disease (Singh et al., 2016). EGCG decreases beta-amyloid levels and plaques via ADAM10-mediated promotion of the alpha-secretase proteolytic pathway, and modulates tau-profiles leading to cognitive improvements (Rezai-Zadeh et al., 2008). This effect is mediated via an estrogen receptor-a (ERa)/phosphoinositide 3-kinase/Ak-transforming dependent mechanism (Fernandez et al., 2010). EGCG also conjugates directly with not folded natural peptides to inhibit the formation of toxic intermediate products of α-synaptic nucleoprotein and amyloid protein and form a nontoxic and disordered oligomer of these two proteins (Ehrnhoefer et al., 2008). The heterogeneous effects of EGCG make it difficult to fully identify and understand the underlying molecular therapeutic mechanisms, but most studies show an amelioration of neural plasticity (De Toma et al., in press; Xie et al., 2008). 3.1 Dyrk1A inhibition as one mechanism of action of green tea extracts containing EGCG In regards to the treatment of DS cognitive impairment, possibly one of the most interesting property of EGCG is its capability to inhibit the kinase activity of DYRK1A, a member of the Dual-specificity tyrosine phosphorylation-regulated kinase (DYRK) family, belonging to the CGMC kinome group. DYRK1A is one of the major candidate genes to explain DS phenotypes. In DS, the triplication of human chromosome 21 leads to approximately 1.5-fold higher DYRK1A levels compared to the general population (Dowjat et al., 2007; Guimerá et al., 1996; Guimera et al., 1999) and its overproduction has been linked to the neuronal phenotypes and 275 276 CHAPTER 9 Plasticity as a therapeutic target cognitive deficits associated with DS (Altafaj et al., 2001). DYRK1A is expressed in the developing and adult brain and is implicated in cell proliferation and neuronal development (Dierssen and Martı́nez de Lagrán, 2006; H€ammerle et al., 2003). Its overexpression leads to disturbances in a wide range of signaling pathways involved in neural progenitor proliferation and differentiation. This may explain proliferation deficits (H€ammerle et al., 2011), dendritic branching defects (Martinez de Lagran et al., 2012; Tejedor and H€ammerle, 2011), or the imbalance of excitation/inhibition (Souchet et al., 2014) that leads to impaired oscillatory activity in the prefrontal cortex (Ruiz-Mejias et al., 2016). DYRK1A is also involved in neurodegeneration and neuronal loss appearing in AD (Ferrer et al., 2005) through hyperphosphorylation of Tau (Ryoo et al., 2007) and APP (Kimura et al., 2007; Ryoo et al., 2008), proteins at the origin of senile plaques and neurofibrillary tangles. Given the deleterious effects of DYRK1A overexpression, a plausible therapeutic strategy for DS would entail controlled inhibition of its expression or the activity of DYRK1A kinase. Ortiz-Abalia et al. (2008) using inhibitory RNA against Dyrk1A delivered by bilateral intra-striatal injections of adeno-associated virus type 2 (AAVshDyrk1A) in 2–3 month-old TgDyrk1A mice, led to a reversal of corticostriatal-dependent phenotypes, as revealed by the attenuation of their hyperactive behavior, the restoration of motor-coordination defects in the treadmill test, and an improvement in sensorimotor gating, as detected in the prepulse inhibition of startle reflex. Using the same strategy in Ts65Dn mice, led to attenuation of the hippocampal synaptic plasticity defects, accompanied by a normalized thigmotactic behavior in the Morris water maze (MWM), indicating partial improvement of their hippocampal-dependent search strategy, but with no positive effects in the escape latency or distance traveled (Altafaj et al., 2013). Normalization of the copy number of Dyrk1A attained by crossing Ts65Dn with heterozygote Dyrk1A +/ mice, resulted in an improvement of the MWM, fear conditioning test and LTP, but did not rescue behavioral alterations (hyperactivity/attention) nor it improved the density of mature hippocampal granule cells in the dentate gyrus (Garcı́aCerro et al., 2014). In most studies, normalization of Dyrk1A improves the behavioral and neural phenotypes of mice overexpressing Dyrk1A but also of Ts65Dn mice. However, the level rescue is variable depending on the age of the mice, and the method used for dosage normalization (shRNA or crossings with Dyrk1A +/). These somewhat positive results obtained encouraged the use of strategies that could be more translational, as is the use of DYRK1A kinase inhibitors. For this reason, DYRK1A has become a target for DS drug development (Duchon and Herault, 2016) and several molecules have been identified and/or developed to inhibit DYRK1A activity, including harmine, EGCG, INDY, FINDY, leucettine L41 and CX-4945 (Adayev et al., 2011; Darwish et al., 2018a,b; Fant et al., 2014; Naert et al., 2015; Ogawa et al., 2010; Kii et al., 2016; Kim et al., 2016; Stringer et al., 2017a,b). Harmine, for example, prevents premature neuronal maturation of trisomic NPCs from the periventricular zone of newborn Ts65Dn mice (Mazur-Kolecka et al., 2012). These molecules have only recently been developed and have not undergone extensive preclinical testing and some may have limited pharmacotherapeutic value 3 Dendritic remodelers in Down syndrome due to side effects [e.g., harmine has side effects associated with monoamine oxidase A inhibition; (Kim et al., 1997)]. Chronic DYRK1A inhibition using DYR219, a potent and selective small molecule inhibitor, reduces insoluble forms of amyloid beta peptides (Aβ) and hyper-phosphorylated tau associated with dramatic delay in the onset of both amyloid plaques and NFTs (Velazquez et al., 2019). In human studies, the overexpression of DYRK1A was reported to suppress the activity of neprilysin (NEP), which is a major Aβ-degrading enzyme in the brain, and phosphorylation at the NEP cytoplasmic domain. NEP activity was markedly reduced in fibroblasts derived from DS patients compared to healthy controls. This impaired activity of NEP was rescued by DYRK1A inhibition (Asai et al., 2017). EGCG is a natural inhibitor of the kinase activity of DYRK1A through noncompetitive inhibition against ATP binding site (Adayev et al., 2006), with an apparent IC50 of 0.33 μM (Bain et al., 2003; Wang et al., 2012). There are several studies showing the efficacy of green tea extracts containing EGCG in transgenic mice, which only overexpress Dyrk1A in an otherwise disomic genetic context. In postweaning (3 week-old) TgDyrk1A mice, 1-month oral treatment with a green tea extract containing EGCG (2–3 mg/day) induced normalization of the excessive proliferation and accelerated cell cycle exit in the granular cellular layer of the DG (Pons-Espinal et al., 2013), and the same dose rescued the deficient recognition memory in 3-month old TgDyrk1A mice as measured with the novel object recognition (NOR) test (De la Torre et al., 2014). These changes were accompanied by a normalization of hippocampal DYRK1A kinase activity levels, suggesting a potential pharmacological role of EGCG to tackle DS altered neurodevelopment and adult phenotypes. The same treatment in 3–4 month-old mice mBACtgDyrk1a at a higher dose (120–200 mg/kg/day EGCG), for 4–6 weeks led to improvement of spine density in prefrontal cortex pyramidal neurons and normalization of LTP (Thomazeau et al., 2014). In a more recent study in 3–4 month-old mBACTgDyrk1A mice, green tea extracts containing EGCG at doses of 60 mg/kg/day for 4 weeks rebalanced the excitation/inhibition since it rescued of glutaminergic expression in the cerebral cortex and the hippocampus, along with an improvement of spontaneous alternation (Souchet et al., 2015). Interestingly, when green tea extracts containing EGCG were given to pregnant mBACTgDyrk1A females, it prevented the alterations in brain volume and cognitive deficits of their pups (Guedj et al., 2009). Also in the YACtg152F7 an equivalent dose of 0.6–1 mg/day EGCG using polyphenon 60 a green tea extract containing EGCG from gestation to adulthood, rescued brain weight and volume of hypothalamus/thalamus and led to improvement of recognition memory in the NOR test. More recent work has confirmed that classes of synthetic DYRK1A inhibitors, the leucettines, which cross the blood–brain barrier and selectively inhibit DYRK1A (Nguyen et al., 2018) leads to normalization of DYRK1A activity and corrects the novel object cognitive impairment observed in several DS mouse models (Tg(Dyrk1a), Ts65Dn, Dp1Yey). Intriguingly, leucettines and green tea extracts containing EGCG only inhibit the fraction of DYRK1A that is overexpressed, and most native, basal DYRK1A is not inhibited. This finding is encouraging in terms of potential therapeutic implications, 277 278 CHAPTER 9 Plasticity as a therapeutic target as complete inhibition of DYRK1A is not desired. Although the mechanism of such selectivity in the inhibitory action is not understood, it has been suggested that this selective effect on DYRK1A overexpression could be linked to the accumulation of excess DYRK1A and DYRK1A inhibitors in specific cellular compartments (Nguyen et al., 2018). Taken together, these results suggested that, in mice, EGCG could partially rescue some neural and behavioral phenotypes and DYRK1A kinase inhibition is possibly an important mechanism in the EGCG effects in DS. In fact, if green tea extracts containing EGCG optimally normalizes DYRK1A activity, additional imbalances of some encoded proteins relevant to DS phenotypic features, such as APP, SYNJ1 and RCAN could be prevented, as those are phosphorylation substrates of DYRK1A. Part of those effects could explain the therapeutic effect of green tea extracts containing EGCG on AD-like phenotypes in DS, also partially mediated by enhanced tau exon 10 inclusion, leading to an increase in 4R-tau/3R-tau ratio in differentiated-human neuronal progenitors and in the neonatal rat brains (Smith et al., 2012). In fact, treatment with EGCG from gestation to adulthood suppressed 3R-tau expression and rescued anxiety and memory deficits in Ts65Dn mouse brains (Yin et al., 2017). Remarkably, EGCG also potentiates the effects of environmental enrichment (Catuara-Solarz et al., 2015, 2016). All these results indicate that it is rather unlikely that the benefits of green tea extracts containing EGCG in DS are limited to the inhibition of DYRK1A kinase activity and the beneficial effects observed in Ts65Dn and other partial trisomic mice are most probably contributed by some of the mechanisms delineated above. 3.2 Effects of green tea extracts containing EGCG on the Ts65Dn model of Down syndrome Although certainly important, the fact that green tea extracts containing EGCG normalized the phenotypes derived from single overexpression of Dyrk1A did not allow to assume whether it would be also beneficial in an inherently complex trisomic context, with hundreds of over- or under-expressed genes. Already 10 years ago Xie et al. showed that it was possible to rescue levels of LTP at the Schaffer collateral-CA1 synapse in hippocampal slices from Ts65Dn mice incubated with EGCG (10 μM) (Xie et al., 2008). Such rescue of synaptic plasticity suggested a potential benefit of EGCG as pro-cognitive therapy for DS. More recently in NPCs isolated from the hippocampus of Ts65Dn mice Valenti et al. (2016) showed that EGCG (20 μM) restored oxidative phosphorylation efficiency and mitochondrial biogenesis, and improved NPC proliferation, suggesting that EGCG has the potential to improve neurogenesis alterations in DS, as shown in transgenic TgDyrk1A mice (Pons-Espinal et al., 2013). The first study describing the effects of EGCG in vivo in the Ts65Dn model of DS was performed in adult mice, and showed beneficial effects of green tea extracts containing 45% EGCG on learning and memory, assessed using the Morris water maze and novel object recognition tests, in adult (3 months of age) Ts65Dn mice 3 Dendritic remodelers in Down syndrome (De la Torre et al., 2014). Another study using polyphenon 60 in drinking water at 225 mg/kg/day, containing 27% EGCG (60 mg/kg/day) for 6 weeks in 3–4 monthold trisomic mice confirmed the cognitive amelioration, and have suggested the rescue of the excitation inhibition imbalance as a possible underlying mechanism (Souchet et al., 2015). In 5–6 month-old mice decaffeinated green tea extracts containing 45% EGCG in drinking water (EGCG dose of 30 mg/kg/day) administered for 1 month, produced some improvement of the Gallagher index and the thigmotaxis along learning sessions, suggesting amelioration of the learning strategy, but with no impact on the latency to reach the escape platform (Catuara-Solarz et al., 2015). This may suggest that the effects of EGCG can vary with age and that the dosage range or the bioavailability of EGCG in different preparations may have an impact of the effects detected. However, not all the studies provide the same positive results. Two studies by the same research group, using different doses (high and low; 20 and 50 mg/kg/day) of EGCG (Sigma Aldrich, >95% purity) showed that neither the short- nor long-term treatment improved performance in a battery of behavioral tasks and even led to detrimental effects on skeletal phenotypes (Stringer et al., 2015, 2017a,b) even though previous studies by the same group showed beneficial effects of EGCG on skeletal phenotypes. Treating Ts65Dn mice at weaning for 3 weeks with the same concentration of EGCG used in human studies (9 mg/kg/day) led to a “substantial improvement in the postnatal femoral phenotype” at 6 weeks of age, although it did not significantly lower the DYRK1A activity level, similar to that found by crossing Ts65Dn with Dyrk1a +/ mice (Blazek et al., 2015). Given that EGCGcontaining supplements are widely available, in a later work, the authors analyzed six commercially available supplements containing EGCG, and compared two of those with pure EGCG for their impact on skeletal deficits in a DS mouse model. The results showed differential effects of commercial supplements on correcting skeletal abnormalities in Ts65Dn mice, suggesting that the dose of EGCG and composition of EGCG-containing supplements may be important. Given the neurodevelopmental load in DS, treatments in the neonatal period would possibly more efficacious in rescuing brain-related defects of DS. Daily injection of pure EGCG (25 mg/kg) in postnatal days P3–P15 was found restoration of neurogenesis, cellularity and connectivity in the hippocampus and neocortex of Ts65Dn mice, but those effects did not last after treatment cessation (Stagni et al., 2016). Also, another study in which EGCG was administered to Ts65Dn females in drinking water (3 mg/day) from mating to weaning of the pups and then, the pups were fed with EGCG-water, found that EGCG rescued anxiety and memory deficits in 2.5 months Ts65Dn mice and suppressed 3R-tau and promoted 4R-tau expression, rescuing the dysregulated 4R-tau/3R-tau ratio in Ts65Dn mouse brains (Yin et al., 2017). A more recent study (Souchet et al., 2019) showed that prenatal treatment with EGCG-complemented food pellets (50 mg/kg) restored VGAT1/VGLUT1 balance, and rescued density of GAD67 interneurons in Dp(16)1Yey mice, trisomic for 140 orthologs of chromosome 21, and improved novel object recognition memory, but not the performance of the Y maze paradigm in the adult (Souchet et al., 2019). 279 280 CHAPTER 9 Plasticity as a therapeutic target A very important aspect is that some of the molecular targets of EGCG, such as DYRK1A, or epigenetic factors, are also modulated by environmental enrichment, suggesting that EGCG can be considered an “environ-mimetic” drug boosting activity-dependent plasticity mechanisms. As mentioned above, a recent study showed that the combination of EE and EGCG acts synergistically in ameliorating learning alterations and age-related cognitive decline in DS (Catuara-Solarz et al., 2015), underlining the potential of combinatorial therapeutic approaches. In summary, the majority of the preclinical studies performed in mouse models of DS have reported improved behavioral outcomes with EGCG supplements, which can be variable in the behavioral domains affected and the degree of improvement. However, there are still many doubts and questions that can only be verified by well-designed preclinical studies evaluated by multidisciplinary teams. It is necessary to understand why some studies have shown that EGCG, even at high doses, does not improve behavioral outcome of Ts65Dn mice and why it can have even deleterious consequences to bone phenotypes. Several factors can account for these discrepancies: (i) the composition of the treatment (pure EGCG vs EGCG in combination with other polyphenols of green tea extracts); (ii) EGCG dosage and bioavailability; (iii) route of administration (food or drinking water); (iv) duration of the treatment; (v) age of the mice; (vi) species and strains; (vii) methods used to evaluate cognitive endpoints; (viii) possible effects of EGCG metabolites or products of its degradation; (ix) the relatively small sample size in conjunction with the intrinsic phenotypic variability of Ts65Dn mice. Other possible explanations for the divergent results obtained with EGCG compounds in adult trisomic mice could also be the different environmental conditions to which mice had been exposed. Given that Ts65Dn are mainly subdominant (Martı́nez-Cue et al., 2005), showing less aggressive behavior, one could think that social defeat or stressful environments such as isolation could certainly have a deleterious impact. This might impede the effects of EGCG containing compounds or other therapies to be found in a consistent way, and may contribute to the lack of effects detected in some studies (Stringer et al., 2015, 2017a,b). Thus, environmental conditions may impact the outcomes and comparability of the studies and would also explain the lack of a clear dosage dependency, given that the effects are the product of a combinatorial action of many molecular cascades, and also depend on the basal state, in a kind of “homeostatic” systems effect. In fact, most of these concerns could be applied to most preclinical studies not only in DS but also in other intellectual disabilities. We certainly need to gain a more systematic view, to understand the variation in the behavioral and brain neuropathological deviations in DS mouse models, which will certainly impact the effects of therapy. This can only be achieved by profiling the effects of the drugs in different mouse strains and DS models in parallel in the same lab, at different time points and with different doses. Given that DS still has no therapy to ameliorate intellectual disability, it is certainly worth continuing these studies. 3 Dendritic remodelers in Down syndrome 3.3 Translational value of preclinical studies with EGCG: Effects in individuals with Down syndrome Since EGCG is readily available at reasonable costs, its promising effects in preclinical studies prompted several clinical trials and clinical studies. At the cellular level, studies in human NPCs and neurons derived from DS-iPSCs treatment with 10 μM EGCG the same effects detected in mouse cellular models of improved proliferation and decrease of apoptosis were also detected (Hibaoui et al., 2014). In lymphoblast and fibroblast cultures from subjects with DS treatment with EGCG (20 μM) rescued mitochondrial function and promoted mitochondrial biogenesis (Valenti et al., 2013). This study prompted a case study in a child (10-year and 3-month-old) with DS who was treated with ECCG (10 mg/kg/day) plus fish oil daily for 6 months (Vacca and Valenti, 2015). This treatment was safe and improved mitochondrial function. However, since EGCG was co-administered with fish oil, the extent to which fish oil contributes to this effect remains to be established. To the best of our knowledge, only one research group (Hospital del Mar; Spain) has led the randomized clinical studies using green tea extracts containing EGCG. In a first pilot double-blind randomized, placebo-controlled phase I clinical trial (ClinicalTrials.gov ID: NCT01699711, De la Torre et al., 2016), young adults with DS (29 subjects) aged 14–29 years were treated with either green tea extracts in capsule form (Mega Green Tea Extract, Decaffeinated, Life Extension®, USA) containing 45% EGCG (mean EGCG oral dose of 9 mg/kg/day; 6 females, 7 males) or a placebo (8 females, 8 males) for 3 months. The most important aspect of this first clinical trial was to determine the safety and toxicity of the treatment, since DS individuals may be more sensitive than euploids to some adverse effects. Even so, some neuropsychological and quality of life tests were performed after 3 months of treatment and 3 months after treatment discontinuation. The trial was also intended to identify DYRK1A activity surrogate biomarkers comparing plasma homocysteine (hcy) levels (Noll et al., 2009, 2012) at baseline and at the end of treatment (3 months). Importantly, the biochemical results allowed establishing a significant correlation between memory improvement and Hcy levels, suggesting a putative dependence of cognitive improvement on DYRK1A kinase activity normalization. An important concern before initiating the clinical trial was hepatic toxicity that had been shown in several reports, although mainly at higher doses. In the Phase I clinical study in DS carried out in 2014–2016, no alteration of hepatic function markers (AST and ALT) was observed. Supporting these observations, in 2018, the European food safety authority (EFSA) scientific panel on food additives and nutrient sources added to food (ANS) published a report (Question number: EFSA-Q-2016-00627; Younes et al., 2018). After reviewing the evidence from 38 intervention studies, which included data on effects of green tea extracts and infusions on serum transaminases, the panel concluded that exposure to green tea extracts at doses at or above 800 mg EGCG/day for 4 months or longer, are only associated with elevations of ALT and AST in a small percentage (usually less than 10%) of the 281 282 CHAPTER 9 Plasticity as a therapeutic target population. Moderate or more severe abnormalities in any liver function were observed in 5.1% of the treated subjects in a study with more than 500 subjects treated with 843 mg EGCG/day for 1 year (Yu et al., 2017). Thus, EFSA experts considered green tea catechins, including EGCG, to be safe and the rare cases of liver injury that have been reported after consumption of green tea infusions, to be most probably due to an idiosyncratic reaction. Animal studies had also reported EGCG to be involved in redox cycling and quinone formation having both antioxidant and pro-oxidative activities that may lead to oxidative stress (Dostal et al., 2015; Sang et al., 2005). However, in the Phase I clinical trial no increase of biomarkers of oxidative stress (oxLDL and GSH-Px activity) was found, but instead a reduction of lipid oxidation in subjects treated with EGCG was detected. This improvement of the oxidative/ antioxidative status combined with a healthy lipid profile (Xicota et al., 2019), as shown by the total cholesterol and LDL cholesterol concentrations, is certainly considered beneficial. This is important since a recent study has carried out an anonymous survey about attitudes and usage of green tea extracts containing EGCG in individuals with DS completed by caregivers, in which most caregivers who did not use green tea extracts containing EGCG reported concerns about potential side effects and lack of effectiveness (Long et al., 2019). Regarding the cognitive and behavioral effects, after 3 months of treatment, EGCG-treated individuals showed a significantly higher percentage of correct answers in visual memory recognition compared with those who had been given placebo. Three months after treatment discontinuation this effect declined, and treated subjects returned to baseline scores. This indicated that treatment with EGCG has a positive effect on cognition in DS, albeit moderate and transitory. In a subsequent clinical study, and based on preclinical studies showing that green tea extracts containing EGCG potentiated the effects of environmental enrichment (Catuara-Solarz et al., 2016), it was examined whether EGCG could potentiate the effects of cognitive stimulation, by comparing the effect of cognitive training with placebo or cognitive training plus green tea extract supplement in capsule form containing 45% EGCG (Life Extension Decaffeinated Mega Green Tea Extract; Life Extension ®, USA). The mean EGCG oral dose was the same as in the phase I trial (9 mg/kg/day). Of course this study does not allow disentangling the effects of EGCG, but from the therapeutic point of view it was the most promising for the patients. After a power analysis to calculate the sample size, in this double-blind randomized phase II clinical trial (ClinicalTrials.gov Identifier: NCT01699711), a larger group (87 subjects) of adults (age: 16–34 years) with DS was enrolled and treatment lasted 12 months. Including 1 month of treatment with a placebo allowed to control for the placebo effect, which is very relevant in this population. No placebo effect was detected in either group (R. De la Torre et al., unpublished). Subjects were periodically tested with a battery of neuropsychological tests during the 12 months of treatment, immediately following treatment cessation, and at 6 months after treatment discontinuation. Primary outcome measures were cognitive evaluation, including cognitive domains such as episodic memory, executive function and adaptive behavior and quality of life. The secondary outcome included neuroimaging in a 3 Dendritic remodelers in Down syndrome subset of patients measuring regional brain morphology and volume (FLAIR) sequence, and intrinsic functional organization (i.e., functional connectivity) in the resting-state within the neural systems, and biochemical biomarkers. Baseline values of many techniques/variables used in these DS clinical trials had to be validated before evaluating their value for assessing therapeutic effects. For example, resting-state whole-brain connectivity degree maps generated in 20 DS individuals and 20 control subjects identified higher regional connectivity in a ventral brain system involving the amygdala/anterior temporal region and the ventral aspect of both the anterior cingulate and frontal cortices, and lower functional connectivity in dorsal executive networks involving dorsal prefrontal and anterior cingulate cortices and posterior insula (Pujol et al., 2015). Both correlated with DS scoring on adaptive behavior related to communication skills. After 12 months of treatment with EGCG, better performance was detected in two of the 15 cognitive performance tests and in one of the nine adaptive skills. Specifically, participants treated with EGCG and cognitive training had significantly higher scores in visual recognition memory (pattern recognition memory test immediate recall, adjusted mean difference: 6.23 percentage points (P ¼ 0.039), inhibitory control (cats and dogs total score, P ¼ 0.041 and total response time, P ¼ 0.024) and improved performance of daily tasks requiring basic literacy (ABAS-II functional academics score, P ¼ 0.002). This last finding was encouraging since benefits in functional academics imply an improvement in the use of basic literacy skills (reading, writing, and mathematics), allowing a better daily independent functioning (i.e., recognize the time, be able to read and write small notes, recognize small quantities when paying, and performing small operations). Even though these effects were small and of subclinical magnitude, they were accompanied by a marked enhancement of regional functional connectivity with significant increases in the functional integration of cortical and subcortical distributed networks, including the frontal cortex, Wernicke area, the precuneus, occipitotemporal and somatosensory cortices, and basal ganglia, in the resting-state whole-brain connectivity degree maps (fcMRI) measurements. Besides, cortical excitability normalization was detected in the noninvasive paired pulse transcranial magnetic stimulation (TMS) studies. These effects could also contribute to the improved cognitive abilities in individuals treated with EGCG and cognitive training, since treatment-related changes in functional connectivity within frontal networks were significantly correlated with the increase in ABAS-II functional academic skills and cognitive deficits in DS have been proposed to result from an excitation or inhibition imbalance with an excess of synaptic inhibition in the hippocampus and increased excitation in the cerebral cortex. It should be remarked that the 2016 study by De la Torre et al., has several limitations: (i) treatment with EGCG was combined with cognitive training and no group received EGCG only. This was due to the reduced capability of recruiting individuals willing to participate in a study in which they could be receiving only placebo without offering any active treatment. Of course this limits the study to determine whether controlled cognitive stimulation had an effect, and whether EGCG could potentiate it. Thus, it remains to be established whether EGCG worked by itself in terms of 283 284 CHAPTER 9 Plasticity as a therapeutic target improving scores in cognitive measures in individuals with DS or whether it has to be combined with cognitive training; (ii) In addition, since the green tea extracts contain various polyphenols in addition to EGCG, the contribution of these polyphenols to cognitive improvement remains to be clarified and it cannot be ruled out that the effects ascribed to EGCG were due to interactions with other constituents in the extracts; (iii) the study was not large enough to assess the gender- and/or age-dependent effects of the treatments. However, gender and age were important co-factor, since women performed significantly better than men of the same age and IQ in most cognitive tests (De Sola et al., 2015), with the most consistent differences occurring in memory and executive functioning and negative trends emerged on quality of life linked to the effect of age after adjusting for IQ and gender, although this was not frequent. Other important factors may also influence the treatment outcome, such as genetic variants (Del Hoyo et al., 2016), the progression of neurodegenerative phenotypes in adults (Del Hoyo et al., 2015; Fenoll et al., 2017), quality of sleep (Horne et al., 2019), self-regulation, psychopathology, daily living/adaptive behavior, and maladaptive behavior. Finally, factors such as diet, years of education, or thyroid status, are also important. Running the trials raised some methodological challenges and questioned the prevailing methodology used to evaluate cognitive functioning of DS individuals. In fact previous studies had indicated the notable lack of consistency and reproducibility of ratings in some neuropsychological tests used in intellectual disability (Silverman et al., 2010). This was the motivation to devise and validate the TESDAD Battery (De Sola et al., 2015) that uses comparison with age-matched typically developed adults. In DS population the TESDAD battery allowed a quantitative assessment of cognitive defects, which indicated language dysfunction and deficits in executive function, as the most important contributors to other cognitive and adaptive behavior outcomes as predictors of functional change in DS. Concretely, verbal comprehension and functional academics showed the highest potential as end-point measures of therapeutic intervention for clinical trials: the former as a cognitive key target for therapeutic intervention, and the latter as a primary functional outcome measure of clinical efficacy. Later on, to address this challenge, the National Institutes of Health (NIH) assembled leading clinicians and scientists to review existing measures and identify those that are appropriate for trials (Esbensen et al., 2017). The available data in adult individuals with DS confirmed some benefits on cognitive performance indicating that the preclinical studies had indeed predictive validity. This is relevant because the validity of the Ts65Dn strain is severly questioned, because of the presence of an extra copy of non-HSA21 genes. However, it is important to note that it is treatment with EGCG plus cognitive training for 12 months, which is more effective and leads to increased functional connectivity and normalization of the excitability in the cerebral cortex. Cognitive training alone had only marginal effects, suggesting that treatments that are ineffective or scarcely effective if administered alone may elicit some benefits when they are given in combination with other drug or with cognitive stimulation. 4 Environmental enrichment 4 Environmental enrichment One of the systems to promote in vivo neuroplasticity in rodents is the use of an enriched environment (EE) that provides animals an increased physical exercise, learning experiences, and social interaction. In the context of DS, nowadays, active care programs are one of the most successful therapeutic interventions used in DS individuals. Moreover early intervention programs in DS children are the only effective have been developed in an attempt to improve cognitive capabilities. However, despite resulting in positive results, those are limited and temporary. Environmental enrichment (EE) in animals mimics a stimulating lifestyle at the laboratory level and has demonstrated beneficial effects on various aspects of brain structure, function, behavior and cognition and it leads to functional compensation in different brain disorders. During developmental stages, it mimics the effects of “early intervention” involving educational and neuroprotection strategies aimed at enhancing brain development. A large number of studies have highlighted the fact that EE modifies the behavior of animals, leading to improvement in complex cognitive functions, particularly learning and memory, and positively affecting the animal’s emotional and stress reactivity (Bayne, 2018). The basic experimental setup has hardly changed over decades: animals, normally rats or mice, are kept in larger groups and in a larger cage, often equipped with additional toys, nesting material and tubes to hide, and are compared with what are considered “standard housing” animals in the laboratory, which in fact could be a paradigm of impoverished environment. EE typically includes: (i) increased physical activity, usually provided by a running wheel and larger cages, (ii) increased social grouping, and (iii) increased opportunities for exploration (i.e., larger environments relative to standard housing conditions) and interactions with novel objects such as toys (Sztainberg and Chen, 2010; Wood et al., 2011). Some of these components, such as physical activity, per se seem to provide most of the beneficial effects (Brown et al., 2003; Kobilo et al., 2011; Lazarov et al., 2010; Van Praag et al., 1999). Voluntary exercise is beneficial for cognition in both normal rodents and mouse models of altered cognition (Paylor et al., 1992; Van Praag, 2008; Van Praag et al., 1999). It has been suggested that these beneficial effects could be mediated, at least in part, by enhanced hippocampal neurogenesis (Clark et al., 2008). The first study of EE in a DS mouse model used a paradigm that included an activity wheel, and lasted for 7 weeks after weaning (Martı́nez-Cue et al., 2002). The study analyzed 86 female and 75 male Ts65Dn mice, and tested circadian spontaneous activity, exploratory behavior, locomotor activity in the open field and spatial memory in the Morris water maze paradigm (MWM). However, in these experiments gender was a relevant factor in determining the influence of enrichment on behavior and learning so that EE improved performance in trisomic females, but deteriorated it in trisomic males. Such negative influence of EE on Ts65Dn males was interpreted as derived from the change of the hierarchical organization leading to increased levels of aggressiveness and stress that could interfere indirectly with their learning 285 286 CHAPTER 9 Plasticity as a therapeutic target processes in a rather aversive circumstance, such as the MWM. This was further confirmed in a study using different enrichment conditions, housing in large social groups or social condition and enrichment in social condition, compared to standard housing (Martı́nez-Cue et al., 2005). Neither social nor physical stimulation alone had positive effects, but also did not disturb Ts65Dn mice performance; however, the combination of both social and physical stimulation deteriorated spatial learning in trisomic mice that showed subordinate behavior associated to stress, as supported by the corticosterone levels. Other studies in Ts65Dn mice have shown that voluntary physical exercise improved the cognitive abilities of trisomic mice and enhanced neurogenesis (Llorens-Martı́n et al., 2010; Parrini et al., 2017). Exposure of young adult Ts65Dn mice (P60) to EE for 6 weeks induced a marked recovery of both cognitive and visual functions, reduced brain inhibition and promoted hippocampal synaptic plasticity, but effects in male and female did not differ (Begenisic et al., 2011). This lack of gender-specific effects may depend on the differences in the age of onset of EE, but could have also been prevented by using a protocol of EE in which only 2–3 Ts65Dn males from the same litter were reared together to avoid an excess of social stimulation that could disturb their behavioral and learning skills. Using a different paradigm, in which Ts65Dn and control mothers were breed in enriched or standard conditions, EE resulted in a robust increase in maternal care levels displayed by Ts65Dn mothers (Begenisic et al., 2015). Maternal care is obviously a very potent stimuli, but living from birth in enriching conditions also gives the animals the opportunity for enhanced social, cognitive and motor stimulation autonomously experienced by the developing animals once their reach the weaning age. This EE paradigm led to a normalization of declarative memory abilities and hippocampal plasticity and rescue of visual system maturation in trisomic offspring. The beneficial EE effects were accompanied by increased BDNF and correction of overexpression of the GABA vesicular transporter vGAT (Begenisic et al., 2015). Moreover, exposure to EE increases hippocampal neurogenesis and the integration of newly born cells into functional circuits. In Ts65Dn mice, both short-term (P18–P30) and long-term (P18–P45) EE enhanced cell proliferation and neurogenesis in the DG and SVZ both in male and female (Chakrabarti et al., 2011). This result seems contradictory to the study of Martı́nez-Cue et al. (2002; see above) in which performance of trisomic males was deteriorated, and was interpreted as differences in the EE design due to the presence of running wheels. However, in fact both experiments incorporated activity wheels, but given that Chakrabarti et al. did not study cognitive performance; direct conclusions about the involvement of neurogenesis in the cognitive effects of EE cannot be extracted. Rodents living in EE conditions display prominent changes at the anatomical level, with robust increments in cortical thickness and weight and modifications of neuronal morphology, in terms of increased dendritic arborization, number of dendritic spines, synaptic density and postsynaptic thickening, occurring in several regions of the brain, particularly in the cerebral cortex and hippocampus (Baroncelli et al., 2010; Hirase and Shinohara, 2014). While 3-week exposure to EE during late development 4 Environmental enrichment (P30) correlated with 32% higher number of spines in the dendritic arbors of layer III pyramidal cells of 1-year-old enriched controls, enriched Ts65Dn mice only had 3% more spines (Dierssen et al., 2003). This suggested that EE does not have long-term structural plasticity effects in pyramidal cells of the Ts65Dn mouse or, alternatively, that trisomic mice lose a higher proportion of spines during aging. At the molecular level, EE in trisomic mice causes a significant restoration of the G-protein-associated signal transduction systems, which are altered in Ts65Dn mice (Dierssen et al., 1996) and in DS (Lumbreras et al., 2006) affecting the cholinergic and noradrenergic systems, which could be beneficial in DS (Dierssen et al., 1997). EE also significantly increased cyclic AMP production in Ts65Dn mice, while it was not modified in control animals (Baamonde et al., 2011). Similarly, EE increased phospholipase C activity in Ts65Dn mice, in response to carbachol and calcium. DYRK1A is also regulated by EE that normalize DYRK1A levels (Pons-Espinal et al., 2013). EE has been also found to either prevent or reverse many neuropathological and behavioral signs of AD in several transgenic mouse models of this pathology (Sale et al., 2014; Sansevero and Sale, 2017). In aged Ts65Dn mice, long-term exposure to EE from P60 until 12 month of age prevents the age-dependent hippocampal increase of low molecular weight Aβ oligomer isoforms which trigger synapse failure and memory impairment in AD-like dementia (Sansevero et al., 2016). Aged enriched Ts65Dn mice, compared to controls reared in standard conditions, showed learning and memory performances that did not differ from those achieved by euploid controls (Sansevero et al., 2016). Interestingly, many of the effects reported for EE, such as neuroplasticity enhancement, antioxidant activity, anti-inflammatory function, neuroprotection and promotion of the nonamyloidogenic proteolytic pathway of APP are similar to those observed upon EGCG treatment. Combined EE-EGCG treatment improved corticohippocampal-dependent learning and memory. Cognitive improvements were accompanied by a rescue of CA1 dendritic spine density and a normalization of the proportion of excitatory and inhibitory synaptic markers in CA1 and dentate gyrus (Catuara-Solarz et al., 2016). EE has also been shown to normalize the expression levels and the kinase activity of DYRK1A (Pons-Espinal et al., 2013), and rescued adult neurogenesis alterations in in mice overexpressing Dyrk1A (TgDyrk1A). However, it is not known whether the same effects are present also in Ts65Dn, although the levels of APP and MAP2ab are reduced and the levels of SOD1 were increased upon voluntary exercise, with a slight (10%) reduction of DYRK1A (Kida et al., 2013). In spite of the overall positive effect of EE reported in trisomic mice, there are several contradictory results across labs, and many open questions remain to be addressed. One confounding factor is that the designs used by laboratories for social, physical, somatosensory enrichment are generally not uniform. Studies also often differ in animal sex, and types of controls used (e.g., isolated vs group standardhoused controls) that can further complicate findings and lead to varied outcomes between laboratories. An important confounder of the social vs nonsocial aspects 287 288 CHAPTER 9 Plasticity as a therapeutic target of the enrichment effects is the fact that Ts65Dn males experience higher social stress when reared in large social groups. It is not surprising then that studies have given ambiguous and sometimes contradictory results on the efficacy and effectiveness of interventions. 5 Future perspectives Targeting plasticity impairments in DS has revealed as a promising strategy to promote cellular mechanisms involved in learning and memory within, and could lead to improved connectivity among key cognitive brain regions. Promising pharmacological, environmental, and neurostimulation interventions may open a whole new range of possibilities. The potential of modulating abnormal plasticity patterns in DS is more promising during the first years of life when the brain is still under construction. Activitydependent plasticity during the postnatal period helps establishing connectivity patterns driving the brain maldevelopment. However, it should be taken into account that plasticity is not equally amenable for therapy in all brain regions and in all lifetime periods. The type of the neural circuit (low-level or high level, single or parallel processing) dictates the degree of plasticity or stability in response to perturbation during sensitive or critical periods of development (Harrison et al., 2005). An interesting possibility is that adult rescue of genetic defects would alleviate or correct developmental phenotypes by tapping into adult mechanisms of cellular, structural, and behavioral plasticity. In DS, however, the concept of “re-opening” the critical period for neural plasticity has not yet been explored and could be of great interest. Many preclinical studies have shown that this approach has beneficial effects at the behavioral, cellular and molecular levels. However, not all have translated into successful clinical trials. Important considerations for the translational value of this preclinical work are worth to note: (i) when interpreting beneficial preclinical treatment effects, we should not only consider the statistical significance of the effect but also if the magnitude is sufficient to translate it into a clinically meaningful improvement; and (ii) most of the studies on DS mouse models have been performed in male mice. In general, all these aspects may lead to both over- and underestimation of treatment effects and should be pondered when reading the preclinical studies presented below. Several guidelines outlining important measures to avoid bias have been prepared including the ARRIVE guidelines that should be embraced by the DS research community. References Adayev, T., Chen-Hwang, M.C., Murakami, N., Wegiel, J., Hwang, Y.W., 2006. Kinetic properties of a MNB/DYRK1A mutant suitable for the elucidation of biochemical pathways. Biochemistry 45, 12011–12019. Adayev, T., Wegiel, J., Hwang, Y.W., 2011. Harmine is an ATP-competitive inhibitor for dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). Arch. Biochem. Biophys. 507, 212–218. References Altafaj, J., et al., 2001. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down’s syndrome. Hum. Mol. Genet. 10, 1915–1923. Altafaj, X., Martı́n, E.D., Ortiz-Abalia, J., Valderrama, A., Lao-Peregrı́n, C., Dierssen, M., Fillat, C., 2013. Normalization of Dyrk1A expression by AAV2/1-shDyrk1A attenuates hippocampal-dependent defects in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 52, 117–127. Asai, M., Kawakubo, T., Mori, R., Iwata, N., 2017. Elucidating pathogenic mechanisms of early-onset Alzheimer’s disease in Down syndrome patients. Yakugaku Zasshi 137 (7), 801–805. Ash, J.A., Velazquez, R., Kelley, C.M., Powers, B.E., Ginsberg, S.D., Mufson, E.J., Strupp, B.J., 2014. Maternal choline supplementation improves spatial mapping and increases basal forebrain cholinergic neuron number and size in aged Ts65Dn mice. Neurobiol. Dis. 70, 32–42. Baamonde, C., Martı́nez-Cue, C., Flórez, J., Dierssen, M., 2011. G-protein-associated signal transduction processes are restored after postweaning environmental enrichment in Ts65Dn, a Down syndrome mouse model. Dev. Neurosci. 33 (5), 442–450. Bahn, S., Mimmack, M., Ryan, M., et al., 2002. Neuronal target genes of the neuron-restrictive silencer factor in neurosperes derived from fetuses with Down’s syndrome: a gene expression study. Lancet 359, 310–315. Bain, J., McLauchlan, H., Elliott, M., Cohen, P., 2003. The specificities of protein kinase inhibitors: an update. Biochem. J. 371, 199–204. Baroncelli, L., Braschi, C., Spolidoro, M., Begenisic, T., Sale, A., Maffei, L., 2010. Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ. 17, 1092–1103. Bayne, K., 2018. Environmental enrichment and mouse models: current perspectives. Anim. Model Exp. Med. 1 (2), 82–90. Begenisic, T., Spolidoro, M., Braschi, C., Baroncelli, L., Milanese, M., Pietra, G., Fabbri, M.E., Bonanno, G., Cioni, G., Maffei, L., Sale, A., 2011. Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome. Front. Cell. Neurosci. 5, 29. Begenisic, T., Sansevero, G., Baroncelli, L., Cioni, G., Sale, A., 2015. Early environmental therapy rescues brain development in a mouse model of Down syndrome. Neurobiol. Dis. 82, 409–419. Benavides-Piccione, R., Ballesteros-Yáñez, I., de Lagrán, M.M., Elston, G., Estivill, X., Fillat, C., Defelipe, J., Dierssen, M., 2004. On dendrites in Down syndrome and DS murine models: a spiny way to learn. Prog. Neurobiol. 74, 111–126. Bianchi, P., Ciani, E., Guidi, S., et al., 2010a. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for down syndrome. J. Neurosci. 30, 8769–8779. Bianchi, P., Ciani, E., Contestabile, A., Guidi, S., Bartesaghi, R., 2010b. Lithium restores neurogenesis in the subventricular zone of the Ts65Dn mouse, a model for Down syndrome. Brain Pathol. 20, 106–118. Blazek, J.D., Abeysekera, I., Li, J., Roper, R.J., 2015. Rescue of the abnormal skeletal phenotype in Ts65Dn Down syndrome mice using genetic and therapeutic modulation of trisomic Dyrk1a. Hum. Mol. Genet. 24, 5687–5696. Boldrini, M., Fulmore, C.A., Tartt, A.N., Simeon, L.R., Pavlova, I., Poposka, V., Rosoklija, G.B., Stankov, A., Arango, V., Dwork, A.J., Hen, R., Mann, J.J., 2018. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599.e5. 289 290 CHAPTER 9 Plasticity as a therapeutic target Brown, J., Cooper-Kuhn, C.M., Kempermann, G., Van Praag, H., Winkler, J., Gage, F.H., Kuhn, H.G., 2003. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 17, 2042–2046. Catuara-Solarz, S., Espinosa-Carrasco, J., Erb, I., Langohr, K., Notredame, C., Gonzalez, J.R., Dierssen, M., 2015. Principal component analysis of the effects of environmental enrichment and (-)-epigallocatechin-3-gallate on age-associated learning deficits in a mouse model of Down syndrome. Front. Behav. Neurosci. 9, 330. Catuara-Solarz, S., Espinosa-Carrasco, J., Erb, I., Langohr, K., Gonzalez, J.R., Notredame, C., Dierssen, M., 2016. Combined treatment with environmental enrichment and (-)-epigallocatechin-3-gallate ameliorates learning deficits and hippocampal alterations in a mouse model of Down syndrome. eNeuro. 3 (5), pii: ENEURO.0103-16.2016. Chakrabarti, L., Galdzicki, Z., Haydar, T.F., 2007. Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J. Neurosci. 27, 11483–11495. Chakrabarti, L., Best, T.K., Cramer, N.P., et al., 2010. Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat. Neurosci. 13, 927–934. Chakrabarti, L., Scafidi, J., Gallo, V., Haydar, T.F., 2011. Environmental enrichment rescues postnatal neurogenesis defect in the male and female Ts65Dn mouse model of Down syndrome. Dev. Neurosci. 33, 428–441. Chambers, C.D., Hernandez-Diaz, S., Van Marter, L.J., Werler, M.M., Louik, C., Jones, K.L., Mitchell, A.A., 2006. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 354, 579–587. Choudhury, S.R., et al., 2011. (-)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis 32, 1525–1532. Chu, K.O., Wang, C.C., Chu, C.Y., Choy, K.W., Pang, C.P., Rogers, M.S., 2007. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 22, 280–287. Clark, S., Schwalbe, J., Stasko, M.R., Yarowsky, P.J., Costa, A.C.S., 2006. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Exp. Neurol. 200, 256–261. Clark, P.J., Brzezinska, W.J., Thomas, M.W., Ryzhenko, N.A., Toshkov, S.A., Rhodes, J.S., 2008. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155, 1048–1058. Contestabile, A., Fila, T., Ceccarelli, C., et al., 2007. Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with Down syndrome and in Ts65Dn mice. Hippocampus 17, 665–678. Contestabile, A., Greco, B., Ghezzi, D., Tucci, V., Benfenati, F., Gasparini, L., 2013. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J. Clin. Invest. 123, 348–361. Corrales, A., Vidal, R., Garcı́a, S., Vidal, V., Martı́nez, P., Garcı́a, E., Flórez, J., SanchezBarceló, E.J., Martı́nez-Cue, C., Rueda, N., 2014. Chronic melatonin treatment rescues electrophysiological and neuromorphological deficits in a mouse model of Down syndrome. J. Pineal Res. 56, 51–61. Dang, V., Medina, B., Das, D., Moghadam, S., Martin, K.J., Lin, B., Naik, P., Patel, D., Nosheny, R., Wesson Ashford, J., Salehi, A., 2014. Formoterol, a long-acting β2 adrenergic agonist, improves cognitive function and promotes dendritic complexity in a mouse model of Down syndrome. Biol. Psychiatry 75, 179–188. References Darwish, S.S., Abdel-Halim, M., Salah, M., Abadi, A.H., Becker, W., Engel, M., 2018a. Development of novel 2,4-bispyridyl thiophene-based compounds as highly potent and selective Dyrk1A inhibitors. Part I: benzamide and benzylamide derivatives. Eur. J. Med. Chem. 157, 1031–1050. Darwish, S.S., Abdel-Halim, M., ElHady, A.K., Salah, M., Abadi, A.H., Becker, W., Engel, M., 2018b. Development of novel amide-derivatized 2,4-bispyridyl thiophenes as highly potent and selective Dyrk1A inhibitors. Part II: identification of the cyclopropylamide moiety as a key modification. Eur. J. Med. Chem. 158, 270–285. Das, I., Park, J.M., Shin, J.H., Jeon, S.K., Lorenzi, H., Linden, D.J., Worley, P.F., Reeves, R.H., 2013. Hedgehog agonist therapy corrects structural and cognitive deficits in a Down syndrome mouse model. Sci. Transl. Med. 5, 201ra120. De la Torre, R., De Sola, S., Pons, M., Duchon, A., de Lagran, M.M., Farre, M., Fitó, M., Benejam, B., Langohr, K., Rodriguez, J., Pujadas, M., Bizot, J.C., Cuenca, A., Janel, N., Catuara, S., Covas, M.I., Blehaut, H., Herault, Y., Delabar, J.M., Dierssen, M., 2014. Epigallocatechin-3-gallate, a dyrk1a inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol. Nutr. Food Res. 58, 278–288. De la Torre, R., de Sola, S., Hernandez, G., Farre, M., Pujol, J., Rodriguez, J., Espadaler, J.M., Langohr, K., Cuenca-Royo, A., Principe, A., Xicota, L., Janel, N., Catuara-Solarz, S., Sanchez-Benavides, G., Blehaut, H., Dueñas-Espı́n, I., Del Hoyo, L., Benejam, B., Blanco-Hinojo, L., Videla, S., Fitó, M., Delabar, J.M., Dierssen, M., 2016. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 5, 801–810. De Sola, S., de la Torre, R., Sánchez-Benavides, G., Benejam, B., Cuenca-Royo, A., Del Hoyo, L., Rodrı́guez, J., Catuara-Solarz, S., Sanchez-Gutierrez, J., Dueñas-Espin, I., Hernandez, G., Peña-Casanova, J., Langohr, K., Videla, S., Blehaut, H., Farre, M., Dierssen, M., TESDAD Study Group, 2015. A new cognitive evaluation battery for Down syndrome and its relevance for clinical trials. Front. Psychol. 6, 708. De Toma, I., Manubens-Gil, L., Ossowski, S., Dierssen, M., 2016. Where environment meets cognition: a focus on two developmental intellectual disability disorders. Neural Plast. 2016, 4235898. De Toma, I., Ortega, M., Aloy, P., Sabidó, E., Dierssen, M., 2019. DYRK1A overexpression alters cognition and neural-related proteomic pathways in the hippocampus that are rescued by green tea extract and/or environmental enrichment. Front. Mol. Neurosci. in press. Del Hoyo, L., Xicota, L., Sánchez-Benavides, G., Cuenca-Royo, A., de Sola, S., Langohr, K., Fagundo, A.B., Farre, M., Dierssen, M., de la Torre, R., 2015. Semantic verbal fluency pattern, dementia rating scores and adaptive behavior correlate with plasma Aβ42 concentrations in Down syndrome young adults. Front. Behav. Neurosci. 9, 301. Del Hoyo, L., Xicota, L., Langohr, K., Sánchez-Benavides, G., de Sola, S., Cuenca-Royo, A., Rodriguez, J., Rodrı́guez-Morató, J., Farre, M., Dierssen, M., de la Torre, R., TESDAD Study Group, 2016. VNTR-DAT1 and COMTVal158Met genotypes modulate mental flexibility and adaptive behavior skills in Down syndrome. Front. Behav. Neurosci. 10, 193. Dierssen, M., 2012. Down syndrome: the brain in trisomic mode. Nat. Rev. Neurosci. 13, 844–858. Dierssen, M., Martı́nez de Lagrán, M.M., 2006. DYRK1A (dual-specificity tyrosinephosphorylated and -regulated kinase 1A): a gene with dosage effect during development and neurogenesis. ScientificWorldJournal 6, 1911–1922. 291 292 CHAPTER 9 Plasticity as a therapeutic target Dierssen, M., Ramakers, G.J., 2006. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes Brain Behav. 5, 48–60. Dierssen, M., Vallina, I.F., Baamonde, C., Lumbreras, M.A., Martı́nez-Cue, C., Calatayud, S.G., Flórez, J., 1996. Impaired cyclic AMP production in the hippocampus of a Down syndrome murine model. Brain Res. Dev. Brain Res. 95, 122–124. Dierssen, M., Vallina, I.F., Baamonde, C., Garcı́a-Calatayud, S., Lumbreras, M.A., Flórez, J., 1997. Alterations of central noradrenergic transmission in Ts65Dn mouse, a model for Down syndrome. Brain Res. 749, 238–244. Dierssen, M., Benavides-Piccione, R., Martı́nez-Cue, C., Estivill, X., Flórez, J., Elston, G.N., DeFelipe, J., 2003. Alterations of neocortical pyramidal cell phenotype in the Ts65Dn mouse model of Down syndrome: effects of environmental enrichment. Cereb. Cortex 13, 758–764. Dostal, A.M., Samavat, H., Bedell, S., Torkelson, C., Wang, R., Swenson, K., Le, C., Wu, A.H., Ursin, G., Yuan, J.M., Kurzer, M.S., 2015. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 83, 26–35. Dowjat, W.K., et al., 2007. Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome. Neurosci. Lett. 413, 77–81. Duchon, A., Herault, Y., 2016. DYRK1A, a dosage-sensitive gene involved in neurodevelopmental disorders, is a target for drug development in Down syndrome. Front. Behav. Neurosci. 10, 104. Ehrnhoefer, D.E., Bieschke, J., Boeddrich, A., et al., 2008. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 15, 558–566. Eriksson, P.S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A.M., Nordborg, C., Peterson, D.A., Gage, F.H., 1998. Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. Esbensen, A.J., Hooper, S.R., Fidler, D., Hartley, S.L., Edgin, J., d’Ardhuy, X.L., Capone, G., Conners, F.A., Mervis, C.B., Abbeduto, L., Rafii, M.S., SJ, K.-M.H., Urv, T., Outcome Measures Working Group, 2017. Outcome measures for clinical trials in Down syndrome. Am. J. Intellect. Dev. Disabil. 122, 247–281. Esposito, G., Imitola, J., Lu, J., et al., 2008. Genomic and functional profiling of human Down syndrome neural progenitors implicates S100B and aquaporin 4 in cell injury. Hum. Mol. Genet. 17, 440–457. Fang, M.Z., et al., 2003. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 63, 7563–7570. Fant, X., Durieu, E., Chicanne, G., Payrastre, B., Sbrissa, D., Shisheva, A., Limanton, E., Carreaux, F., Bazureau, J.P., Meijer, L., 2014. cdc-like/dual-specificity tyrosine phosphorylation-regulated kinases inhibitor leucettine L41 induces mTOR-dependent autophagy: implication for Alzheimer’s disease. Mol. Pharmacol. 85, 441–450. Fenoll, R., Pujol, J., Esteba-Castillo, S., de Sola, S., Ribas-Vidal, N., Garcı́a-Alba, J., SánchezBenavides, G., Martı́nez-Vilavella, G., Deus, J., Dierssen, M., Novell-Alsina, R., de la Torre, R., 2017. Anomalous white matter structure and the effect of age in Down syndrome patients. J. Alzheimers Dis. 57, 61–70. Fernandez, J.W., Rezai-Zadeh, K., Obregon, D., Tan, J., 2010. EGCG functions through estrogen receptor-mediated activation of ADAM10 in the promotion of nonamyloidogenic processing of APP. FEBS Lett. 584, 4259–4267. References Ferrer, I., et al., 2005. Constitutive DYRK1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. Neurobiol. Dis. 20, 392–400. Fung, S.T., Ho, C.K., Choi, S.W., Chung, W.Y., Benzie, I.F., 2013. Comparison of catechin profiles in human plasma and urine after single dosing and regular intake of green tea (Camellia sinensis). Br. J. Nutr. 109, 2199–2207. Furu, K., Kieler, H., Haglund, B., Engeland, A., Selmer, R., Stephansson, O., Valdimarsdottir, U.A., Zoega, H., Artama, M., Gissler, M., Malm, H., Nørgaard, M., 2015. Selective serotonin reuptake inhibitors and venlafaxine in early pregnancy and risk of birth defects: population based cohort study and sibling design. BMJ 350, h1798. Garcı́a-Cerro, S., Martı́nez, P., Vidal, V., Corrales, A., Flórez, J., Vidal, R., Rueda, N., Arbones, M.L., Martı́nez-Cue, C., 2014. Overexpression of Dyrk1A is implicated in several cognitive, electrophysiological and neuromorphological alterations found in a mouse model of Down syndrome. PLoS One 9, e106572. Giacomini, A., Stagni, F., Trazzi, S., Guidi, S., Emili, M., Brigham, E., Ciani, E., Bartesaghi, R., 2015. Inhibition of APP gamma-secretase restores sonic hedgehog signaling and neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 82, 385–396. Giacomini, A., Stagni, F., Emili, M., Guidi, S., Salvalai, M.E., Grilli, M., Vidal-Sanchez, V., Martinez-Cue, C., Bartesaghi, R., 2018. Treatment with corn oil improves neurogenesis and cognitive performance in the Ts65Dn mouse model of Down syndrome. Brain Res. Bull. 140, 378–391. Guedj, F., Sebrie, C., Rivals, I., Ledru, A., Paly, E., Bizot, J.C., Smith, D., Rubin, E., Gillet, B., Arbones, M., Delabar, J.M., 2009. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One 4 (2), e4606. Guidi, S., Bonasoni, P., Ceccarelli, C., et al., 2008. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 18, 180–197. Guidi, S., Ciani, E., Bonasoni, P., Santini, D., Bartesaghi, R., 2011. Widespread proliferation impairment and hypocellularity in the cerebellum of fetuses with down syndrome. Brain Pathol. 21, 361–373. Guidi, S., Stagni, F., Bianchi, P., Ciani, E., Giacomini, A., De Franceschi, M., Moldrich, R., Kurniawan, N., Mardon, K., Giuliani, A., Calzà, L., Bartesaghi, R., 2014. Prenatal pharmacotherapy rescues brain development in a Down’s syndrome mouse model. Brain 137, 380–401. Guimerá, J., Casas, C., Pucharcòs, C., Solans, A., Domènech, A., Planas, A.M., Ashley, J., Lovett, M., Estivill, X., Pritchard, M.A., 1996. A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum. Mol. Genet. 5 (9), 1305–1310. Guimera, J., Casas, C., Estivill, X., Pritchard, M., 1999. Human minibrain homologue (MNBH/DYRK1): characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics 57, 407–418. Guo, Y., Zhao, Y., Nan, Y., Wang, X., Chen, Y., Wang, S., 2017. (-)-Epigallocatechin-3-gallate ameliorates memory impairment and rescues the abnormal synaptic protein levels in the frontal cortex and hippocampus in a mouse model of Alzheimer’s disease. Neuroreport 28, 590–597. 293 294 CHAPTER 9 Plasticity as a therapeutic target H€ammerle, B., Carnicero, A., Elizalde, C., Ceron, J., Martinez, S., Tejedor, F.J., 2003. Expression patterns and subcellular localization of the Down syndrome candidate protein MNB/DYRK1A suggest a role in late neuronal diffferentiation. Eur. J. Neurosci. 17, 2277–2286. H€ammerle, B., Ulin, E., Guimera, J., Becker, W., Guillemot, F., Tejedor, F.J., 2011. Transient expression of Mnb/Dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling. Development 138 (12), 2543–2554. Harrison, R.V., Gordon, K.A., Mount, R.J., 2005. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev. Psychobiol. 46, 252–261. Hayes, R.M., Wu, P., Shelton, R.C., Cooper, W.O., Dupont, W.D., Mitchel, E., Hartert, T.V., 2012. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am. J. Obstet. Gynecol. 207, 49.e1-9. Hewitt, C.A., Ling, K.H., Merson, T.D., et al., 2010. Gene network disruptions and neurogenesis defects in the adult Ts1Cje mouse model of Down syndrome. PLoS One 5, e11561. Hibaoui, Y., Grad, I., Letourneau, A., Sailani, M.R., Dahoun, S., Santoni, F.A., Gimelli, S., Guipponi, M., Pelte, M.F., Bena, F., et al., 2014. Modelling and rescuing neurodevelopmental defect of Down syndrome using induced pluripotent stem cells from monozygotic twins discordant for trisomy 21. EMBO Mol. Med. 6, 259–277. Hirase, H., Shinohara, Y., 2014. Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 280, 282–298. Horne, R.S., Wijayaratne, P., Nixon, G.M., Walter, L.M., 2019. Sleep and sleep disordered breathing in children with down syndrome: effects on behaviour, neurocognition and the cardiovascular system. Sleep Med. Rev. 44, 1–11. Ishihara, K., Amano, K., Takaki, E., et al., 2010. Enlarged brain ventricles and impaired neurogenesis in the Ts1Cje and Ts2Cje mouse models of Down syndrome. Cereb. Cortex 20, 1131–1143. Kazim, S.F., Blanchard, J., Bianchi, R., Iqbal, K., 2017. Early neurotrophic pharmacotherapy rescues developmental delay and Alzheimer’s-like memory deficits in the Ts65Dn mouse model of Down syndrome. Sci. Rep. 7, 45561. Kelley, C.M., Ash, J.A., Powers, B.E., Velazquez, R., Alldred, M.J., Ikonomovic, M.D., Ginsberg, S.D., Strupp, B.J., Mufson, E.J., 2016. Effects of maternal choline supplementation on the septohippocampal cholinergic system in the Ts65Dn mouse model of Down syndrome. Curr. Alzheimer Res. 13, 84–96. Kentrup, H., Becker, W., Heukelbach, J., et al., 1996. Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J. Biol. Chem. 271, 3488–3495. Kida, E., Rabe, A., Walus, M., Albertini, G., Golabek, A.A., 2013. Long-term running alleviates some behavioral and molecular abnormalities in Down syndrome mouse model Ts65Dn. Exp. Neurol. 240, 178–189. Kii, I., Sumida, Y., Goto, T., Sonamoto, R., Okuno, Y., Yoshida, S., Kato-Sumida, T., Koike, Y., Abe, M., Nonaka, Y., Ikura, T., Ito, N., Shibuya, H., Hosoya, T., Hagiwara, M., 2016. Selective inhibition of the kinase DYRK1A by targeting its folding process. Nat. Commun. 7, 11391. Kim, H., Sablin, S.O., Ramsay, R.R., 1997. Inhibition of monoamine oxidase A by beta-carboline derivatives. Arch. Biochem. Biophys. 337, 137–142. References Kim, H., Lee, K.S., Kim, A.K., Choi, M., Choi, K., Kang, M., Chi, S.W., Lee, M.S., Lee, J.S., Lee, S.Y., Song, W.J., Yu, K., Cho, S., 2016. A chemical with proven clinical safety rescues Down-syndrome-related phenotypes in through DYRK1A inhibition. Dis. Model. Mech. 9, 839–848. Kimura, R., et al., 2007. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum. Mol. Genet. 16, 15–23. Knoth, R., Singec, I., Ditter, M., Pantazis, G., Capetian, P., Meyer, R.P., Horvat, V., Volk, B., Kempermann, G., 2010. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 5, e8809. Kobilo, T., Liu, Q.R., Gandhi, K., Mughal, M., Shaham, Y., van Praag, H., 2011. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn. Mem. 18, 605–609. Krasuski, J.S., Alexander, G.E., Horwitz, B., Rapoport, S.I., Schapiro, M.B., 2002. Relation of medial temporal lobe volumes to age and memory function in nondemented adults with Down’s syndrome: implications for the prodromal phase of Alzheimer’s disease. Am. J. Psychiatry 159, 74–81. Laffaire, J., Rivals, I., Dauphinot, L., et al., 2009. Gene expression signature of cerebellar hypoplasia in a mouse model of Down syndrome during postnatal development. BMC Genomics 10, 138. Lazarov, O., Mattson, M.P., Peterson, D.A., Pimplikar, S.W., van Praag, H., 2010. When neurogenesis encounters aging and disease. Trends Neurosci. 33, 569–579. Lee, M.J., Wang, Z.Y., Li, H., Chen, L., Sun, Y., Gobbo, S., Balentine, D.A., Yang, C.S., 1995. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomark. Prev. 4, 393–399. Lin, L.C., Wang, M.N., Tseng, T.Y., Sung, J.S., Tsai, T.H., 2007. Pharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 55, 1517–1524. Llorens-Martı́n, M.V., Rueda, N., Tejeda, G.S., Flórez, J., Trejo, J.L., Martı́nez-Cue, C., 2010. Effects of voluntary physical exercise on adult hippocampal neurogenesis and behavior of Ts65Dn mice, a model of Down syndrome. Neuroscience 171, 1228–1240. Long, R., Drawbaugh, M.L., Davis, C.M., Goodlett, C.R., Williams, J.R., Roper, R.J., 2019. Usage of and attitudes about green tea extract and epigallocathechin-3-gallate (EGCG) as a therapy in individuals with Down syndrome. Complement. Ther. Med. 45, 234–241. López-Hidalgo, R., Ballestı́n, R., Vega, J., Blasco-Ibáñez, J.M., Crespo, C., Gilabert-Juan, J., Nácher, J., Varea, E., 2016. Hypocellularity in the murine model for Down syndrome Ts65Dn is not affected by adult neurogenesis. Front. Neurosci. 2, 10–75. Lorenzi, H.A., Reeves, R.H., 2006. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 1104, 153–159. Lott, I.T., Dierssen, M., 2010. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 9, 623–633. Lu, Q.R., Sun, T., Zhu, Z., et al., 2002. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109, 75–86. Lumbreras, M., Baamonde, C., Martı́nez-Cue, C., Lubec, G., Cairns, N., Salles, J., Dierssen, M., Flórez, J., 2006. Brain G protein-dependent signaling pathways in Down syndrome and Alzheimer’s disease. Amino Acids 31, 449–456. 295 296 CHAPTER 9 Plasticity as a therapeutic target Malberg, J.E., Eisch, A.J., Nestler, E.J., Duman, R.S., 2000. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110. Martinez de Lagran, M., Benavides-Piccione, R., Ballesteros-Yañez, I., Calvo, M., Morales, M., Fillat, C., Defelipe, J., Ramakers, G.J., Dierssen, M., 2012. Dyrk1A influences neuronal morphogenesis through regulation of cytoskeletal dynamics in mammalian cortical neurons. Cereb. Cortex 22, 2867–2877. Martı́nez-Cue, C., Baamonde, C., Lumbreras, M., et al., 2002. Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behav. Brain Res. 134, 185–200. Martı́nez-Cue, C., Rueda, N., Garcı́a, E., Davisson, M.T., Schmidt, C., Flórez, J., 2005. Behavioral, cognitive and biochemical responses to different environmental conditions in male Ts65Dn mice, a model of Down syndrome. Behav. Brain Res. 163 (2), 174–185. Martı́nez-Cue, C., Martı́nez, P., Rueda, N., Vidal, R., Garcı́a, S., Vidal, V., Corrales, A., Montero, J.A., Pazos, Á., Flórez, J., Gasser, R., Thomas, A.W., Honer, M., Knoflach, F., Trejo, J.L., Wettstein, J.G., Hernández, M.C., 2013. Reducing GABAA α5 receptor-mediated inhibition rescues functional and neuromorphological deficits in a mouse model of Down syndrome. J. Neurosci. 33, 3953–3966. Mazur-Kolecka, B., Golabek, A., Kida, E., Rabe, A., Hwang, Y.W., Adayev, T., Wegiel, J., Flory, M., Kaczmarski, W., Marchi, E., Frackowiak, J., 2012. Effect of DYRK1A activity inhibition on development of neuronal progenitors isolated from Ts65Dn mice. J. Neurosci. Res. 90, 999–1010. Menghini, D., Costanzo, F., Vicari, S., 2011. Relationship between brain and cognitive processes in Down syndrome. Behav. Genet. 41, 381–393. Milenkovic, D., et al., 2012. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: a new mechanism of the action of polyphenols. PLoS One 7, e29837. Moldrich, R.X., Dauphinot, L., Laffaire, J., et al., 2009. Proliferation deficits and gene expression dysregulation in Down’s syndrome (Ts1Cje) neural progenitor cells cultured from neurospheres. J. Neurosci. Res. 87, 3143–3152. Moon, J., Chen, M., Gandhy, S.U., Strawderman, M., Levitsky, D.A., Maclean, K.N., Strupp, B.J., 2010. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav. Neurosci. 124 (3), 346–361. Morè, L., Lauterborn, J.C., Papaleo, F., Brambilla, R., 2019. Enhancing cognition through pharmacological and environmental interventions: examples from preclinical models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2019.02.003. pii: S0149-7634(18)30292-6. Moreno-Jimenez, E.P., Flor-Garcı́a, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., Ávila, J., Llorens-Martı́n, M., 2019. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560. Moses-Kolko, E.L., Bogen, D., Perel, J., Bregar, A., Uhl, K., Levin, B., Wisner, K.L., 2005. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 293, 2372–2383. Naert, G., Ferre, V., Meunier, J., Keller, E., Malmstr€ om, S., Givalois, L., Carreaux, F., Bazureau, J.P., Maurice, T., 2015. Leucettine L41, a DYRK1A-preferential DYRKs/CLKs inhibitor, prevents memory impairments and neurotoxicity induced by oligomeric Aβ25-35 peptide administration in mice. Eur. Neuropsychopharmacol. 25, 2170–2182. References Nakano-Kobayashi, A., Awaya, T., Kii, I., Sumida, Y., Okuno, Y., Yoshida, S., Sumida, T., Inoue, H., Hosoya, T., Hagiwara, M., 2017. Prenatal neurogenesis induction therapy normalizes brain structure and function in Down syndrome mice. Proc. Natl. Acad. Sci. U. S. A. 114, 10268–10273. Navarro-Romero, A., Vázquez-Oliver, A., Gomis-González, M., Garzón-Montesinos, C., Falcón-Moya, R., Pastor, A., Martı́n-Garcı́a, E., Pizarro, N., Busquets-Garcia, A., Revest, J.M., Piazza, P.V., Bosch, F., Dierssen, M., de la Torre, R., Rodrı́guez-Moreno, A., Maldonado, R., Ozaita, A., 2019. Cannabinoid type-1 receptor blockade restores neurological phenotypes in two models for Down syndrome. Neurobiol. Dis. 125, 92–106. Nguyen, T.L., Duchon, A., Manousopoulou, A., Loaëc, N., Villiers, B., Pani, G., Karatas, M., Mechling, A.E., Harsan, L.A., Limanton, E., Bazureau, J.P., Carreaux, F., Garbis, S.D., Meijer, L., Herault, Y., 2018. Correction of cognitive deficits in mouse models of Down syndrome by a pharmacological inhibitor of DYRK1A. Dis. Model. Mech. 11 (9), pii: dmm035634. Noll, C., Planque, C., Ripoll, C., Guedj, F., Diez, A., Ducros, V., Belin, N., Duchon, A., Paul, J.L., Badel, A., de Freminville, B., Grattau, Y., Blehaut, H., Herault, Y., Janel, N., Delabar, J.M., 2009. DYRK1A, a novel determinant of the methioninehomocysteine cycle in different mouse models overexpressing this Down-syndromeassociated kinase. PLoS One 4, e7540. Noll, C., Tlili, A., Ripoll, C., Mallet, L., Paul, J.L., Delabar, J.M., Janel, N., 2012. Dyrk1a activates antioxidant NQO1 expression through an ERK1/2-Nrf2 dependent mechanism. Mol. Genet. Metab. 105, 484–488. Ogawa, Y., Nonaka, Y., Goto, T., Ohnishi, E., Hiramatsu, T., Kii, I., Yoshida, M., Ikura, T., Onogi, H., Shibuya, H., Hosoya, T., Ito, N., Hagiwara, M., 2010. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 1, 86. Olivier, J.D., Akerud, H., Kaihola, H., Pawluski, J.L., Skalkidou, A., H€ ogberg, U., Sundstr€ omPoromaa, I., 2013. The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring. Front. Cell. Neurosci. 7, 73. Ortiz-Abalia, J., Sahún, I., Altafaj, X., Andreu, N., Estivill, X., Dierssen, M., Fillat, C., 2008. Targeting Dyrk1A with AAVshRNA attenuates motor alterations in TgDyrk1A, a mouse model of Down syndrome. Am. J. Hum. Genet. 83, 479–488. Parrini, M., Ghezzi, D., Deidda, G., Medrihan, L., Castroflorio, E., Alberti, M., Baldelli, P., Cancedda, L., Contestabile, A., 2017. Aerobic exercise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci. Rep. 7, 16825. Paylor, R., Morrison, S.K., Rudy, J.W., Waltrip, L.T., Wehner, J.M., 1992. Brief exposure to an enriched environment improves performance on the Morris water task and increases hippocampal cytosolic protein kinase C activity in young rats. Behav. Brain Res. 52, 49–56. Pons-Espinal, M., Martinez de Lagran, M., Dierssen, M., 2013. Environmental enrichment rescues dyrk1a activity and hippocampal adult neurogenesis in tgdyrk1a. Neurobiol. Dis. 60, 18–31. Pujol, J., del Hoyo, L., Blanco-Hinojo, L., de Sola, S., Macià, D., Martı́nez-Vilavella, G., Amor, M., Deus, J., Rodrı́guez, J., Farre, M., Dierssen, M., de la Torre, R., 2015. Anomalous brain functional connectivity contributing to poor adaptive behavior in Down syndrome. Cortex 64, 148–156. Ramakrishna, N., Meeker, H.C., Brown, W.T., 2016. Novel epigenetic regulation of alpha-synuclein expression in Down syndrome. Mol. Neurobiol. 53, 155–162. 297 298 CHAPTER 9 Plasticity as a therapeutic target Reefhuis, J., Devine, O., Friedman, J.M., Louik, C., Honein, M.A., National Birth Defects Prevention Study, 2015. Specific SSRIs and birth defects: bayesian analysis to interpret new data in the context of previous reports. BMJ 351, h3190. Rezai-Zadeh, K., et al., 2008. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 1214, 177–187. Roper, R.J., Baxter, L.L., Saran, N.G., Klinedinst, D.K., Beachy, P.A., Reeves, R.H., 2006. Defective cerebellar response to mitogenic Hedgehog signaling in Down’s syndrome mice. Proc. Natl. Acad. Sci. U. S. A. 103, 1452–1456. Roy, N.S., Wang, S., Jiang, L., Kang, J., Benraiss, A., Harrison-Restelli, C., Fraser, R.A., Couldwell, W.T., Kawaguchi, A., Okano, H., Nedergaard, M., Goldman, S.A., 2000. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat. Med. 6, 271–277. Ruiz-Mejias, M., Martinez de Lagran, M., Mattia, M., Castano-Prat, P., Perez-Mendez, L., Ciria-Suarez, L., Gener, T., Sancristobal, B., Garcı́a-Ojalvo, J., Gruart, A., Delgado-Garcı́a, J.M., Sanchez-Vives, M.V., Dierssen, M., 2016. Overexpression of Dyrk1A, a Down syndrome candidate, decreases excitability and impairs gamma oscillations in the prefrontal cortex. J. Neurosci. 36, 3648–3659. Ryoo, S.R., et al., 2007. DYRK1A-mediated hyperphosphorylation of tau: a functional link between Down syndrome and Alzheimer disease. J. Biol. Chem. 282, 34850–34857. Ryoo, S.R., et al., 2008. Dual-specificity tyrosine(γ)-phosphorylation regulated kinase 1A-mediated phosphorylation of amyloid precursor protein: evidence for a functional link between Down syndrome and Alzheimer’s disease. J. Neurochem. 104, 1333–1344. Saldanha, S.N., Kala, R., Tollefsbol, T.O., 2014. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 324, 40–53. Sale, A., Berardi, N., Maffei, L., 2014. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol. Rev. 94, 189–234. Sang, S., Hou, Z., Lambert, J.D., Yang, C.S., 2005. Redox properties of tea polyphenols and related biological activities. Antioxid. Redox Signal. 7, 1704–1714. Sansevero, G., Sale, A., 2017. Environment as therapy: neuroscience for intellectual disability and dementia. Oncotarget 8, 5682–5683. Sansevero, G., Begenisic, T., Mainardi, M., Sale, A., 2016. Experience-dependent reduction of soluble β-amyloid oligomers and rescue of cognitive abilities in middle-age Ts65Dn mice, a model of Down syndrome. Exp. Neurol. 283, 49–56. Sanz, E.J., De-las-Cuevas, C., Kiuru, A., Bate, A., Edwards, R., 2005. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 365, 482–487. Scarpa, E.S., Ninfali, P., 2015. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int. J. Mol. Sci. 16, 15727–15742. Shi, W., Li, L., Ding, Y., Yang, K., Chen, Z., Fan, X., Jiang, S., Guan, Y., Liu, Z., Xu, D., Wu, L., 2018. The critical role of epigallocatechin gallate in regulating mitochondrial metabolism. Future Med. Chem. 10, 795–809. Shichiri, M., Yoshida, Y., Ishida, N., Hagihara, Y., Iwahashi, H., Tamai, H., et al., 2011. α-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic. Biol. Med. 50, 1801–1811. Shors, T.J., Townsend, D.A., Zhao, M., Kozorovitskiy, Y., Gould, E., 2002. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584. References Silverman, W., Miezejeski, C., Ryan, R., Zigman, W., Krinsky-McHale, S., Urv, T., 2010. Stanford-Binet & WAIS IQ differences and their implications for adults with intellectual disability (aka mental retardation). Dermatol. Int. 38, 242–248. Singh, B.N., Shankar, S., Srivastava, R.K., 2011. Green tea catechin, epigallocatechin-3gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 82, 1807–1821. Singh, N.A., Mandal, A.K., Khan, Z.A., 2016. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 15, 60. Smith, B., Medda, F., Gokhale, V., Dunckley, T., Hulme, C., 2012. Recent advances in the design, synthesis, and biological evaluation of selective DYRK1A inhibitors: a new avenue for a disease modifying treatment of Alzheimer’s? ACS Chem. Nerosci. 3, 857–872. Sorrells, S.F., Paredes, M.F., Cebrian-Silla, A., Sandoval, K., Qi, D., Kelley, K.W., James, D., Mayer, S., Chang, J., Auguste, K.I., Chang, E.F., Gutierrez, A.J., Kriegstein, A.R., Mathern, G.W., Oldham, M.C., Huang, E.J., Garcia-Verdugo, J.M., Yang, Z., AlvarezBuylla, A., 2018. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. Souchet, B., Guedj, F., Sahún, I., Duchon, A., Daubigney, F., Badel, A., Yanagawa, Y., Barallobre, M.J., Dierssen, M., Yu, E., Herault, Y., Arbones, M., Janel, N., Creau, N., Delabar, J.M., 2014. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol. Dis. 69, 65–75. Souchet, B., Guedj, F., Penke-Verdier, Z., Daubigney, F., Duchon, A., Herault, Y., Bizot, J.C., Janel, N., Creau, N., Delatour, B., et al., 2015. Pharmacological correction of excitation/ inhibition imbalance in Down syndrome mouse models. Front. Behav. Neurosci. 9, 267. Souchet, B., Duchon, A., Gu, Y., Dairou, J., Chevalier, C., Daubigney, F., Nalesso, V., Creau, N., Yu, Y., Janel, N., Herault, Y., Delabar, J.M., 2019. Prenatal treatment with EGCG enriched green tea extract rescues GAD67 related developmental and cognitive defects in Down syndrome mouse models. Sci. Rep. 9, 3914. Spalding, K.L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H.B., Bostrom, E., Westerlund, I., Vial, C., Buchholz, B.A., Possnert, G., Mash, D.C., Druid, H., Frisen, J., 2013. Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. Stagni, F., Giacomini, A., Guidi, S., Ciani, E., Ragazzi, E., Filonzi, M., De Iasio, R., Rimondini, R., Bartesaghi, R., 2015. Long-term effects of neonatal treatment with fluoxetine on cognitive performance in Ts65Dn mice. Neurobiol. Dis. 74, 204–218. Stagni, F., Giacomini, A., Emili, M., Trazzi, S., Guidi, S., Sassi, M., Ciani, E., Rimondini, R., Bartesaghi, R., 2016. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience 333, 277–301. Stagni, F., Giacomini, A., Guidi, S., Emili, M., Uguagliati, B., Salvalai, M.E., Bortolotto, V., Grilli, M., Rimondini, R., Bartesaghi, R., 2017. A flavonoid agonist of the TrkB receptor for BDNF improves hippocampal neurogenesis and hippocampus-dependent memory in the Ts65Dn mouse model of DS. Exp. Neurol. 298, 79–96. Stringer, M., Abeysekera, I., Dria, K.J., Roper, R.J., Goodlett, C.R., 2015. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacol. Biochem. Behav. 138, 70–79. Stringer, M., Abeysekera, I., Thomas, J., LaCombe, J., Stancombe, K., Stewart, R.J., Dria, K.J., Wallace, J.M., Goodlett, C.R., Roper, R.J., 2017a. Epigallocatechin-3-gallate (EGCG) consumption in the Ts65Dn model of Down syndrome fails to improve behavioral deficits and is detrimental to skeletal phenotypes. Physiol. Behav. 177, 230–241. 299 300 CHAPTER 9 Plasticity as a therapeutic target Stringer, M., Goodlett, C.R., Roper, R.J., 2017b. Targeting trisomic treatments: optimizing Dyrk1a inhibition to improve Down syndrome deficits. Mol. Genet. Genomic Med. 5 (5), 451–465. Sztainberg, Y., Chen, A., 2010. An environmental enrichment model for mice. Nat. Protoc. 5, 1535–1539. Takebayashi, H., Yoshida, S., Sugimori, M., et al., 2000. Dynamic expression of basic helix-loop-helix Olig family members: implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech. Dev. 99, 143–148. Teipel, S.J., Alexander, G.E., Schapiro, M.B., M€ oller, H.J., Rapoport, S.I., Hampel, H., 2004. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain 127, 811–824. Tejedor, F.J., H€ammerle, B., 2011. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 278 (2), 223–235. Thakur, V.S., Gupta, K., Gupta, S., 2012. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int. J. Oncol. 41, 353–361. Thomazeau, A., Lassalle, O., Iafrati, J., Souchet, B., Guedj, F., Janel, N., Chavis, P., Delabar, J., Manzoni, O.J., 2014. Prefrontal deficits in a murine model overexpressing the Down syndrome candidate gene Dyrk1a. J. Neurosci. 34, 1138–1147. Trazzi, S., Mitrugno, V.M., Valli, E., et al., 2011. APP-dependent up-regulation of Ptch1 underlies proliferation impairment of neural precursors in Down syndrome. Hum. Mol. Genet. 20, 1560–1573. Vacca, R.A., Valenti, D., 2015. Green tea EGCG plus fish oil omega-3 dietary supplements rescue mitochondrial dysfunctions and are safe in a Down’s syndrome child. Clin. Nutr. 34, 783–784. Valenti, D., De Rasmo, D., Signorile, A., Rossi, L., de Bari, L., Scala, I., Granese, B., Papa, S., Vacca, R.A., 2013. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim. Biophys. Acta 1832, 542–552. Valenti, D., de Bari, L., de Rasmo, D., Signorile, A., Henrion-Caude, A., Contestabile, A., Vacca, R.A., 2016. The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochim. Biophys. Acta 1862, 1093–1104. Van Praag, H., 2008. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 10, 128–140. Van Praag, H., Kempermann, G., Gage, F.H., 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270. Velazquez, R., Ash, J.A., Powers, B.E., Kelley, C.M., Strawderman, M., Luscher, Z.I., Ginsberg, S.D., Mufson, E.J., Strupp, B.J., 2013. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 58, 92–101. Velazquez, R., Meechoovet, B., Ow, A., Foley, C., Shaw, A., Smith, B., Oddo, S., Hulme, C., Dunckley, T., 2019. Chronic Dyrk1 inhibition delays the onset of AD-like pathology in 3xTg-AD mice. Mol. Neurobiol. 56, 8364–8375. https://doi.org/10.1007/s12035-01901684-9. Vink, J., Incerti, M., Toso, L., Roberson, R., Abebe, D., Spong, C.Y., 2009. Prevention of developmental delays in a Down syndrome mouse model. Obstet. Gynecol. 112, 1242–1251. References Wang, D., Wang, F., Tan, Y., Dong, L., Chen, L., Zhu, W., Wang, H., 2012. Discovery of potent small molecule inhibitors of DYRK1A by structure-based virtual screening and bioassay. Bioorg. Med. Chem. Lett. 22, 168–171. Wood, N.I., Glynn, D., Morton, A.J., 2011. “Brain training” improves cognitive performance and survival in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 42, 427–437. Wyganowska-Świa˛tkowska, M., Matthews-Kozanecka, M., Matthews-Brzozowska, T., Skrzypczak-Jankun, E., Jankun, J., 2018. Can EGCG alleviate symptoms of Down syndrome by altering proteolytic activity? Int. J. Mol. Sci. 19, 248. pii: E248. Xicota, L., Rodriguez-Morato, J., Dierssen, M., de la Torre, R., 2017. Potential role of (-)-epigallocatechin-3-Gallate (EGCG) in the secondary prevention of Alzheimer disease. Curr. Drug Targets 18, 174–195. Xicota, L., Rodrı́guez, J., Langohr, K., Fitó, M., Dierssen, M., de la Torre, R., TESDAD study group, 2019. Effect of epigallocatechin gallate on the body composition and lipid profile of down syndrome individuals: implications for clinical management. Clin. Nutr. 5614, 30255–30259. Xie, W., Ramakrishna, N., Wieraszko, A., Hwang, Y.W., 2008. Promotion of neuronal plasticity by (-)-epigallocatechin-3-gallate. Neurochem. Res. 33, 776–783. Yabut, O., Domagauer, J., D’Arcangelo, G., 2010. Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. J. Neurosci. 30, 4004–4014. Yang, E.J., Ahn, Y.S., Chung, K.C., 2001. Protein kinase Dyrk1 activates cAMP response element-binding protein during neuronal differentiation in hippocampal progenitor cells. J. Biol. Chem. 276, 39819–39824. Yin, X., Jin, N., Shi, J., Zhang, Y., Wu, Y., Gong, C.X., Iqbal, K., Liu, F., 2017. Dyrk1A overexpression leads to increase of 3R-tau expression and cognitive deficits in Ts65Dn Down syndrome mice. Sci. Rep. 7, 619. Younes, M., Aggett, P., Aguilar, F., Crebelli, R., Dusemund, F., Filipic, M., Frutos, M.J., Galtier, P., Gott, D., Gundert-Remy, U., Lambre, C., Leblanc, J.C., Lillegaard, I.T., Moldeus, P., Mortensen, A., Oskarsson, A., Stankovic, I., Waalkens-Berendsen, I., Woutersen, R.A., Andrade, R.J., Fortes, C., Mosesso, P., Restani, P., Arcella, D., Pizzo, F., Smeraldi, C., Wright, M., EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), 2018. Scientific opinion on the safety of green tea catechins. EFSA J. 16, 5239. Yu, Z., Samavat, H., Dostal, A., Wang, R., Torkelson, C.J., Yang, C.S., Butler, L.M., Kensler, T.W., Wu, A.H., Kurzer, M.S., Yuan, J.M., 2017. Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer Prev. Res. 10, 571–579. Zhou, Q., Anderson, D.J., 2002. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73. Zhou, J., Mao, L., Xu, P., Wang, Y., 2018. Effects of (-)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice fed a high-fat diet. Nutrients 10, pii: E1681. Zhou, W.B., Miao, Z.N., Zhang, B., Long, W., Zheng, F.X., Kong, J., Yu, B., 2019. Luteolin induces hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neural Regen. Res. 14, 613–620. 301 302 CHAPTER 9 Plasticity as a therapeutic target Further reading Guidi, S., Stagni, F., Bianchi, P., Ciani, E., Ragazzi, E., Trazzi, S., Grossi, G., Mangano, C., Calzà, L., Bartesaghi, R., 2013. Early pharmacotherapy with fluoxetine rescues dendritic pathology in the Ts65Dn mouse model of Down syndrome. Brain Pathol. 23, 129–143. Kempermann, G., Kuhn, H.G., Gage, F.H., 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. Kempermann, G., Gast, D., Gage, F.H., 2002. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 52, 135–143. Pernet, V., Schwab, M.E., 2012. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 349, 97–104. Pinter, J.D., Eliez, S., Schmitt, J.E., Capone, G.T., Reiss, A.L., 2001. Neuroanatomy of Down’s syndrome: a high-resolution MRI study. Am. J. Psychiatry 158, 1659–1665. Raz, N., Torres, I.J., Briggs, S.D., Spencer, W.D., Thornton, A.E., Loken, W.J., Gunning, F.M., McQuain, J.D., Driesen, N.R., Acker, J.D., 1995. Selective neuroanatomic abnormalities in Down’s syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology 45, 356–366. Rigoldi, C., Galli, M., Condoluci, C., Carducci, F., Onorati, P., Albertini, G., 2009. Gait analysis and cerebral volumes in Down’s syndrome. Funct. Neurol. 24, 147–152. Stagni, F., Magistretti, J., Guidi, S., Ciani, E., Mangano, C., Calzà, L., Bartesaghi, R., 2013. Pharmacotherapy with fluoxetine restores functional connectivity from the dentate gyrus to field CA3 in the Ts65Dn mouse model of Down syndrome. PLoS One 8 (4), e61689. Van Praag, H., Schinder, A.F., Christle, B.R., Toni, N., Palmer, T.D., Gage, F.H., 2002. Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034.