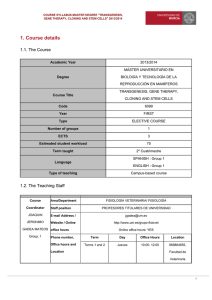
Oxidative Stress in Plants and Its Management Sachin Teotia and Deepali Singh Abstract We all live in an oxygen-rich environment which has to deal with the danger of oxidative stress. During normal cell metabolism, reactive oxygen species (ROS) are constantly produced, mainly by respiratory and photosynthetic components. These species mainly include superoxide radicals (O2!), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radical (OH!). The others are hydroperoxyl radical (HO2˙), alkoxy radical (RO˙), peroxyl radicals (ROO˙), and excited carbonyl (RO). But during stress conditions like salinity, drought, metal toxicity, herbicides, fungicides, air pollutants, hypoxia, and abnormal conditions of light, temperature, and topography, ROS are produced in excess amount. These highly reactive molecules can react with many cellular biomolecules and other components and damage DNA, proteins, and lipids. Thus, their concentration has to be tightly controlled. To counter the deleterious effects of ROS, aerobic organisms are equipped with antioxidant systems to scavenge ROS from the cells. Enzymatic antioxidants are mainly superoxide dismutase (SOD), catalase, ascorbate peroxidase, glutathione peroxidase, glutathione S-transferases, and peroxiredoxin, while the nonenzymatic antioxidants are mainly ascorbate, glutathione, proline, tocopherol, flavonoids, and carotenoids. These antioxidants protect against the oxidative damage by inhibiting or quenching free radicals and ROS. When the balance between the production of ROS and the quenching activities of antioxidants is disturbed, the cell faces the risk of oxidative stress and damage. These ROS creating stresses are numerous and often species or area specific. These stresses cause significant crop losses. There is a growing need to develop crops which can be resistant to the effects of various oxidative stresses. One such way is to develop transgenic plants overexpressing one or more S. Teotia (*) " D. Singh School of Biotechnology, Gautam Buddha University, Greater Noida 201312, India e-mail: sachin25@gmail.co R.K. Gaur and P. Sharma (eds.), Approaches to Plant Stress and their Management, DOI 10.1007/978-81-322-1620-9_13, # Springer India 2014 227 228 S. Teotia and D. Singh antioxidants, which can confer resistance towards particular stress. Another way is to develop mutants which are resistant towards certain stresses. Keywords Reactive oxygen species " Oxidative stress tolerance " Enzymatic Antioxidants " Nonenzymatic antioxidants " Transgenic plants and mutants Introduction Oxygen is the primary source of life. In an oxygen atmosphere, the generation of ROS, especially under metabolic stress, is unavoidable. ROS are also produced continuously as by-products of various metabolic pathways localized in different cellular compartments such as chloroplast, mitochondria, peroxisomes, and apoplast (del Rio et al. 2006; Panieri et al. 2013). Many metabolic processes normally produce ROS, which comprise mainly of superoxide radical, hydrogen peroxide, hydroxyl radical, and singlet oxygen. ROS may also be produced by abiotic stress such as salinity, drought, heavy metals, nutrient deficiency, herbicides, fungicides, air pollutants, ozone, light, temperature, topography, and hypoxia and by biotic factors such as pathogen attacks. Oxidative stress is programmed to be a regulated process, and the equilibrium between ROS and its quenching determines the well-being of a plant. Oxidative damage of cells happens when the balance between the production of ROS and their quenching by antioxidants reaches the state of disequilibrium. The extent of oxidative stress depends on the type of ROS that is produced, the concentration and the site where it is released, their interaction with other molecules in the cell, and the developmental stage and potential of the cell (Moller et al. 2007). The enhanced and prolonged production of ROS can cause significant damage to cell structures in plants like lipid peroxidation, protein oxidation, damage to nucleic acids, enzyme inhibition, and programmed cell death (Mittler 2002; Perez-Perez et al. 2012) (Fig. 1). However, plants have evolved protective scavenging systems in response to these ROS. High levels of ROS are kept in check by dynamic and synergistic mechanisms of ROS-scavenging antioxidants that control the concentration of intracellular ROS (Apel and Hirt 2004). An antioxidant is described as a compound capable of scavenging ROS without itself undergoing conversion to a destructive radical (Noctor and Foyer 1998). These antioxidants can be conveniently divided into two groups: enzymatic and nonenzymatic antioxidants (Fig. 1). Reactive Oxygen Species (ROS) ROS are mostly free radicals produced as byproducts of redox reactions. ROS is formed as a natural by-product of the normal metabolism in the presence of oxygen. In plant cells, ROS are continuously produced as a consequence of aerobic metabolism in all of the the intracellular organelles; as by-products in the electron transport chains of chloroplasts, mitochondria, and the plasma membrane (cytochrome b-mediated electron transfer); and in peroxisomes (Apel and Hirt 2004; Asada 1999). Several apoplastic enzymes may also lead to ROS production under normal and stress conditions. ROS include a number of molecules derived from oxygen, such as oxygen ions, free radicals, and peroxides. All of these molecules are highly reactive due to the presence of unpaired electrons at valence shell. ROS have important roles in cell signaling, cellular homeostasis, and oxidative stress (Kotchoni and Gachomo 2006; Neill et al. 2002). ROS play two main divergent roles in plants; when present in low concentrations, they act as signaling molecules mediating several responses in plant, Oxidative Stress in Plants and Its Management 229 Fig. 1 Diagrammatic representation of various agents generating ROS in plants and various antioxidants scavenging those ROS molecules including growth and development, and responses under stresses, whereas when present in high concentrations, they cause extensive damage to cellular components. However, as described above, during times of various environmental stresses, ROS levels can increase dramatically. Thus, ROS has multiple roles in cells, and therefore, it is not feasible to eliminate them completely, but at the same time, it is extremely necessary to control them tightly to avoid any oxidative damage. High levels of ROS can cause damage to cellular structures, nucleic acids, lipids, and proteins (Bergamini et al. 2004; Wiseman and Halliwell 1996). ROS is quenched by a number of enzymatic antioxidants and molecules (see next section) (Fig. 1). The problem arises in stressful conditions, when there is disequilibrium between ROS production and its quenching. Then, modulation of ROS by the antioxidants is necessary for the survival of the cell. When the level of ROS in a cell exceeds the antioxidative capacity of the antioxidants, a cell is said to be in a state of oxidative stress. Superoxide Radicals (O2!) In plants, photosynthesis takes place in chloroplasts, and oxygen is generated which can accept electrons passing through the photosystems, thus forming O2!. O2! is mainly produced in the thylakoid membrane-bound primary electron acceptor of photosystem I. Normally O2 is converted to H2O by transfer of four electrons, but occasionally O2 can react with other electron transfer chain components and only one electron is transferred, producing the O2! (Giba et al. 1998). With one unpaired electron, O2! is a free radical (Forman et al. 2004). The generation of O2! may trigger the formation of more reactive ROS like OH!, 1O2, and also H2O2 (Halliwell 2006). 230 Singlet Oxygen (1O2) Singlet oxygen, 1O2, is the first excited electronic state of O2 and is not related to electron transfer to O2. During photosynthesis in plants, sometimes insufficient energy dissipation can lead to the formation of a chlorophyll triplet state that can transfer its excitation energy to ground-state O2 to form 1O2 (Halliwell 2006). This can oxidize chloroplast molecules and also trigger cell death. Additionally, 1O2 is formed by low intercellular CO2 concentration in the chloroplast resulting from the closed stomata because of various abiotic stresses such as salinity and drought. 1O2 production has also been produced as a mechanism of resistance in plant–pathogen interactions through the production of phytoalexins (Flors and Nonell 2006; Flors et al. 2006). Formation of 1O2 during photosynthesis has a substantial damaging effect on essential components of the whole photosynthetic machinery. 1O2 activates a signaling cascade that can stimulate a specific gene expression response and can interact with signal cascades of other ROS, thereby activating several stressresponse pathways (op den Camp et al. 2003). 1 O2 is highly diffusive and destructive, as it reacts with most biomolecules and rapidly oxidizes amino acids, lipids, pigments, and DNA (Agnez-Lima et al. 2012; Fischer et al. 2013). It reacts with nitric oxide (NO) to form peroxynitrite (ONOO!). Hydrogen Peroxide (H2O2) H2O2 is mainly produced by dismutation of O2! by superoxide dismutase (SOD), NADPH oxidase, cell-wall peroxidase, amino oxidase, oxalate oxidase, and flavin-containing oxidase (Neill et al. 2002). Excess of H2O2 in the plant cells leads to the occurrence of oxidative stress. H2O2 is moderately reactive, relatively stable, and highly diffusible. At low concentrations, H2O2 acts as a signaling molecule involved in mediating the acquisition of tolerance to both biotic and abiotic stresses (Desikan et al. 2004), while at high concentrations, it leads to programmed cell death (Quan et al. 2008). H2O2 oxidizes the thiol groups of enzymes and thereby S. Teotia and D. Singh inactivates them. It plays a role as an intermediate in the formation of other ROS, including hypochlorous acid (HOCl) and •OH. Hydroxyl Radicals (OH!) This radical is formed from H2O2 in a reaction catalyzed by metal ions (Fe2+ or Cu+), often present in complex with different proteins or other molecules. This is known as the Fenton reaction: Fe2+ + H2O2 ! Fe3+ + •OH + OH!. OH! can also be produced from O2! and H2O2 at neutral pH and ambient temperatures by the iron-catalyzed O2!. This is called the Haber–Weiss reaction: O2! + H2O2 ! •OH + OH! + O2. OH! is the most reactive among all ROS and dangerous as it can potentially react with all biomolecules like DNA, proteins, lipids, and almost any constituent of cells and ultimately leads to cell death (Halliwell 2006). It causes lipid peroxidation, protein damage, and membrane destruction. Effects of ROS On Metabolism Lipid peroxidation: When higher ROS levels are reached, lipid peroxidation takes place in both cellular and organelle membranes, thereby not only directly affecting normal cellular functioning but also aggravating the oxidative stress through production of lipid-derived radicals (Catala 2010). Hydroperoxyl radical (HO2˙) which is formed from O2! by protonation in aqueous solutions can subtract hydrogen atoms from polyunsaturated fatty acids (PUFAs) and lipid hydroperoxides and cause lipid autooxidation. Because of the presence of double bonds, PUFAs are excellent targets for attack by free radicals (particularly 1O2 and OH!), forming mixture of lipid hydroperoxides (LOOH) (Moller et al. 2007). Extensive PUFA peroxidation decreases the membrane fluidity, increases leakiness, and causes secondary damage to membrane proteins (Moller et al. 2007). Oxidative Stress in Plants and Its Management Protein oxidation: It is defined as covalent modification of a protein which can be induced by ROS or components of oxidative stress. Reaction of proteins with ROS causes modifications in the form of the following: (i) side chains oxidation mainly at cysteine, methionine, and tryptophan; (ii) carbonylation; (iii) nitrosylation; and (iv) interaction with products of PUFA oxidation (Moller et al. 2007). Formation of a disulfide between two cysteine residues to form cystine and oxidation of methionine to form methionine sulfoxide are some of the common modifications. Other amino acids can also be target of redox modifications in proteins. Protein nitrosylation mainly involves the covalent attachment of a nitric oxide (NO) group to the thiol side chain of select cysteine residues. This attachment impacts the protein function. But among all modifications, protein carbonylation is the main feature of protein oxidation. Carbonylation of amino acid residues like arginine, lysine, threonine, or proline is one of the most commonly occurring oxidative modifications of proteins. This modification might lead to alteration in protein activity, its proteolytic breakdown or aggregate formation (Debska et al. 2012; Moller et al. 2007). On Growth and Development Oxidative stress has marked effect on growth and development of plants. The common stressassociated phenotypes seen under various abiotic stresses have been termed stress-induced morphogenetic response (SIMR; (Potters et al. 2007)). Typical SIMR responses include decreases in root length, stem height, and leaf area, altered xylem development, and redistribution of cell division and elongation (reviewed by (Potters et al. 2009)). DNA Damage Exposure to various biotic and abiotic stress factors might damage the DNA and exerts genotoxic stress (Balestrazzi et al. 2011; Tuteja 231 et al. 2009). OH! is most reactive and damages both purine and pyrimidine bases and also deoxyribose backbone of the DNA molecule (Cooke et al. 2003). This can induce cleavage of DNA, base deletion, formation of pyrimidine dimers, DNA–protein cross-links, and alkylation and oxidation of bases of DNA (Tuteja et al. 2001). 1O2 primarily attacks guanine and converts it into eight-hydroxyguanine (Britt 1996). In addition to mutations, oxidative DNA modifications can lead to changes in the methylation of cytosines, which is important for regulating gene expression (Moller et al. 2007). Cellular Protection Against Oxidative Stress by Scavenging of ROS Under normal conditions, the ROS molecules are scavenged by various antioxidants (Foyer and Noctor 2005). In order to control ROS levels and protect the cells from oxidative damage, plants deploy a complex antioxidant defense system which scavenges the ROS. These antioxidant systems include various enzymes and nonenzymatic metabolites that may also play a significant role in ROS signaling in plants (Vranova et al. 2002). Many of these enzymes and molecules are overexpressed and accumulate in various stressed conditions. Enzymatic Antioxidants Superoxide Dismutase (SOD) SOD is the most effective intracellular enzymatic antioxidant which is ubiquitously found in all cellular compartments of organisms (Table 1). SODs are metalloproteins that catalyze dismutation of superoxide radical (O2!) into O2 and H2O2. SODs are classified by their metal cofactors into three known families: CuZnSODs, localized in cytosol or in plastids; MnSOD, mainly restricted to mitochondria; and FeSOD, localized in the plastid (Asensio et al. 2012). These SODs can be tissue specific such as CuZnSOD (Ogawa et al. 1997; Karlsson et al. 2005) or MnSOD (Corpas et al. 2006). SOD acts Table 1 ROS-scavenging enzymes and molecules Antioxidant enzymes Superoxide dismutase Catalase Ascorbate peroxidase Monodehydroascorbate reductase Dehydroascorbate reductase Guaiacol-type peroxidase Peroxiredoxin Glutathione peroxidase Glutathione reductase Glutathione Stransferases Methionine sulfoxide reductase Thioredoxin E. C. number 1.15.1.1 Subcellular localization Cyt, Chl, Mit, Per 1.11.1.6 Gly, Cyt, Mit, Per 1.11.1.11 Cyt, Per, Chl, Mit 1.6.5.4 Chl stroma, Mit, Cyt 1.8.5.1 Cyt, Chl, Mit Reaction(s) catalyzed 2O2! + 2H+ ! 2H2O2 + O2 2H2O2 ! O2 + 2H2O 2AsA + H2O2 ! 2MDHA + 2H2O NADPH + 2MDHA ! NADP+ + 2AsA 2GSH + DHA ! GSSG + AsA Vac, Chl, Cyt, Donor + H2O2 ! oxidized donor + 2H2O Mit, ER 1.11.1.15 Cyt, Chl, Mit, ROOH + 2RSH ! ROH + RSSR + H2O Nuc 1.11.1.12 Cyt, Mit, Chl GSH + ROOH ! GSSG + ROH + 2H2O 1.11.1.9 GSH + H2O2 ! GSSG + 2H2O 1.6.4.2 Cyt, Chl, Mit NADPH + GSSG ! NADP+ + GSH 2.5.1.18 Cyt, Mit, Chl RX + GSH ! HX + R-S-GSH 1.11.1.7 1.8.4.11 Cyt, Chl, Mit, ER, SP 1.8.1.9 Cyt, Chl, Mit, Nuc 1.20.4.1 Cyt, Chl, Mit, Apo Subcellular localization Chl, Apo, Cyt, Vac, Mit, Per Met. + thioredoxin disulfide ! Met.(S)-S oxide + Thioredoxin Thioredoxin + NADP+ ! thioredoxin disulfide + NADPH Arsenate + glutaredoxin ! arsenite + glutaredoxin disulfide + H2O Antioxidant molecules Reaction(s) catalyzed Ascorbate (vitamin C) O2! + AsA + H+ ! A.! + H2O2 1 O2 + AsA + H+ ! H2O2 + DHA H2O2 + AsA ! MDHA + 2H2O Glutathione (GSH) Chl, Apo, Cyt, Vac, Mit, ROOH + 2GSH ! ROH + GSSG + H2O Per Scavenges H2O2, OH!, and 1O2 Proline Cyt (normal conditions), Proline + OH!/1O2 ! proline nitroxide/proline peroxide Chl (stressed conditions) α-Tocopherol Cell and Chl memb α-Tocopherol + 1O2 ! α-tocopherylquinone (vitamin E) Also, scavenges OH, ROO,·and ROOH (lipid peroxyl) radicals in thylakoid membranes Carotenoids (ß-Carotene Chl, chromoplast, Quench 1O2 by involving their own oxidation and forming and zeaxanthin) elaioplast, and β-carotene endoperoxide in high-light conditions, amyloplast modulate formation of triplet chlorophyll, and prevent formation of 1O2 Flavonoids Vac, Nuc, Chl, Apo, Cyt, Scavenge H2O2 and OH! ER, Cwl Polyamines Nuc, Chl, Mit, Cyt, Vac, Scavenge O2!, 1O2, OH! Cwl Glycine betaine Chl Stabilizes PS II repair proteins and alleviate lipid peroxidation Glutaredoxin O2 singlet oxygen, A.!, ascorbate free radical, AsA ascorbate, Apo apoplast, APX ascorbate peroxidase, Cyt cytosol, Chl chloroplast, Cwl cell wall, DHA dehydroascorbate, DHAR dehydroascorbate reductase, ER endoplasmic reticulum, Gly glyoxysomes, GSH glutathione, GSSG oxidized glutathione, GSTs glutathione S-transferases, H+ hydrogen ion, H2O water, H2O2 hydrogen peroxide, Met methionine, MDHA monodehydroascorbate, MDHAR monodehydroascorbate reductase, Mit mitochondria, NADP+ nicotinamide adenine dinucleotide phosphate, NADPH reduced NADP+, Nuc nucleus, O2 oxygen, O2! superoxide radical, OH˙ hydroxyl radical, Per peroxisomes, PSII photosystem II, R may be an aliphatic, aromatic, or heterocyclic group, ROO! peroxy radical, ROOH organic peroxide, S sulfide, SP secreted pathway, Vac vacuole, X may be a sulfate, nitrite, or halide group 1 Oxidative Stress in Plants and Its Management 233 in the first line of defense against the toxic effects of elevated levels of ROS. The SODs remove superoxide radical and hence decrease the risk of OH! formation. SODs are upregulated in response to many abiotic and biotic stresses and have a crucial role in the survival of plants under stressed conditions (Alscher et al. 2002; Raychaudhuri and Deng 2000). There have been many reports of the production of both biotic and abiotic stress-tolerant transgenic plants overexpressing different SODs (Table 2). MDHAR has a high specificity for monodehydroascorbate (MDHA) as the electron acceptor and NADPH as the electron donor. Oxidation of ascorbate (AsA) leads to the formation of MDHA. If MDHA is not reduced again to AsA by MDHAR, it will spontaneously convert into AsA and dehydroascorbate (DHA). Therefore, MDHAR rapidly reduce MDHA to AsA using NADPH. This rapid regeneration is necessary in order to maintain the antioxidative capacity of AsA. Catalase (CAT) CAT is a heme-coordinated tetrameric protein encoded by nuclear genes that plays an important role in maintaining cellular concentration of hydrogen peroxide to a level, necessary for all aspects of normal plant growth and development. CATs are located mostly in peroxisomes and glyoxysomes, where they play a key role in the removal of H2O2 generated by various oxidases. CAT directly dismutates H2O2 into H2O and O2 and are required for ROS detoxification during stressed conditions (Mittler et al. 2004). CAT also reacts with some hydroperoxides. Dehydroascorbate Reductase (DHAR) DHAR is a monomeric thiol enzyme which regenerates ascorbate from dehydroascorbate (DHA) (Foyer and Mullineaux 1998). DHAR catalyzes the reduction of DHA to AsA using GSH as the reducing substrate. This is a key in conferring tolerance to various abiotic stresses which produce ROS. Stresses such as drought, chilling, ozone, and metal toxicity increase the activity of the DHAR in plants (Maheshwari and Dubey 2009; Yoshida et al. 2006). It has also been found that DHAR overexpression also enhances plant tolerance against various abiotic stresses (Lee et al. 2007) (Table 2). Thus, MDHAR and DHAR are equally important in regulating the level of AsA and its redox state under oxidative stress (Eltayeb et al. 2006, 2007). Ascorbate Peroxidase (APX) The APX are heme-containing enzymes. The APX family has many isoforms located at different subcellular locations like thylakoid (tAPX), glyoxisome membrane (gmAPX), chloroplast stroma (sAPX), and cytosol (cAPX) (Da˛browska et al. 2007). APX is involved in scavenging of H2O2. APX catalyzes the reduction of H2O2 to water and uses ascorbate as a reductant for this reaction (Shigeoka et al. 2002; Asada 1999). The scavenging of H2O2 by APX is the first step of the ascorbate–glutathione (ASH-GSH) cycle. APX activity is enhanced in plants in response to different abiotic stresses. In Arabidopsis thaliana APX activity increased during exposure of plants to ozone, sulfur dioxide, chilling, and UV-B (Kubo et al. 1995; Rao et al. 1996). Guaiacol Peroxidase (GPOX) GPOX is a heme-containing protein. GPOX has a role in the biosynthesis of lignin and defense against biotic stresses by consuming H2O2. GPOX in plants use guaiacol and pyrogallol as a reducing substrate to oxidize many substrates in the presence of H2O2 (Vianello et al. 1997). This means that GPOX oxidize certain substrates at the expense of H2O2 and rid the cell of excess peroxide produced, especially under stress conditions. They are also effective quenchers of reactive intermediary forms of O2 and peroxyl radicals. Monodehydroascorbate Reductase (MDHAR) MDHAR is a flavin adenine dinucleotide (FAD) enzyme, present in cytosol and chloroplast. Peroxiredoxins (PRXs) Peroxiredoxins are non-heme containing peroxidases, which have to rely on an external electron donor to reduce H2O2, alkyl hydroperoxide, and 234 S. Teotia and D. Singh Table 2 Transgenic plants accumulating various antioxidant enzymes and molecules Gene and source Superoxide dismutase (SOD) FeSOD from Arabidopsis thaliana Transgenic organism Nicotiana tabacum Cu/ZnSOD from Hevea brasiliensis Hevea brasiliensis Chloroplastic Cu/ZnSOD from Pisum sativum Nicotiana tabacum MnSOD from Triticum aestivum Brassica napus MnSOD from Nicotiana plumbaginifolia MnSOD from Pisum sativum Medicago sativa Oryza sativa Cu/Zn SOD from Spinacia oleracea Malus domestica Cu/Zn SOD from Oryza sativa Nicotiana tabacum Cu/Zn SOD from Avicennia marina Oryza sativa Cu/Zn SOD from Solanum lycopersicum Cytosolic Cu/ZnSOD from Solanum lycopersicum Solanum tuberosum MnSOD from Tamarix androssowii Populus davidiana Catalase (CAT) Catalase from Broccoli Arabidopsis thaliana Catalase gene, katE, from E. coli Oryza sativa Catalase gene, Cat2, from Zea mays Nicotiana tabacum Catalase gene, Cat2, from Nicotiana tabacum Catalase gene from Triticum aestivum Solanum tuberosum Beta vulgaris Oryza sativa Ascorbate peroxidase (APX) Ascorbate peroxidase-like 1 gene from Nicotiana tabacum Capsicum annuum tAPX from Brassica napus Brassica napus Response of transgenic against various stresses Reference Enhanced tolerance to oxidative stress induced by methyl viologen (MV) Protection against ROS and tolerance to water deficit Resistance against MV-mediated oxidative stress (Van Camp et al. 1996) Tolerance against heat stress by removing H2O2 Tolerance against salt stress by removing H2O2 Tolerance to MV-mediated oxidative stress and resistance to bacterial pathogen Enhanced resistance to fungal pathogen, P. infestans Increased resistance to lowtemperature stress by removing H2O2 (Chiang et al. 2013) (Nagamiya et al. 2007) (Polidoros et al. 2001) (Leclercq et al. 2012) (Gupta et al. 1993a; Kwon et al. 2002) Resistance to aluminum (Basu et al. 2001) Resistance to cold stress (McKersie et al. 1999) Resistance to drought and MV and (Wang et al. polyethylene glycol (PEG)-induced 2005) oxidative stress Resistance to high and freezing (Artlip et al. temperatures 2009) Enhanced tolerance to salt, water, (Badawi et al. and PEG stresses 2004) Tolerance to MV-mediated (Prashanth oxidative stress, salinity and et al. 2008) drought stress Elevated tolerance to MV (Perl et al. 1993) Increased tolerance to MV and to (Tertivanidis leaf infection with the fungus et al. 2004) Cercospora beticola Enhanced salt tolerance (Wang et al. 2010) Increased tolerance to MVmediated oxidative stress and to the oomycete pathogen, Phytophthora nicotianae Increases resistance to salt stress and drought, and reduced accumulation of H2O2 (Yu et al. 1999) (Matsumuraa et al. 2002) (Sarowar et al. 2005) (Wang et al. 2011) (continued) Oxidative Stress in Plants and Its Management 235 Table 2 (continued) Gene and source cAPX from Pisum sativum APX3 from Arabidopsis thaliana Two cytosolic ascorbate peroxidases from Oryza sativa Glutathione reductase (GR) GR from bacteria Transgenic organism Solanum lycopersicum Nicotiana tabacum Arabidopsis thaliana GR from E. coli A poplar hybrid, Populus tremula x Populus alba Nicotiana tabacum GR from Pisum sativum Nicotiana tabacum Monodehydroascorbate reductase (MDHAR) cMDHAR from Solanum lycopersicum Solanum lycopersicum MDAR1 gene from Arabidopsis Nicotiana tabacum thaliana Dehydroascorbate reductase (DHAR) DHAR from Homo sapiens Nicotiana tabacum DHAR from Oryza sativa Arabidopsis thaliana cDHAR from Arabidopsis thaliana Nicotiana tabacum cDHAR from Arabidopsis thaliana Nicotiana tabacum Glutathione S-transferases (GSTs) GST and GPX from tobacco Nicotiana tabacum GST from cotton Nicotiana tabacum GST from maize Triticum aestivum GST from rice Oryza sativa GST from Trichoderma virens Nicotiana tabacum GST, from wild soybean (Glycine soja) Nicotiana tabacum GST from maize Nicotiana tabacum Response of transgenic against various stresses Reference Tolerance to chilling and salt stress (Wang et al. 2005) Increases protection against (Wang et al. oxidative stress 1999) Increases protection against salt (Lu et al. 2007) stress Tolerance to oxidative stress caused by MV (Foyer et al. 1995) (Lederer and Tolerance to oxidative stress caused by MV, H2O2, heavy metal Boger 2003; stress, and UV-B radiation Poage et al. 2011) Tolerance to oxidative stress (Creissen et al. caused by MV 1994) Resistant to salt- and PEG-induced osmotic stress, showing lower level of H2O2, higher APX activity. Enhanced tolerance to temperature and MV-mediated oxidative stresses Enhanced tolerance against ozone, salt, and PEG stress (Li et al. 2010a; Li et al. 2012b) Enhanced tolerance to MV, H2O2, low temperature, and salt Enhanced resistance to salt stress (Kwon et al. 2003) (Ushimaru et al. 2006) (Yin et al. 2010) (Eltayeb et al. 2006) Enhanced tolerance to aluminum stress Enhanced tolerance to ozone, drought, salt, and PEG stresses Enhanced tolerance to oxidative, chilling, and salt stress Enhanced tolerance to oxidative stress induced by a low concentration of MV Tolerant to herbicide (Eltayeb et al. 2007) (Roxas et al. 2000, 1997) (Yu et al. 2003) (Milligan et al. 2001) Enhanced tolerance to low (Takesawa temperature et al. 2002) Enhanced tolerance to cadmium (Dixit et al. 2011) Enhanced tolerance to drought and (Ji et al. 2010) salt Higher tolerance to alachlor (Karavangeli et al. 2005) (continued) 236 S. Teotia and D. Singh Table 2 (continued) Gene and source GST from Prosopis juliflora Transgenic organism Nicotiana tabacum GST (GSTL1) from Oryza sativa GST (GSTL2) from Oryza sativa Oryza sativa Arabidopsis thaliana Peroxiredoxins (PRXs) 2-Cys PRX from Arabidopsis thaliana Response of transgenic against various stresses Enhanced tolerance to drought Enhanced tolerance to herbicides Enhanced tolerance for heavy metals and other abiotic stresses like cold, osmotic stress and salt Reference (George et al. 2010) (Hu et al. 2009) (Kumar et al. 2013) Tall fescue (Festuca Increased tolerance against heat arundinacea) and and MV Solanum tuberosum L. cv. Atlantic Nicotiana tabacum Improved resistance to MV and Botrytis infection Arabidopsis thaliana Increased tolerance to salt and cold stress Nicotiana tabacum Increased tolerance to oxidative stress (Kim et al. 2010, 2011) GRX5 from Pteris vittata Solanum lycopersicum Arabidopsis thaliana Enhanced tolerance to oxidative and heat stress More tolerance to arsenic (Wu et al. 2012) (Sundaram et al. 2009) Thioredoxin (TRX) TRX-h1 from Oryza sativa Oryza sativa More tolerance to salt stress (Zhang et al. 2011) (Li et al. 2010b) PRX-Q from Gentiana triflora cv. Yahaba Y514 PRX-Q from Suaeda salsa 1-Cys- PRX from Oryza sativa Glutaredoxin (GRX) AtGRXS17 from Arabidopsis thaliana P-TRX from Phalaris coerulescens Hordeum vulgare Methionine sulfoxide reductase (MSR) Peptide MSR from Arabidopsis Arabidopsis thaliana thaliana MSRB2 gene from Capsicum annuum Solanum lycopersicum MSRB3 from Arabidopsis thaliana Arabidopsis thaliana Cytosolic MSRB7 and MSRB8 from Arabidopsis thaliana MSRA4.1 from Oryza sativa Arabidopsis thaliana Oryza sativa Glutathione Peroxidase (GPX) GPX from Chlamydomonas Nicotiana tabacum GST and GPX from Nicotiana tabacum Nicotiana tabacum GPX2 from Synechocystis Arabidopsis thaliana Increased aluminum resistance (Kiba et al. 2005) (Jing et al. 2006) (Lee et al. 2000) Enhanced tolerance to MV and high light in cold conditions Showed reduced production of H2O2 and resistance towards Phytophthora capsici and Phytophthora infestans Enhanced tolerance to MV and cold stress Enhanced tolerance to MV and H2O2 treatment Enhanced tolerance to salt stress (Romero et al. 2004) (Oh et al. 2010) Increased tolerance to oxidative stress caused by MV, cold, and salt stress Increased tolerance to cold and salt stress Enhanced tolerance to oxidative damage caused by H2O2, MV, Fe ions, and other stresses such as chilling, high salinity, or drought (Yoshimura et al. 2004) (Kwon et al. 2007) (Li et al. 2012a) (Guo et al. 2009) (Roxas et al. 1997) (Gaber et al. 2006) Ascorbate (AsA) L-Gulono-gamma-lactone oxidase from Solanum tuberosum L. Increased levels of AsA in transgenic (Hemavathi rat cv. Taedong Valley plants leading to enhanced tolerance et al. 2010) to MV, salt, and mannitol (continued) Oxidative Stress in Plants and Its Management 237 Table 2 (continued) Gene and source D-Galacturonic acid reductase from strawberry (this enzyme converts Dgalacturonic acid into L-Galactonic acid which is converted to L-galactono-1,4lactone, the immediate precursor of AsA) Glutathione (GSH) Glutathione synthetase (GS) from E. coli Glutathione synthetase enzyme from Streptococcus thermophilus γ-Glutamylcysteine synthetase and Glutathione synthetase from Brassica juncea γ-Glutamylcysteine synthetase (γ-ECS) from Oryza sativa γ-Glutamylcysteine synthetase (γ-ECS) from E. coli Response of transgenic against Transgenic organism various stresses Reference Solanum tuberosum L. Increased levels of AsA in (Hemavathi cv. Taedong Valley transgenic plants leading to et al. 2009) enhanced tolerance to MV, salt, and mannitol Brassica juncea Enhanced tolerance to cadmium Nicotiana tabacum Enhancing tolerance to abiotic stress Enhanced tolerance to atrazine; 1chloro-2, 4-dinitrobenzene; phenanthrene; and metolachlor Enhanced tolerance to salt and MV (Choe et al. 2013) Enhanced tolerance to (Gullner et al. chloroacetanilide herbicides, 2001) acetochlor, and metolachlor Brassica juncea Oryza sativa Poplar hybrid (Populus tremula X Populus alba) Proline (Pro) P5CS from Vigna aconitifolia L. Nicotiana tabacum P5CS from Vigna aconitifolia L. Medicago truncatula P5CS from Vigna aconitifolia L. Cicer arietinum Osmotin gene Nicotiana tabacum P5CS from Vigna aconitifolia P5CS from Vigna aconitifolia Oryza sativa L. ssp. indica cv. ADT 43 Triticum aestivum P5CR from Triticum aestivum Arabidopsis thaliana P5CR from Arabidopsis thaliana Glycine max Ornithine-δ-aminotransferase (δ-OAT) from Arabidopsis thaliana Oryza sativa L. ssp. japonica cv. Zhongzuo 321 Arabidopsis thaliana Antisense of proline dehydrogenase (ProDH) from Arabidopsis thaliana Tocopherols (TOCs) Tocopherol cyclase (VTE1) from Arabidopsis thaliana VTE2.1 from Solanum chilense Nicotiana tabacum Nicotiana tabacum Carotenoids (CARs) Beta-carotene ketolase gene (bkt) from Daucus carota green algae (Liang Zhu et al. 1999) (Liedschulte et al. 2010) (Flocco et al. 2004) Increased tolerance to osmotic stress Enhanced tolerance to osmotic stress Enhanced tolerance to salt stress (Kishor et al. 1995) (Verdoy et al. 2006) (Kiran Kumar Ghanti et al. 2011) Enhanced tolerance to salinity and (Barthakur drought et al. 2001) Enhanced tolerance to salt stress (Karthikeyan et al. 2011) Enhanced tolerance to drought (Vendruscolo et al. 2007) Enhanced stress tolerance (Ma et al. 2008) Enhanced tolerance to heat and (De Ronde drought stress et al. 2004) Accumulation of proline and (Liangqi et al. enhanced tolerance to drought and 2003) salt stress Proline accumulation and more (Nanjo et al. tolerance to freezing and high 1999) salinity Enhanced tolerance to drought induced oxidative stress Increased tolerance to oxidative stress damage as evidenced by reduced lipid peroxidation and delayed leaf senescence (Liu et al. 2008) (Espinoza et al. 2013) Plants accumulate ketocarotenoids and show enhanced tolerance to UV-B radiation, H2O2, and MV (Jayaraj and Punja 2008) (continued) 238 S. Teotia and D. Singh Table 2 (continued) Gene and source chyB gene that encodes beta-carotene hydroxylase to make zeaxanthin from Arabidopsis thaliana chyB gene that encodes beta-carotene hydroxylase from Arabidopsis thaliana DSM2 gene which encodes β-carotene hydroxylase (BCH) from Oryza sativa Flavonoids (FLVs) Anthocyanidin synthase (ANS) from Oryza sativa Flavonol synthase 1 (FLS1) from Zea mays Polyamines (PAs) S-Adenosyl-l-methionine decarboxylase (SAMDC) from yeast Arginine decarboxylase (ADC) from Datura stramonium Spermidine synthase (SPDS) from apple (Malus domestica) Spermidine synthase (SPDS) from Cucurbita ficifolia ADC from oat (Avena sativa) Transgenic organism Arabidopsis thaliana Nicotiana tabacum Oryza sativa Oryza sativa mutant Nootripathu (NP) Arabidopsis thaliana Solanum lycopersicum Oryza sativa Nicotiana tabacum var. Xanthi SAMDC from carnation (Dianthus caryophyllus L.) flower SPDS from Cucurbita ficifolia Nicotiana tabacum Choline oxidase (codA) from Arthrobacter globiformis Choline monooxygenase (CMO) from Atriplex hortensis Choline dehydrogenase (CDH), encoded by betA from E. coli Choline monooxygenase (CMO) from Spinacia oleracea Betaine aldehyde dehydrogenase (BADH) from Spinacia oleracea A chloroplastic BADH from Spinacia oleracea More tolerance to drought stress, reduced lipid peroxidation Increased resistance to drought and oxidative stresses and increase of the xanthophylls and nonphotochemical quenching (Zhao et al. 2013) (Du et al. 2010) Accumulation of a mixture of flavonoids and anthocyanins, with increased antioxidant potential Increased resistance to UV-B radiation (Reddy et al. 2007) Enhanced tolerance to hightemperature stress Enhanced tolerance to drought (Cheng et al. 2009) (Capell et al. 2004) (Wen et al. 2008) (Kasukabe et al. 2004) European pear (Pyrus Enhanced tolerance to salinity, communis L. ‘Ballad’) osmotic, and heavy metal stress Arabidopsis thaliana Enhanced tolerance to chilling, freezing, salinity, hyperosmosis, drought, and MV Oryza sativa Enhanced tolerance to salt stress SAMDC from human Glycine betaine (GB) Choline oxidase (codA) from Arthrobacter globiformis Response of transgenic against various stresses Reference More tolerance to high light, high (Davison et al. temperature, and lipid peroxidation 2002) (Emiliani et al. 2013) (Roy and Wu 2001) (Waie and Rajam 2003) Enhanced tolerance to salinity, drought, and fungal pathogens (V. dahliae and F. oxysporum) Enhanced tolerance to salt, cold, acidic, and abscisic acid stress Increased tolerance to chilling, heat, and MV (Wi et al. 2006) (Kasukabe et al. 2006) Solanum lycopersicum Mill. cv. Moneymaker Solanum tuberosum L. cv. Superior Gossypium hirsutum Enhanced tolerance to chilling, high salt, and oxidative stresses (Park et al. 2007) Enhanced tolerance to salt, drought, and MV Increased tolerance to salt stress Nicotiana tabacum Improved tolerance to salinity and cold stress Enhanced tolerance to salt stress and temperature stress Protection of Rubisco activity in high-temperature stress Improved tolerance to salt, oxidative stress, and low temperature (Ahmad et al. 2008b) (Zhang et al. 2009) (Holmstrom et al. 2000) (Shirasawa et al. 2006) (Yang et al. 2005) (Fan et al. 2012) Ipomoea batatas, cv. Kokei 14 Oryza sativa Nicotiana tabacum Ipomoea batatas, cv.-Sushu-2 (continued) Oxidative Stress in Plants and Its Management 239 Table 2 (continued) Gene and source Others Aldose/aldehyde reductase from Medicago sativa Transgenic organism Oxalate oxidase (OxO) from wheat Nicotiana tabacum Nicotiana tabacum Apolipoprotein D ortholog (AtTIL) from Arabidopsis thaliana Arabidopsis thaliana Aldehyde dehydrogenase ALDH3I1 and Arabidopsis thaliana ALDH7B4 from Arabidopsis thaliana BcZAT12 from Brassica carinata Solanum lycopersicum, cv. H-86 Serotonin N-acetyltransferase (NAT) Oryza sativa from sheep, producing more melatonin Isoprene synthase from Populus alba, producing more isoprene Nicotiana tabacum other peroxides. This electron donor is often reduced thioredoxin; hence, PRXs are often called thioredoxin peroxidases. The PRX family in plants can be divided into four groups (A to D) (Dietz 2011). A-type PRX are 2-Cys peroxiredoxin (2-CysPRX), the B-type PRX are 1-Cys peroxiredoxin (1-CysPRX), the C-type PRX are peroxiredoxin Q (PRX-Q), and the D-type PRXs are type II peroxiredoxins (PRXII). These four groups can be further divided depending on their subcellular locations. In plants, 2-Cys-PRXs are the most abundant PRXs and are located in chloroplasts. The 2-Cys-PRXs reduce peroxides through a thiol-based mechanism. During catalysis, these enzymes are sometimes inactivated by the substrate-dependent oxidation of the catalytic cysteine to the sulfinic acid (!SO2H) form and are reactivated by reduction carried by sulfiredoxin (SRX) (Jonsson et al. 2008). Glutathione Peroxidase (GPX) GPXs are considered as a fifth class of PRX, but evolutionary PRX and GPX are considered two different protein families. GPXs are a large family of diverse isozymes (Rodriguez Milla et al. 2003). GPXs help plants alleviate oxidative stress by reducing a broad range of hydroperoxides, including H2O2 and organic and lipid hydroperoxides Response of transgenic against various stresses Reference Tolerance against oxidative damage caused by paraquat, heavy metal treatment, and drought Increased tolerance to MV or high light-induced oxidative stress Enhances tolerance to freezing and oxidative stress Enhances tolerance to osmotic and oxidative stress Tolerance to heat-shock (HS)-induced oxidative stress (Oberschall et al. 2000) Increased resistance to the singletoxygen-generating peroxidizing herbicide butafenacil and increased SOD and CAT activity Increased resistance to ozoneinduced oxidative damage and high temperature (Park et al. 2013) (Wan et al. 2009) (Charron et al. 2008) (Kotchoni et al. 2006) (Shah et al. 2013) (Vickers et al. 2009) (LOOH) (Arthur 2000). They use GSH to do this function. They help prevent lipid peroxidation of cellular membranes by removing free peroxide in the cell. GPX also functions as an oxidative signal transducer (Miao et al. 2006). GPXs are reduced by thioredoxins. Glutathione Reductase (GR) GR is a flavoprotein oxidoreductase and is a key player against ROS defense by maintaining the reduced status of glutathione (GSH). It is localized mainly in chloroplasts but also in small amounts in mitochondria and cytosol (Edwards et al. 1990). GR catalyzes the conversion of GSSG into GSH (Meister and Anderson 1983). GR transfers electrons from NADPH to GSSG to generate GSH. Thus, GR maintains a high ratio of GSH/GSSG in plant cells which is important for scavenging H2O2. GR and GSH play a crucial role in determining the tolerance of a plant under various stresses (Foyer et al. 1997). In rice, expression of GR was found to be induced by ABA and chilling, drought, and salinity (Kaminaka et al. 1998). Glutathione S-Transferases (GSTs) Plant GSTs can be divided into eight classes of: phi, tau, theta, zeta, lambda, dehydroascorbate reductase 240 (DHAR), EF1Bγ and tetrachlorohydroquinone dehalogenase (TCHQD). Arabidopsis encodes about 55 GSTs (Dixon and Edwards 2010). GSTs catalyze the transfer of the tripeptide glutathione (γglutamyl-cysteinyl-glycine; GSH) to a cosubstrate (R-X) containing a reactive electrophilic center to form a polar S-glutathionylated reaction product (RSG). GSTs detoxify electrophilic herbicides and other xenobiotics by catalyzing their conjugation with GS, to produce less toxic and more watersoluble conjugates. Apart from herbicide detoxification, plant GSTs are known to function in hormone homeostasis, tyrosine metabolism, hydroxyperoxide detoxification, and plant responses to various stresses. GST activity in plants is induced in response to many abiotic stresses (Dixon et al. 2010). GSTs safeguard proteins from oxidative damage and maintain redox homeostasis by regenerating AsA from DHA (Dixon and Edwards 2010). S. Teotia and D. Singh are composed of six well-defined types (TRXs f, m, x, y, h, and o) that reside in different cell compartments and function in different processes (Meyer et al. 2005). TRX can exist either in reduced (dithiol) or in oxidized (disulfide) form. Reduced TRX acts to directly reduce protein disulfides and cysteine sulfenic acid (Meyer et al. 2012). Land plants contain a large GRX family. GRXs are small, nearly ubiquitous, oxidoreductases constituting an alternative reducing system to TRXs. GRXs catalyze the reduction of disulfide bonds of their substrate proteins in the presence of glutathione (GSH) and help binding of iron–sulfur clusters (Rouhier 2010). GRXs are important proteins for the response of plants to oxidative stress because they help in regenerating antioxidant enzymes. GRXs are directly reduced by GSH to produce GSSG. Nonenzymatic Antioxidants Methionine Sulfoxide Reductase (MSR) In proteins, methionine (Met) residues are especially sensitive to oxidation, as ROS can oxidize them to form S and R methionine sulfoxide (MetSO) diastereoisomers. Thus, Met residues form Met-S-sulfoxide or Met-R-sulfoxide, causing inactivation or malfunction of the proteins. To rescue the proteins, the oxidized forms of methionine, S-MetSO and R-MetSO, are reduced back to Met by the MetSO reductases, MsrA and MsrB, respectively (Sharov and Schoneich 2000). These proteins catalyze the thioredoxindependent reduction of MetSO back to Met (Brot et al. 1981), thereby repairing proteins. MSRs are proposed to act as a last-chance antioxidants and importantly repair proteins damaged from oxidative stress (Cabreiro et al. 2006). Glutaredoxin (GRX) and Thioredoxin (TRX) Thioredoxins (TRX) and glutaredoxins (GRX) constitute families of thiol oxidoreductases, furnishing reducing power to PRX, MSR, and arsenate reductases, which are key players for the plant response to the oxidative environment. TRXs are small redox proteins, widely distributed, and function in redox regulation in a broad spectrum of cellular reactions. Plant TRXs Ascorbate (AsA) AsA is the most potent and abundant antioxidant that protects the cell from the damage caused by ROS in plants (Foyer and Noctor 2011; Smirnoff 2007). Exogenous application of AsA renders the plants to be resistant to salt stress as shown in case of durum wheat (Azzedine et al. 2011). AsA can directly scavenge O2!, OH!, and 1O2 and reduce H2O2 to water via ascorbate peroxidase reaction (Noctor and Foyer 1998). APX requires a reducing substrate, ascorbate, which is then oxidized to monodehydroascorbate (MDHA). Transgenic plants overexpressing genes leading to increased ascorbate content confer resistance to oxidative and other stresses (Table 2). AsA is a reduced form, while its oxidized forms are MDHA and DHA. Regeneration of AsA is catalyzed by either MDHAR (from MDHA) or DHAR (from DHA) by using NADPH or reduced glutathione (GSH), respectively. AsA also maintains α-tocopherol in a reduced state. AsA regenerate tocopherol from tocopheroxyl radicals, thus providing protection to the membranes. Exogenous application of AsA positively influences the activity of many antioxidative enzymes and minimizes the oxidative damage (Shalata and Neumann 2001). Oxidative Stress in Plants and Its Management Glutathione (GSH) Reduced glutathione (GSH) is a major watersoluble antioxidant in plant cells, localized in all cell compartments (Table 1) (Moran et al. 2000). GSH is a tripeptide (γ-glutamylcysteinyl-glycine), which is synthesized from Cys and that exists interchangeably with the oxidized form, GSSG, and is vital for normal cellular function. Two sequential ATPdependent reactions allow the synthesis of γglutamylcysteine (γ-EC) from L-glutamate and L-cysteine, followed by the formation of GSH by addition of glycine to the C-terminal end of γ-EC (Meister 1988). These reactions are catalyzed by γ-glutamylcysteine synthetase (γ-ECS) and glutathione synthetase (GS). Glutathione plays important roles in protecting cells from biotic and abiotic stress. In a cell, it is the major antioxidant and major cellular redox buffer which directly scavenges most free radicals and reactive oxygen species (Noctor and Foyer 1998). GSH is a key ROS scavenger and can protect macromolecules like proteins, lipids, and DNA by acting as a proton donor forming GSSG. The reduced state of cells brought by GSH counteracts the effects of oxidative stress (Meyer 2008) by scavenging 1O2, H2O2, and OH! (Alscher 1989). Additionally, GSH is critical in regenerating another antioxidant like ascorbate (AsA), via the ASH-GSH cycle (Foyer and Halliwell 1976). Biosynthesis of glutathione is stimulated under oxidative stress conditions, as GSH gets converted to GSSG. In oxidative stress, GSH prevents the denaturation of proteins caused by the oxidation of protein thiol groups. Moreover, GSH acts as a substrate for GPX and GST, which are also involved in the removal of ROS (Noctor et al. 2002). The AsA-GSH cycle constitutes one of the most important antioxidant systems in plants. In this cycle, the ascorbate and the glutathione are utilized as reducers which are recycled through consuming the ATP and NADPH by the action of four enzymes: APX, MDHAR, DHAR, and GR. Proline (Pro) Pro is also considered as a potent antioxidant and potential inhibitor of adverse effects of ROS (Krishnan et al. 2008; Matysik et al. 2002; 241 Szabados and Savoure 2010). In plants the synthesis of L-Pro takes place from L-glutamic acid by the action of enzymes D1-pyrroline-5carboxylate synthetase (P5CS) and D1pyrroline-5-carboxylate reductase (P5CR) (Verbruggen and Hermans 2008). Following salt, drought, and metal stress, there is a dramatic accumulation of Pro. Free Pro has been proposed to act as an osmoprotectant, a protein stabilizer, a metal chelator, maintainer of redox homeostasis, and OH! and 1O2 scavenger (Ashraf and Foolad 2007; Matysik et al. 2002). Pro appeared as an effective scavenger of OH! (Smirnoff and Cumbes 1989). The constitutive or stressinducible expression of P5CS cDNA in plants leads to Pro accumulation and confers tolerance to various abiotic stresses (Hmida-Sayari et al. 2005; Su and Wu 2004) (Table 2). Tocopherols (TOCs) TOCs are lipid-soluble antioxidant and are potential scavengers of ROS (Shao et al. 2007). TOCs are considered general antioxidants for protection of membrane stability, including quenching or scavenging ROS like 1O2 and OH!(KriegerLiszkay and Trebst 2006). TOCs are localized in plants in the thylakoid membrane of chloroplasts. Out of the four isomers of TOCs (α, β, γ, δ) found in plants, α- and γ-tocopherol are predominant. αtocopherol has the highest antioxidant activity, which together with the hydrophilic antioxidants, glutathione and ascorbate participates in the detoxification of ROS (Kamal-Eldin and Appelqvist 1996). TOCs also reduce lipid peroxyl radicals (LOO!) to their corresponding hydroperoxides (Maeda et al. 2005). TOCs also participate in cell signaling. Oxidative stress activates the expression of genes responsible for the synthesis of TOCs in higher plants (Ahmad et al. 2008a). Tocopherol cyclase (VTE1) catalyzes the penultimate step of TOC synthesis Porfirova et al. 2002. Carotenoids (CARs) CARs are the most abundant pigmented plantderived compounds. CARs are considered to be the first line of defense of plants against toxicity. Like TOCs, CARs are lipid-soluble antioxidants that play a role in oxidative stress tolerance (Edge et al. 1997). Oxygenated CARs are known as 242 xanthophylls. Examples of these compounds are zeaxanthin and lutein. In all photosynthetic organisms, the carotenoids β-carotene and zeaxanthin, together with TOCs play a photoprotective role, either by dissipating excess excitation energy as heat or by scavenging ROS and suppressing lipid peroxidation. They play a role of an antioxidant by preventing the formation of singlet oxygen by quenching the triplet chlorophylls (Chl3) and other harmful free radicals which are naturally formed during photosynthesis (Ramel et al. 2012). Flavonoids (FLVs) FLVs are a group of polyphenolic compounds produced as secondary metabolites by plants. FLVs occur widely in the plant kingdom and accumulate in the plant vacuole as glycosides and also as exudates on the surface of leaves and other aerial plant parts. FLVs serve as ROS scavengers by neutralizing harmful radicals under adverse environmental conditions (Agati et al. 2007). FLVs absorb UV light, and plants able to synthesize these compounds were more tolerant to high UV irradiation than mutants impaired in the flavonoid pathway (Emiliani et al. 2013). FLVs play a key role in quenching free radicals, 1O2, and decomposing peroxides (Vieyra et al. 2009). Many flavonoid biosynthetic genes are induced under stress conditions (Kim et al. 2012). Polyamines (PAs) PAs are a group of natural compounds with aliphatic nitrogen structure and present in almost all living organisms. Putrescine, spermidine, and spermine are the most commonly found PAs in higher plants and could be present in free, soluble-conjugated, and insoluble-bound forms. PAs play important roles in plant growth and development (Kusano et al. 2008). They are also potent ROS scavengers and inhibitors of lipid peroxidation (Belle et al. 2004). The accumulation of conjugated and free polyamines in plants is very important for their protection against oxidative stress induced by abiotic factors (Jang et al. 2012; Nayyar and Chander 2004). Among the common polyamines, putrescine appears to be the most sensitive for external stress. Plant PAs are involved in imparting tolerance to such stresses such as cold, heat, salinity, hyperosmosis, hypoxia, and S. Teotia and D. Singh atmospheric pollutants (Liu et al. 2007). An exogenous supply of polyamines can protect plant against ozone damage (Bors et al. 1989). PAs are powerful OH! scavengers and can also quench O2! at a higher concentrations (Drolet et al. 1986). Spermine or spermidine also can quench 1 O2 at higher concentrations (Das and Misra 2004). Exogenously applied PAs counteracted the toxic effects of paraquat in Arabidopsis (Kurepa et al. 1998a). Glycine Betaine (GB) GB is a nitrogenous compound, a quaternary amine. GB is synthesized by either oxidation of choline or N-methylation of glycine (Chen and Murata 2002). In plants, the enzyme choline monooxygenase (CMO) first converts choline into betaine aldehyde, followed by the action of betaine aldehyde dehydrogenase (BADH) (a NAD+, dependent enzyme), to produce glycine betaine. These enzymes are mainly found in chloroplast stroma. GB biosynthetic genes have been widely used to improve abiotic stress in transgenic plants (Chen and Murata 2011). GB biosynthetic gene, choline oxidase (codA) from Arthrobacter globiformis, has been widely used for GB production in transgenic plants (Ahmad et al. 2008b; Park et al. 2004). codA converts choline into GB in a single step. GB has been implicated in inhibiting ROS accumulation and activation of some stress-related genes. It helps in controlling water balance but can also help to maintain protein and membrane structure. During salt or drought stress, synthesis of proteins involved in PSII repair is affected, leading to photoinhibition. GB stabilizes those PSII repair proteins and thus helps in the repair of PSII, which eventually increases stress tolerance (Li et al. 2013). There have been reports of GB alleviating lipid peroxidation (Li et al. 2013; Cruz et al. 2013). Genetic Engineering of Oxidative Stress Resistance in Plants Development of Transgenic Plants A number of transgenic plants with improved tolerance to various abiotic stresses have been Oxidative Stress in Plants and Its Management achieved through development of plants overexpressing enzymes involved in oxidative protection, such as GPX, SOD, APX, GST, and GR (Gupta et al. 1993a, b; Lee et al. 2007; Lu et al. 2007; Miao et al. 2006; Roxas et al. 1997) (Table 2). Sometimes, overexpression of one gene may not be enough to confer desired stress resistance upon transgenic plants (Lee et al. 2009). In those cases combinations of two or more antioxidants in transgenic plants have shown to have synergistic effects on stress tolerance (Tseng et al. 2008). Therefore, there has been increased emphasis on production of such transgenic plants. Development of Mutants Many plant mutants show reduced tolerance to oxidative stress (Charron et al. 2008; Filkowski et al. 2004; Li et al. 2011; Ning et al. 2010; Shin et al. 2009). But there are several examples of plant mutants which show enhanced tolerance to oxidative stress as summarized in Table 3. Conclusions and Future Perspectives ROS are unavoidable part of cell metabolism. They are generated by electron transport activities of chloroplast, mitochondria, and plasma membrane or as a by-product of various metabolic pathways in different cellular compartments. ROS can also be produced as result of various prolonged abiotic stresses. Under normal environmental conditions, ROS production in various cell compartments is low. These ROS are highly reactive and toxic and ultimately result in oxidative stress and damage to the cell. In oxidative stress ROS or free radicals are generated which can damage the biomolecules and cell structures and homeostasis, including oxidative damage to nucleic acids, lipids, and proteins. This leads to altered membrane properties like fluidity, loss of enzyme activity, protein structures, folding and crosslinking, inhibition of protein synthesis, DNA damage, impaired ion transport, and apoptosis. 243 The free radicals of ROS interact with each other and also with antioxidant systems. If ROS has to play a role of signaling molecules or preventing the spread of pathogens, their localization and concentration needs to be controlled. For this purpose, plant cell and its compartments like chloroplast, mitochondria, and peroxisomes deploy antioxidant defense systems to protect themselves against the oxidative damage caused by ROS. This repertoire of antioxidants comprises of enzymatic and nonenzymatic components. When ROS is produced in excess or when the antioxidant defense system is not properly functioning, the cell faces the danger of oxidative damage. To evaluate the negative role of ROS, it is important to understand mechanisms of its resistance and tolerance in plants. In the recent years, a lot of progress has been done in the field of oxidative stress, but still a lot of gaps in our knowledge of ROS metabolism and their effects on plants are left. Further progress in the fields of genomics, proteomics, and metabolomics will help in untying the knots of hidden biochemical networks involved in establishing oxidative stress in the cell. Knowledge of improved understanding of these, together with the biotechnological tools, will be helpful in producing plants with enhanced levels of tolerance to ROS. Past and ongoing research has already proven that induced expression of various antioxidant enzymes and accumulation of various antioxidant molecules have key roles in detoxification of ROS. Overexpression of ROS-scavenging enzymes like SODs, CAT, APX, GPX, PRX, GR, MDHAR, DHAR, and GST results in abiotic stress tolerance in various crop plants due to increased ROS-scavenging capacity. Significant loss to the yield of crops is done because of the cumulative effect of abiotic stress factors. Therefore, steps for better understanding of the mechanisms of abiotic stress and finding the ways that would increase stress tolerance in plants are crucial for nations’ economy and worldwide agriculture. ROS detoxification system is very complex and controlled at multiple levels in various subcellular locations, and modulating one component of the antioxidative defense system Table 3 Mutants showing tolerance to oxidative stress Response against oxidative stresses Resistance to ROS generating herbicides aminotriazole (AT) and MV Involved in karrikin and Increased tolerance strigolactone signaling to MV and H2O2 NAC-domain transcription Increased tolerance factor regulates senescence in to MV and H2O2 leaves Involved in ethylene signal Increased tolerance transduction to MV and H2O2 Forms subunits of histone Increased tolerance acetyltransferase complex to MV and CsCl Involved in signal Increased tolerance transduction of plant defense to H2O2 and trichome development Involved in photoautotrophic Increased tolerance to salt tolerance MV, high-light intensity Mutated gene Species Function(s) AAL-TOXIN RESISTANT Arabidopsis Not known (ATR) 1, 2, 7, 9 thaliana Reference (Gechev et al. 2008; Qureshi et al. 2011) ORE9 (AT2G42620) (Woo et al. 2004) ORE1 (AT5G39610) ORE3 (AT5G39610) ELONGATOR subuntis, ELP2 and ELP6 HYS1/CPR5 PHOTOAUTOTROPHIC SALT TOLERATE 1 (PST1) RADICAL-INDUCED CELL DEATH1 (RCD1) Arabidopsis thaliana Arabidopsis thaliana Arabidopsis thaliana Arabidopsis thaliana Arabidopsis thaliana Arabidopsis thaliana Arabidopsis A putative PARP protein, thaliana interacts with many stressrelated transcription factors SIMILAR TO RCD ONE 1 Arabidopsis (SRO1) thaliana GIGANTEA Arabidopsis thaliana PARAQUAT RESISTANT2 Arabidopsis thaliana UDP-GLUCOSYL TRANSFERASE 71C1 (UGT71C1) POLY(ADP-RIBOSE) POLYMERASE 1 AND 2 (PARP1 AND PARP2) DET2 Arabidopsis thaliana Arabidopsis thaliana (Woo et al. 2004) (Woo et al. 2004) (Zhou et al. 2009) (Hong-Ying et al. 2010) (Tsugane et al. 1999) (Ahlfors et al. 2004; Overmyer et al. 2000; Fujibe et al. 2004) (Teotia and A putative PARP protein has Increased tolerance to redundant roles with RCD1 H2O2, salt, and osmotic Lamb 2009) stress Promotes flowering under Increased tolerance (Kurepa et al. long days in a circadian clock- to MV 1998b) controlled flowering Encodes an SIncreased tolerance (Chen et al. nitrosoglutathione reductase to MV 2009) that is a key regulator of cell death Glycosylate the 3-OH of Increased tolerance (Lim et al. 2008) hydroxycinnamates and to MV flavonoids Poly (ADP-ribosylation) of Increased tolerance to (De Block et al. target proteins MV, high light, drought, 2005) and heat Involved in the brassinolide Increased tolerance (Cao et al. 2005) biosynthetic pathway to oxidative stress Reduction of α-tocopherol and Increased tolerance (Abbasi et al. increase in γ-tocopherol to MV and sorbitol 2007) Arabidopsis thaliana Silencing of γ-tocopherol Nicotiana methyltransferase (γtabacum TMT) Lysine decarboxylase-like Oryza sativa Accumulation of the 1 polyamines, putrescine, spermidine, and spermine under conditions of oxidative stress GLUTATHIONE SArabidopsis Participates in light signaling and affects GSH and ABA TRANSFERASE U17 thaliana accumulation (GSTU17) Peroxiredoxin Q Arabidopsis Peroxiredoxin Q decomposes (PRX-Q) thaliana peroxides using thioredoxin as an electron donor Increased tolerance to MV, UV-B, freezing, and osmotic stress Reduced accumulation (Jang et al. 2012) of ROS after exposure to oxidative, high salt, and acid stresses Plants were more tolerant to drought and salt stresses Decreased oxidative stress sensitivity (Chen et al. 2012) (Lamkemeyer et al. 2006) Oxidative Stress in Plants and Its Management might not be enough to confer resistance to the whole ROS pathway which may be emanating from multiple stressors. Genetic engineering to develop transgenic crops with gene stacking of different classes of ROS-scavenging enzymes and their isoforms may also be used to obtain synergistic and diversified tolerance to multiple environmental stresses. Similarly, mutants with enhanced tolerance to various stresses are also the answer to the growing demand of developing crops to withstand harsh environmental conditions. Therefore, plants with the ability to control or alleviate ROS levels are the need of the hour and answer to the future to enhance food production. References Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM (2007) Specific roles of alpha- and gammatocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol 143:1720–1738 Agati G, Matteini P, Goti A, Tattini M (2007) Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol 174:77–89 Agnez-Lima LF, Melo JT, Silva AE, Oliveira AH, Timoteo AR, Lima-Bessa KM, Martinez GR, Medeiros MH, Di Mascio P, Galhardo RS, Menck CF (2012) DNA damage by singlet oxygen and cellular protective mechanisms. Mutat Res, 751(1):15–28 Ahlfors R, Lang S, Overmyer K, Jaspers P, Brosche M, Tauriainen A, Kollist H, Tuominen H, Belles-Boix E, Piippo M, Inze D, Palva ET, Kangasjarvi J (2004) Arabidopsis RADICAL-INDUCED CELL DEATH1 belongs to the WWE protein-protein interaction domain protein family and modulates abscisic acid, ethylene, and methyl jasmonate responses. Plant Cell 16:1925–1937 Ahmad P, Sarwat M, Sharma S (2008a) Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 51:167–173 Ahmad R, Kim MD, Back KH, Kim HS, Lee HS, Kwon SY, Murata N, Chung WI, Kwak SS (2008b) Stressinduced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep 27:687–698 Alscher RG (1989) Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant 77:457–464 Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341 Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399 245 Arthur JR (2000) The glutathione peroxidases. Cell Mol Life Sci 57:1825–1835 Artlip TS, Wisniewski ME, Macarisin D, Norelli JL (2009) Ectopic expression of a Spinach SOD gene in young apple trees enhances abiotic stress resistance. Acta Hortic 839:645–650 Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639 Asensio AC, Gil-Monreal M, Pires L, Gogorcena Y, Aparicio-Tejo PM, Moran JF (2012) Two Fe-superoxide dismutase families respond differently to stress and senescence in legumes. J Plant Physiol 169:1253–1260 Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216 Azzedine F, Hocine G, Mebarek B (2011) Improvement of salt tolerance in durum wheat by ascorbic acid application. Plant Physiol Biochem 7:27–37 Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166: 919–928 Balestrazzi A, Confalonieri M, Macovei A, Dona M, Carbonera D (2011) Genotoxic stress and DNA repair in plants: emerging functions and tools for improving crop productivity. Plant Cell Rep 30:287–295 Barthakur S, Babu V, Bansa KC (2001) Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. J Plant Biochem Biotechnol 10:31–37 Basu U, Good AG, Taylor GJ (2001) Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Environ 24:1278–1269 Belle NAV, Dalmolin GD, Fonini G, Rubin MA, Rocha JBT (2004) Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res 1008:245–251 Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10:1611–1626 Bors W, Langebartels C, Michel C, Sandermann H Jr (1989) Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28:1589–1595 Britt AB (1996) DNA damage and repair in plants. Annu Rev Plant Physiol Plant Mol Biol 47:75–100 Brot N, Weissbach L, Werth J, Weissbach H (1981) Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci USA 78:2155–2158 Cabreiro F, Picot CR, Friguet B, Petropoulos I (2006) Methionine sulfoxide reductases: relevance to aging and protection against oxidative stress. Ann N Y Acad Sci 1067:37–44 246 Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzeng H, Zhao H, Kuai B (2005) Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant 123:57–66 Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101:9909–9914 Catala AA (2010) Synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem Biophys Res Commun 399:318–323 Charron JB, Ouellet F, Houde M, Sarhan F (2008) The plant Apolipoprotein D ortholog protects Arabidopsis against oxidative stress. BMC Plant Biol 8:86 Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257 Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20 Chen R, Sun S, Wang C, Li Y, Liang Y, An F, Li C, Dong H, Yang X, Zhang J, Zuo J (2009) The Arabidopsis PARAQUAT RESISTANT2 gene encodes an S-nitrosoglutathione reductase that is a key regulator of cell death. Cell Res 19:1377–1387 Chen JH, Jiang HW, Hsieh EJ, Chen HY, Chien CT, Hsieh HL, Lin TP (2012) Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol 158:340–351 Cheng L, Zou Y, Ding S, Zhang J, Yu X, Cao J, Lu G (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol 51:489–499 Chiang C, Chen S, Chen L, Chiang M, Chien H, Lin K (2013) Expression of the broccoli catalase gene (BoCAT) enhances heat tolerance in transgenic Arabidopsis. J Plant Biochem Biotechnol. DOI:10.1007/s13562-013-0210-1 Choe YH, Kim YS, Kim IS, Bae MJ, Lee EJ, Kim YH, Park HM, Yoon HS (2013) Homologous expression of gamma-glutamylcysteine synthetase increases grain yield and tolerance of transgenic rice plants to environmental stresses. J Plant Physiol 170:610–618 Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214 Corpas FJ, Fernandez-Ocana A, Carreras A, Valderrama R, Luque F, Esteban FJ, Rodriguez-Serrano M, Chaki M, Pedrajas JR, Sandalio LM, del Rio LA, Barroso JB (2006) The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves. Plant Cell Physiol 47:984–994 Creissen GP, Broadbent P, Kular B, Reynolds H, Wellburn AR, Mullineaux PM (1994) Manipulation of glutathione reductase in transgenic plants: implications for plants’ responses to environmental S. Teotia and D. Singh stress. Proc R Soc Edinb Sect B Biol Sci 102:167–175 Cruz FJR, Castro GLS, Silva Júnior DD, FestucciBuselli RA, Pinheiro HA (2013) Exogenous glycine betaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild waterstressed Carapa guianensis plants. Photosynthetica 51:102–108 Da˛browska G, Kata K, Goc A, Szechyńska-Hebda M, Skrzypek E (2007) Characteristics of the plant ascorbate peroxidase family. Acta Biol Cracov Bot 49:7–17 Das K, Misra H (2004) Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol Cell Biochem 262:127–133 Davison PA, Hunter CN, Horton P (2002) Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418:203–206 De Block M, Verduyn C, De Brouwer D, Cornelissen M (2005) Poly(ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J 41:95–106 De Ronde JA, Cress WA, Kruger GH, Strasser RJ, Van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. J Plant Physiol 161:1211–1224 Debska K, Bogatek R, Gniazdowska A (2012) Protein carbonylation and its role in physiological processes in plants. Postepy Biochem 58:34–43 del Rio LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol 141:330–335 Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212 Dietz KJ (2011) Peroxiredoxins in plants and cyanobacteria. Antioxid Redox Signal 15:1129–1159 Dixit P, Mukherjee PK, Ramachandran V, Eapen S (2011) Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS One 6:e16360 Dixon DP, Edwards R (2010) Glutathione transferases. Arabidopsis Book 8:e0131 Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338–350 Drolet G, Dumbroff EB, Legge RL, Thompson JE (1986) Radical scavenging properties of polyamines. Phytochemistry 25:367–371 Du H, Wang N, Cui F, Li X, Xiao J, Xiong L (2010) Characterization of the beta-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol 154:1304–1318 Edge R, McGarvey DJ, Truscott TG (1997) The carotenoids as anti-oxidants — a review. J Photochem Photobiol B Biol 41:189–200 Oxidative Stress in Plants and Its Management Edwards EA, Rawsthorne S, Mullineaux PM (1990) Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 180:278–284 Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K (2006) Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol Plant 127:57–65 Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225:1255–1264 Emiliani J, Grotewold E, Falcone Ferreyra ML, Casati P (2013) Flavonols protect Arabidopsis plants against UV-B deleterious effects. Mol Plant, 6 (4): 1376–1379 Espinoza A, San Martin A, López-Climent M, Ruiz-Lara S, Gómez-Cadenas A, Casaretto JA (2013) Engineered drought-induced biosynthesis of α-tocopherol alleviates stress-induced leaf damage in tobacco. J Plant Physiol 170:1285–1294 Fan W, Zhang M, Zhang H, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7:e37344 Filkowski J, Kovalchuk O, Kovalchuk I (2004) Genome stability of vtc1, tt4, and tt5 Arabidopsis thaliana mutants impaired in protection against oxidative stress. Plant J 38:60–69 Fischer BB, Hideg E, Krieger-Liszkay A (2013) Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid Redox Signal, 18: 2145–2162 Flocco CG, Lindblom SD, Smits EA (2004) Overexpression of enzymes involved in glutathione synthesis enhances tolerance to organic pollutants in Brassica juncea. Int J Phytoremediation 6:289–304 Flors C, Nonell S (2006) Light and singlet oxygen in plant defense against pathogens: phototoxic phenalenone phytoalexins. Acc Chem Res 39:293–300 Flors C, Ogilby PR, Luis JG, Grillo TA, Izquierdo LR, Gentili PL, Bussotti L, Nonell S (2006) Phototoxic phytoalexins. Processes that compete with the photosensitized production of singlet oxygen by 9phenylphenalenones. Photochem Photobiol 82:95–103 Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:C246–C256 Foyer C, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25 Foyer CH, Mullineaux PM (1998) The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett 425:528–529 Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress 247 perception and physiological responses. Plant Cell 17:1866–1875 Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18 Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol 109:1047–1057 Foyer CH, Lopez-Delgado H, Dat JF, Scott I (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100:241–254 Fujibe T, Saji H, Arakawa K, Yabe N, Takeuchi Y, Yamamoto KT (2004) A methyl viologen-resistant mutant of Arabidopsis, which is allelic to ozonesensitive rcd1, is tolerant to supplemental ultravioletB irradiation. Plant Physiol 134:275–285 Gaber A, Yoshimura K, Yamamoto T, Yabuta Y, Takeda T, Miyasaka H, Nakano Y, Shigeoka S (2006) Glutathione peroxidase-like protein of Synechocystis PCC 6803 confers tolerance to oxidative and environmental stresses in transgenic Arabidopsis. Physiol Plant 128:251–262 Gang W, Zhen-Kuan W, Yong-Xiang W, Li-Ye C, HongBo S (2007) The mutual responses of higher plants to environment: physiological and microbiological aspects. Colloids Surf B Biointerfaces 59:113–119 Gechev TS, Ferwerda MA, Mehterov N, Laloi C, Qureshi MK, Hille J (2008) Arabidopsis AAL-toxin-resistant mutant atr1 shows enhanced tolerance to programmed cell death induced by reactive oxygen species. Biochem Biophys Res Commun 375:639–644 George S, Venkataraman G, Parida A (2010) A chloroplast-localized and auxin-induced glutathione S-transferase from phreatophyte Prosopis juliflora confer drought tolerance on tobacco. J Plant Physiol 167:311–318 Giba Z, Todorivic S, Grubisic D, Konjevic R (1998) Occurrence and regulatory roles of superoxide anion radical and nitric oxide in plants. Iugoslav Physiol Pharmacol Acta 34:447–461 Gullner G, Komives T, Rennenberg H (2001) Enhanced tolerance of transgenic poplar plants overexpressing gamma-glutamylcysteine synthetase towards chloroacetanilide herbicides. J Exp Bot 52:971–979 Guo X, Wu Y, Wang Y, Chen Y, Chu C (2009) OsMSRA4.1 and OsMSRB1.1, two rice plastidial methionine sulfoxide reductases, are involved in abiotic stress responses. Planta 230:227–238 Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993a) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90:1629–1633 Gupta AS, Webb RP, Holaday AS, Allen RD (1993b) Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol 103:1067–1073 248 Hagar H, Ueda N, Shah SV (1996) Role of reactive oxygen metabolites in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Am J Physiol 271:209–215 Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322 Hemavathi, Upadhyaya CP, Akula N, Young KE, Chun SC, Kim DH, Park SW (2010) Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol Lett 32:321–330 Hemavathi, Upadhyaya CP, Younga KE, Akulaa N, Kimb HS, Heungb JJ, Ohc OM, Aswathd CR, Chuna SC, Kima DH, Parka SW (2009) Over-expression of strawberry D-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci 177:659–667 Hmida-Sayari A, Gargouri-Bouzid R, Bidani A, Jaoua L, Savourà A, Jaoua S (2005) Overexpression of ÎΔ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci 169:746–752 Holmstrom K-O, Somersalo S, Mandal A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51:177–185 Hong-Ying H, Wen-Ling L, Li-Ying S, Zhan S, ChengWei Y, Chang-Lian P (2010) Increased resistance of Arabidopsis cpr5 mutant to H2O2- induced photooxidation. Pak J Bot 42:1041–1049 Hu T, Qv X, Xiao G, Huang X (2009) Enhanced tolerance to herbicide of rice plants by over-expression of a glutathione S-transferase. Mol Breed 24:409–418 Jang S, Wi S, Choi Y, An G, Park K (2012) Increased polyamine biosynthesis enhances stress tolerance by preventing the accumulation of reactive oxygen species: T-DNA mutational analysis of Oryza sativa lysine decarboxylase-like protein 1. Mol Cells 34:251–262 Jayaraj J, Punja ZK (2008) Transgenic carrot plants accumulating ketocarotenoids show tolerance to UV and oxidative stresses. Plant Physiol Biochem 46:875–883 Ji W, Zhu Y, Li Y, Yang L, Zhao X, Cai H, Bai X (2010) Over-expression of a glutathione S-transferase gene, GsGST, from wild soybean (Glycine soja) enhances drought and salt tolerance in transgenic tobacco. Biotechnol Lett 32:1173–1179 Jing L-W, Chen S-H, Guo X-L, Zhang H, Zhao Y-X (2006) Overexpression of a chloroplast-located peroxiredoxin Q gene, SsPrxQ, increases the salt and low-temperature tolerance of Arabidopsis. J Integr Plant Biol 48:1244–1249 Jonsson TJ, Murray MS, Johnson LC, Lowther WT (2008) Reduction of cysteine sulfinic acid in peroxiredoxin by sulfiredoxin proceeds directly through a sulfinic phosphoryl ester intermediate. J Biol Chem 283:23846–23851 S. Teotia and D. Singh Kamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701 Kaminaka H, Morita S, Nakajima M, Masumura T, Tanaka K (1998) Gene cloning and expression of cytosolic glutathione reductase in rice (Oryza sativa L.). Plant Cell Physiol 39:1269–1280 Karavangeli M, Labrou NE, Clonis YD, Tsaftaris A (2005) Development of transgenic tobacco plants overexpressing maize glutathione S-transferase I for chloroacetanilide herbicides phytoremediation. Biomol Eng 22:121–128 Karlsson M, Melzer M, Prokhorenko I, Johansson T, Wingsle G (2005) Hydrogen peroxide and expression of hipI-superoxide dismutase are associated with the development of secondary cell walls in Zinnia elegans. J Exp Bot 56:2085–2093 Karthikeyan A, Pandian S, Ramesh M (2011) Transgenic indica rice cv. ADT 43 expressing a Δ1-pyrroline-5carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. Plant Cell Tissue Organ Cult (PCTOC) 107:383–395 Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stressregulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722 Kasukabe Y, He L, Watakabe Y, Otani M, Shimada T, Tachibana S (2006) Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotechnol 23:75–83 Kiba A, Nishihara M, Tsukatani N, Nakatsuka T, Kato Y, Yamamura S (2005) A peroxiredoxin Q homolog from gentians is involved in both resistance against fungal disease and oxidative stress. Plant Cell Physiol 46:1007–1015 Kim KH, Alam I, Lee KW, Sharmin SA, Kwak SS, Lee SY, Lee BH (2010) Enhanced tolerance of transgenic tall fescue plants overexpressing 2-Cys peroxiredoxin against methyl viologen and heat stresses. Biotechnol Lett 32:571–576 Kim MD, Kim YH, Kwon SY, Jang BY, Lee SY, Yun DJ, Cho JH, Kwak SS, Lee HS (2011) Overexpression of 2-cysteine peroxiredoxin enhances tolerance to methyl viologen-mediated oxidative stress and high temperature in potato plants. Plant Physiol Biochem 49:891–897 Kim B-G, Lee E-R, Ahn J-H (2012) Analysis of flavonoid contents and expression of flavonoid biosynthetic genes in Populus euramericana Guinier in response to abiotic stress. J Korean Soc Appl Biol Chem 55:141–145 Kiran Kumar Ghanti S, Sujata KG, Vijay Kumar BM, Nataraja Karba N, Janardhan Reddy K, Srinath Rao M, Kavi Kishor PB (2011) Heterologous expression of P5CS gene in chickpea enhances salt tolerance without affecting yield. Biol Plant 55:634–640 Oxidative Stress in Plants and Its Management Kishor PBK, Hong Z, Miao GH, Hu CAA, Verma DPS (1995) Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394 Kotchoni SO, Gachomo EW (2006) The reactive oxygen species network pathways: an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J Biosci 31:389–404 Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D (2006) Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ 29:1033–1048 Krieger-Liszkay A, Trebst A (2006) Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J Exp Bot 57:1677–1684 Krishnan N, Dickman MB, Becker DF (2008) Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med 44:671–681 Kubo A, Saji H, Tanaka K, Kondo N (1995) Expression of Arabidopsis cytosolic ascorbate peroxidase gene in response to ozone or sulfur dioxide. Plant Mol Biol 29:479–489 Kumar S, Asif MH, Chakrabarty D, Tripathi RD, Dubey RS, Trivedi PK (2013) Expression of a rice Lambda class of glutathione S-transferase, OsGSTL2, in Arabidopsis provides tolerance to heavy metal and other abiotic stresses. J Hazard Mater 248–249:228–237 Kurepa J, Smalle J, Montagu MV, Inzé D (1998a) Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiol 39:987–992 Kurepa J, Smalle J, Van Montagu M, Inzé D (1998b) Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J 14:759–764 Kusano T, Berberich T, Tateda C, Takahashi Y (2008) Polyamines: essential factors for growth and survival. Planta 228:367–381 Kwon SY, Jeong YJ, Lee HS, Kim JS, Cho KY, Allen RD, Kwak SS (2002) Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant Cell Environ 25:873–882 Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160:347–353 Kwon SJ, Kwon SI, Bae MS, Cho EJ, Park OK (2007) Role of the methionine sulfoxide reductase MsrB3 in cold acclimation in Arabidopsis. Plant Cell Physiol 48:1713–1723 249 Lamkemeyer P, Laxa M, Collin V, Li W, Finkemeier I, Schottler MA, Holtkamp V, Tognetti VB, IssakidisBourguet E, Kandlbinder A, Weis E, Miginiac-Maslow M, Dietz KJ (2006) Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J 45:968–981 Leclercq J, Martin F, Sanier C, Clement-Vidal A, Fabre D, Oliver G, Lardet L, Ayar A, Peyramard M, Montoro P (2012) Over-expression of a cytosolic isoform of the HbCuZnSOD gene in Hevea brasiliensis changes its response to a water deficit. Plant Mol Biol 80:255–272 Lederer B, Boger P (2003) Antioxidative responses of tobacco expressing a bacterial glutathione reductase. Z Naturforsch C 58:843–849 Lee KO, Jang HH, Jung BG, Chi YH, Lee JY, Choi YO, Lee JR, Lim CO, Cho MJ, Lee SY (2000) Rice 1Cysperoxiredoxin over-expressed in transgenic tobacco does not maintain dormancy but enhances antioxidant activity. FEBS Lett 486:103–106 Lee YP, Kim SH, Bang JW, Lee HS, Kwak SS, Kwon SY (2007) Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep 26:591–598 Lee S-C, Kwon S-Y, Kim S-R (2009) Ectopic expression of a cold-responsive CuZn superoxide dismutase gene, SodCc1, in transgenic rice (Oryza sativa L.). J Plant Biol 52:154–160 Li F, Wu QY, Sun YL, Wang LY, Yang XH, Meng QW (2010a) Overexpression of chloroplastic monodehydroascorbate reductase enhanced tolerance to temperature and methyl viologen-mediated oxidative stresses. Physiol Plant 139:421–434 Li QY, Niu HB, Yin J, Shao HB, Niu JS, Ren JP, Li YC, Wang X (2010b) Transgenic barley with overexpressed PTrx increases aluminum resistance in roots during germination. J Zhejiang Univ Sci B 11:862–870 Li G, Nasar V, Yang Y, Li W, Liu B, Sun L, Li D, Song F (2011) Arabidopsis poly(ADP-ribose) glycohydrolase 1 is required for drought, osmotic and oxidative stress responses. Plant Sci 180:283–291 Li CW, Lee SH, Chieh PS, Lin CS, Wang YC, Chan MT (2012a) Arabidopsis root-abundant cytosolic methionine sulfoxide reductase B genes MsrB7 and MsrB8 are involved in tolerance to oxidative stress. Plant Cell Physiol 53:1707–1719 Li F, Wu QY, Duan M, Dong XC, Li B, Meng QW (2012b) Transgenic tomato plants overexpressing chloroplastic monodehydroascorbate reductase are resistant to salt- and PEG-induced osmotic stress. Photosynthetica 50:120–128 Li M, Li Z, Li S, Guo S, Meng Q, Li G, Yang X (2013) Genetic engineering of glycine betaine biosynthesis reduces heat-enhanced photoinhibition by enhancing antioxidative defense and alleviating lipid peroxidation in tomato. Plant Mol Biol Rep 1–10. DOI:10.1007/s11105-013-0594-z 250 Liang Zhu Y, Pilon-Smits EA, Jouanin L, Terry N (1999) Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol 119:73–80 Liangqi W, Zhanmin F, Lei G, Yongqing L, Wenjing Z, Li-Jia Q, Zhangliang C (2003) Over-expression of an Arabidopsis δ-OAT gene enhances salt and drought tolerance in transgenic rice. Chin Sci Bull 48:2594–2600 Liedschulte V, Wachter A, Zhigang A, Rausch T (2010) Exploiting plants for glutathione (GSH) production: uncoupling GSH synthesis from cellular controls results in unprecedented GSH accumulation. Plant Biotechnol J 8:807–820 Lim CE, Choi JN, Kim IA, Lee SA, Hwang YS, Lee CH, Lim J (2008) Improved resistance to oxidative stress by a loss-of-function mutation in the Arabidopsis UGT71C1 gene. Mol Cells 25:368–375 Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T (2007) Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol 24:117–126 Liu X, Hua X, Guo J, Qi D, Wang L, Liu Z, Jin Z, Chen S, Liu G (2008) Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotechnol Lett 30:1275–1280 Lu Z, Liu D, Liu S (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep 26:1909–1917 Ma L, Zhou E, Gao L, Mao X, Zhou R, Jia J (2008) Isolation, expression analysis and chromosomal location of P5CR gene in common wheat (Triticum aestivum L.). S Afr J Bot 74:705–712 Maeda H, Sakuragi Y, Bryant DA, Dellapenna D (2005) Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol 138:1422–1435 Maheshwari R, Dubey RS (2009) Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul 59:37–49 Matsumuraa T, Tabayashib N, Kamagata Y, Souma C, Saruyama H (2002) Wheat catalase expressed in transgenic rice plants can improve tolerance against low temperature injury. Physiol Plant 116:317–327 Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532 McKersie BD, Bowley SR, Jones KS (1999) Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 119:839–848 Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208 Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760 Meyer AJ (2008) The integration of glutathione homeostasis and redox signaling. J Plant Physiol 165:1390–1403 S. Teotia and D. Singh Meyer Y, Reichheld JP, Vignols F (2005) Thioredoxins in Arabidopsis and other plants. Photosynth Res 86:419–433 Meyer Y, Belin C, Delorme-Hinoux V, Reichheld JP, Riondet C (2012) Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxid Redox Signal 17:1124–1160 Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18:2749–2766 Milligan AS, Daly A, Parry MAJ, Lazzeri PA, Jepson I (2001) The expression of a maize glutathione Stransferase gene in transgenic wheat confers herbicide tolerance, both in planta and in vitro. Mol Breed 7:301–315 Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410 Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498 Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481 Moran JF, Iturbe-Ormaetxe I, Matamoros MA, Rubio MC, Clemente MR, Brewin NJ, Becana M (2000) Glutathione and homoglutathione synthetases of legume nodules. Cloning, expression, and subcellular localization. Plant Physiol 124:1381–1392 Nagamiya K, Motohashi T, Nakao K, Prodhan SH, Hattori E, Hirose S, Ozawa K, Ohkawa Y, Takabe T, Takab T, Komamine A (2007) Enhancement of salt tolerance in transgenic rice expressing an Escherichia coli catalase gene, katE. Plant Biotechnol Rep 1:49–55 Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (1999) Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett 461:205–210 Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in Chickpea. J Agron Crop Sci 190:355–365 Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot 53:1237–1247 Ning J, Li X, Hicks LM, Xiong L (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152:876–890 Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279 Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53:1283–1304 Oxidative Stress in Plants and Its Management Oberschall A, Deak M, Torok K, Sass L, Vass I, Kovacs I, Feher A, Dudits D, Horvath GV (2000) A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. Plant J 24:437–446 Ogawa K, Kanematsu S, Asada K (1997) Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol 38:1118–1126 Oh SK, Baek KH, Seong ES, Joung YH, Choi GJ, Park JM, Cho HS, Kim EA, Lee S, Choi D (2010) CaMsrB2, pepper methionine sulfoxide reductase B2, is a novel defense regulator against oxidative stress and pathogen attack. Plant Physiol 154:245–261 op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332 Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H Jr, Kangasjarvi J (2000) Ozone-sensitive arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12:1849–1862 Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B (2013) Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med 57:176–187 Park J, Gu Y, Lee Y, Yang Z (2004) Phosphatidic acid induces leaf cell death in Arabidopsis by activating the Rho-related small G protein GTPase-mediated pathway of reactive oxygen species generation. Plant Physiol 134:129–136 Park EJ, Jeknic Z, Pino MT, Murata N, Chen TH (2007) Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ 30:994–1005 Park S, Lee D-E, Jang H, Byeon Y, Kim Y-S, Back K (2013) Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J Pineal Res 54:258–263 Perez-Perez ME, Lemaire SD, Crespo JL (2012) Reactive oxygen species and autophagy in plants and algae. Plant Physiol 160:156–164 Perl A, Perl-Treves R, Galili S, Aviv D, Shalgi E, Malkin S, Galun E (1993) Enhanced oxidative-stress defense in transgenic potato expressing tomato Cu, Zn superoxide dismutases. Theor Appl Genet 85:568–576 Poage M, Le Martret B, Jansen MA, Nugent GD, Dix PJ (2011) Modification of reactive oxygen species scavenging capacity of chloroplasts through plastid transformation. Plant Mol Biol 76:371–384 Polidoros AN, Mylona PV, Scandalios JG (2001) Transgenic tobacco plants expressing the maize Cat2 gene have altered catalase levels that affect plant-pathogen interactions and resistance to oxidative stress. Transgenic Res 10:555–569 251 Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105 Potters G, Pasternak TP, Guisez Y, Jansen MA (2009) Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ 32:158–169 Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörmann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99:12495–12500 Prashanth SR, Sadhasivam V, Parida A (2008) Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica rice var Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res 17:281–291 Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50:2–18 Qureshi M, Radeva V, Genkov T, Minkov I, Hille J, Gechev T (2011) Isolation and characterization of Arabidopsis mutants with enhanced tolerance to oxidative stress. Acta Physiol Plant 33:375–382 Ramel F, Birtic S, Cuine S, Triantaphylides C, Ravanat JL, Havaux M (2012) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158:1267–1278 Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-Band ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136 Raychaudhuri SS, Deng XW (2000) The role of superoxide dismutase in combating oxidative stress in higher plants. Bot Rev 66:89–98 Reddy AM, Reddy VS, Scheffler BE, Wienand U, Reddy AR (2007) Novel transgenic rice overexpressing anthocyanidin synthase accumulates a mixture of flavonoids leading to an increased antioxidant potential. Metab Eng 9:95–111 Rodriguez Milla MA, Maurer A, Rodriguez Huete A, Gustafson JP (2003) Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. Plant J 36:602–615 Romero HM, Berlett BS, Jensen PJ, Pell EJ, Tien M (2004) Investigations into the role of the plastidial peptide methionine sulfoxide reductase in response to oxidative stress in Arabidopsis. Plant Physiol 136:3784–3794 Rouhier N (2010) Plant glutaredoxins: pivotal players in redox biology and iron-sulphur centre assembly. New Phytol 186:365–372 Roxas VP, Smith RK Jr, Allen ER, Allen RD (1997) Overexpression of glutathione S-transferase/ glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15:988–991 Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerance in transgenic tobacco 252 seedlings that overexpress glutathione S-transferase/ glutathione peroxidase. Plant Cell Physiol 41:1229–1234 Roy M, Wu R (2001) Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci 160:869–875 Sarowar S, Kim EN, Kim YJ, Ok SH, Kim KD, Hwang BK, Shin JS (2005) Overexpression of a pepper ascorbate peroxidase-like 1 gene in tobacco plants enhances tolerance to oxidative stress and pathogens. Plant Sci 169:55–63 Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211 Shao H, Chu LY, Lu ZH, Kang CM (2007) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14 Shah K, Singh M, Rai AC (2013) Effect of heat-shock induced oxidative stress is suppressed in BcZAT12 expressing drought tolerant tomato. Phytochemistry 95:109–117 Sharov VS, Schoneich C (2000) Diastereoselective protein methionine oxidation by reactive oxygen species and diastereoselective repair by methionine sulfoxide reductase. Free Radic Biol Med 29:986–994 Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319 Shin JH, Yoshimoto K, Ohsumi Y, Jeon JS, An G (2009) OsATG10b, an autophagosome component, is needed for cell survival against oxidative stresses in rice. Mol Cells 27:67–74 Shirasawa K, Takabe T, Kishitani S (2006) Accumulation of glycinebetaine in rice plants that overexpress choline monooxygenase from spinach and evaluation of their tolerance to abiotic stress. Ann Bot 98:565–571 Smirnoff N (2007) Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Smirnoff N (ed) Antioxidants and reactive oxygen species in plants. Blackwell Publishing Ltd, Oxford, UK, pp 53–86 Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060 Su J, Wu R (2004) Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci 166:941–948 Sundaram S, Wu S, Ma LQ, Rathinasabapathi B (2009) Expression of a Pteris vittata glutaredoxin PvGRX5 in transgenic Arabidopsis thaliana increases plant arsenic tolerance and decreases arsenic accumulation in the leaves. Plant Cell Environ 32:851–858 Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97 Takesawa T, Ito M, Hiroyuki Kanzaki H, Kameya N, Nakamura I (2002) Over-expression of ζ glutathione S. Teotia and D. Singh S-transferase in transgenic rice enhances germination and growth at low temperature. Mol Breed 9:93–101 Teotia S, Lamb RS (2009) The paralogous genes RADICAL-INDUCED CELL DEATH1 and SIMILAR TO RCD ONE1 have partially redundant functions during Arabidopsis development. Plant Physiol 151:180–198 Tertivanidis K, Goudoula C, Vasilikiotis C, Hassiotou E, Perl-Treves R, Tsaftaris A (2004) Superoxide dismutase transgenes in sugarbeets confer resistance to oxidative agents and the fungus C. beticola. Transgenic Res 13:225–233 Tseng M-J, Liu C-W, Yiu J-C (2008) Tolerance to sulfur dioxide in transgenic Chinese cabbage transformed with both the superoxide dismutase containing manganese and catalase genes of Escherichia coli. Sci Hortic 115:101–110 Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195–1206 Tuteja N, Singh MB, Misra MK, Bhalla PL, Tuteja R (2001) Molecular mechanisms of DNA damage and repair: progress in plants. Crit Rev Biochem Mol Biol 36:337–397 Tuteja N, Ahmad P, Panda BB, Tuteja R (2009) Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat Res 681:134–149 Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Naito M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184 Van Camp W, Capiau K, Van Montagu M, Inze D, Slooten L (1996) Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts. Plant Physiol 112:1703–1714 Vendruscolo EC, Schuster I, Pileggi M, Scapim CA, Molinari HB, Marur CJ, Vieira LG (2007) Stressinduced synthesis of proline confers tolerance to water deficit in transgenic wheat. J Plant Physiol 164:1367–1376 Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759 Verdoy D, Coba De La Peña T, Redondo FJ, Lucas MM, Pueyo JJ (2006) Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ 29:1913–1923 Vianello A, Zancani M, Nagy G, Macri F (1997) Guaiacol peroxidase associated to soybean root plasma membranes oxidizes ascorbate. J Plant Physiol 150:5–5 Vieyra FE, Boggetti HJ, Zampini IC, Ordonez RM, Isla MI, Alvarez RM, De Rosso V, Mercadante AZ, Oxidative Stress in Plants and Its Management Borsarelli CD (2009) Singlet oxygen quenching and radical scavenging capacities of structurally-related flavonoids present in Zuccagnia punctata Cav. Free Radic Res 43:553–564 Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Nicholas Hewitt C (2009) Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ 32:520–531 Vranova E, Inze D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236 Waie B, Rajam MV (2003) Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci 164:727–734 Wan X, Tan J, Lu S, Lin C, Hu Y, Guo Z (2009) Increased tolerance to oxidative stress in transgenic tobacco expressing a wheat oxalate oxidase gene via induction of antioxidant enzymes is mediated by H2O2. Physiol Plant 136:30–44 Wang J, Zhang H, Allen RD (1999) Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol 40:725–732 Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162:465–472 Wang Y, Wisniewski M, Meilan R, Cui M, Webb R, Fuchigami L (2005) Overexpression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. J Am Soc Hortic Sci 130:167–173 Wang YC, Qu GZ, Li HY, Wu YJ, Wang C, Liu GF, Yang CP (2010) Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol Biol Rep 37:1119–1124 Wang J, Fan Z, Liu Z, Xiang J, Chai L, Li X, Yang Y (2011) Thylakoid-bound ascorbate peroxidase increases resistance to salt stress and drought in Brassica napus. Afr J Biotechnol 10:8039–8045 Wen XP, Pang XM, Matsuda N, Kita M, Inoue H, Hao YJ, Honda C, Moriguchi T (2008) Over-expression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res 17:251–263 Wi S, Kim W, Park K (2006) Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep 25:1111–1121 Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313(Pt 1):17–29 253 Woo HR, Kim JH, Nam HG, Lim PO (2004) The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and ore9 are tolerant to oxidative stress. Plant Cell Physiol 45:923–932 Wu Q, Lin J, Liu JZ, Wang X, Lim W, Oh M, Park J, Rajashekar CB, Whitham SA, Cheng NH, Hirschi KD, Park S (2012) Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol J 10:945–955 Yang X, Liang Z, Lu C (2005) Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol 138:2299–2309 Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621 Yoshida S, Tamaoki M, Shikano T, Nakajima N, Ogawa D, Ioki M, Aono M, Kubo A, Kamada H, Inoue Y, Saji H (2006) Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol 47:304–308 Yoshimura K, Miyao K, Gaber A, Takeda T, Kanaboshi H, Miyasaka H, Shigeoka S (2004) Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J 37:21–33 Yu D, Xie Z, Chen C, Fan B, Chen Z (1999) Expression of tobacco class II catalase gene activates the endogenous homologous gene and is associated with disease resistance in transgenic potato plants. Plant Mol Biol 39:477–488 Yu T, Li YS, Chen XF, Hu J, Chang X, Zhu YG (2003) Transgenic tobacco plants overexpressing cotton glutathione S-transferase (GST) show enhanced resistance to methyl viologen. J Plant Physiol 160:1305–1311 Zhang H, Dong H, Li W, Sun Y, Chen S, Kong X (2009) Increased glycine betaine synthesis and salinity tolerance in AhCMO transgenic cotton lines. Mol Breed 23:289–298 Zhang C-J, Zhao B-C, Ge W-N, Zhang Y-F, Song Y, Sun D-Y, Guo Y (2011) An apoplastic H-type thioredoxin is involved in the stress response through regulation of the apoplastic reactive oxygen species in rice. Plant Physiol 157:1884–1899 Zhao Q, Wang G, Ji J, Jin C, Wu W, Zhao J (2013) Over-expression of Arabidopsis thaliana β-carotene hydroxylase (chyB) gene enhances drought tolerance in transgenic tobacco. J Plant Biochem Biotechnol 1–9. DOI: 10.1007/s13562-013-0201-2 Zhou X, Hua D, Chen Z, Zhou Z, Gong Z (2009) Elongator mediates ABA responses, oxidative stress resistance and anthocyanin biosynthesis in Arabidopsis. Plant J 60:79–90