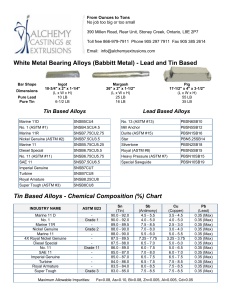
A review on biomedical titanium alloys Recent progress and prospect
Anuncio

REVIEW Titanium Alloys www.aem-journal.com A Review on Biomedical Titanium Alloys: Recent Progress and Prospect Lai-Chang Zhang* and Liang-Yu Chen* stents, heart valves, cardiac simulator, and replacement implants in knees, hips, elbows, shoulders, ears, and dentistry.[1,5] Among these parts, implants employed for hip and knee joint replacements have a great deal of requirements since osteoarthritis (inflammation in the bone joints), osteoporosis (weakening of the bones), and trauma usually lead to pain or loss in function of the tissues.[2] These deteriorating diseases result in gradual degradation in the mechanical properties of the bones because of inordinate loading or loss of the function for the self-healing process. It has been reported that the population of elderly people remarkably increases in the United States and less developed countries after 2010 (from 4.9 million in 2002 to 39.7 million in 2010) and most of them over the age of 40 may have such types of degenerative diseases.[1,6] Therefore, using biomedical artificial implants to recover the function of diseased hard tissues by surgical implantation is the best solution for patients. The data on the total replacement surgery for joints in the United States reveal that by the end of 2030, the total number of replacement surgery will increase by 174% (0.57 million procedures) for hip replacement and by 673% (3.48 million procedures) for knee arthroplasties from the current rate.[7] The reason for the growth in joint replacement is mainly ascribed to the increase in both replacement surgeries and revision surgeries. Unfortunately, the success rates of the revision surgeries are rather low as compared to the first implantation. The revision surgery may cause tremendous pain to the patients. The main reasons for the implant failure can be ascribed to debris generation, metal ions release and foreign body response in the human body, mismatch of modulus between the bones and the implants or too low strength to maintain the load.[3] As such, biomaterial implants employed for load-bearing applications must have many reliable properties, including appropriate mechanical properties, excellent corrosion resistance, high wear resistance, excellent biocompatibility, osseointegration, and non-cytotoxicity to avoid the revision surgery.[8] One of the most important things for designing and selecting the biomedical materials is their medical intentions. Once a biomedical implant is placed, a considerable number of reactions would take place at the interface of the host tissue and the implant. These reactions determine the biocompatibility of an implant, which also determines the success of the implantation.[9] To be biocompatible, toxic elements are not Compared with stainless steel and Co–Cr-based alloys, Ti and its alloys are widely used as biomedical implants due to many fascinating properties, such as superior mechanical properties, strong corrosion resistance, and excellent biocompatibility. After briefly introducing several most commonly used biomedical materials, this article reviews the recent development in Ti alloys and their biomedical applications, especially the low-modulus β-type Ti alloys and their design methods. This review also systemically investigates the recently attractive progress in preparation of biomedical Ti alloys, including additive manufacturing, porous powder metallurgy, and severe plastic deformation, applied in the manufacturing and the influenced microstructures and properties. Nevertheless, there are still some problems with the long-term performance of Ti alloys, and therefore several surface modification methods are reviewed to further improve their biological activity, wear resistance, and corrosion resistance. Finally, the biocompatibility of Ti and its alloys is concluded. Summarizing the findings from literature, future prediction is also conducted. 1. Introduction Biomedical materials, which are made of natural or artificial materials, are commonly used as structures and implants of human beings with the purpose of replacing their lost or diseased biological structure and improving the quality of life.[1–3] With growing aging population problems, the aged people may suffer from diseases such as arthritis and joint pain, and therefore there is a great increase in the demand for replacing dysfunctional hard tissues by artificial instruments made of biomedical materials.[4] Therefore, biomedical materials have gained substantially increasing attention in recent decades. According to the specific application, biomedical materials can be used in various parts in the human body as intravascular Prof. L. C. Zhang School of Engineering Edith Cowan University 270 Joondalup Drive, Joondalup, Perth, WA 6027, Australia E-mail: l.zhang@ecu.edu.au; lczhangimr@gmail.com Dr. L. Y. Chen School of Science Jiangsu University of Science and Technology Mengxi Road 2#, Zhenjiang 212003, P. R. China lychen@just.edu.cn The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adem.201801215. DOI: 10.1002/adem.201801215 Adv. Eng. Mater. 2019, 21, 1801215 1801215 (1 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com expected to be used in biomedical materials.[10] According to previous studies, elements such as Ti, Nb, Mo, Ta, Zr, Au, W, and Sn are highly biocompatible, while Al, V, Cr, Ni, etc. are considered as hazardous elements for human body.[11,12] The detailed information on pure metals and their biocompatibility is shown in Figure 1. As a result, the safe and biocompatible elements are more preferred to be considered when designing biomedical materials.[10] Meanwhile, the mechanical properties of biomedical materials determine the implants for specific applications. As biomedical materials, their elastic modulus, fatigue strength, tensile strength, and elongation are highly important for load-bearing hard tissue replacements. For instance, the strength of implant materials decides their loadbearing capability.[13] Besides fatigue and fracture failure, an implant is required to have a comparable modulus to the replaced bone.[14] Table 1 shows the tensile properties of human cortical bone. Table 2 and 3 list the compressive properties of human cortical bone and cancellous bone. Normally, if an implant has a greater modulus than the replaced bone, stress shielding effect, which prohibits the transfer of needed stress to the adjacent bone, would lead to bone resorption around the implant and the death of bone cells.[15] Therefore, an ideal biomedical material is expected to have the combined properties of low modulus (close to the bone) and high strength to sustain a long-term service period and to eliminate the revision surgery. Furthermore, the service period of the implant is determined by its wear and corrosion resistance.[16] The low wear and corrosion resistance of the implants in the human body lead to the wear debris and/or releases the Lai-Chang Zhang is a Professor of Materials Engineering and the Program Leader–Mechanical Engineering in the School of Engineering at Edith Cowan University. After receiving his PhD in Materials Science and Engineering at the Institute of Metal Research, Chinese Academy of Sciences, Prof. Zhang held several positions at The University of Western Australian, University of Wollongong, IFW Dresden and Technische Universität Darmstadt. His current research interest is focusing on the metal additive manufacturing (e.g. selective laser melting, electron beam melting), titanium alloys and composites, and processing-microstructure-properties in high-performance materials. Liang-Yu Chen is an Associate Professor in the School of Science at Jiangsu University of Science and Technology. He received his PhD degree from Shanghai Jiaotong University in 2016. His current research interest focuses on the biomedical titanium alloys, plasma sprayed coatings and corrosion science of metallic materials. incompatible metal ions, which may cause allergic and toxic reactions.[16] Therefore, the designed biomedical materials are also expected to have both high corrosion and wear resistance, aiming to maximize the longevity of the implants in the human Table 1. Tensile properties of human cortical bones. Figure 1. Pure metals and their biocompatibility. (Reproduced with permission from ref. [11]. Copyright (1998), Elsevier). Adv. Eng. Mater. 2019, 21, 1801215 Bone Age N n σUTS [MPa] E [GPa] emax [%] ρ [g cm3] Fibula 41.5 17 20 100 19.2 2.10 1.91 [59] Fibula 71 17 16 80 15.2 1.19 1.73 [59] Humerus 15–89 64 27 149 15.6 2.20 1.77 [60] Humerus 15–89 64 16 151 16.1 1.90 1.72 [60] Tibia 41.5 17 67 106 18.9 1.76 1.96 [59] Tibia 71 17 34 84 16.2 1.56 1.83 [59] Tibia 20–89 28 123 156 23.8 3.09 – [61] Femur 41.5 17 35 102 14.9 1.32 1.91 [59] Femur 71 17 35 68 13.6 1.07 1.85 [59] Femur 15–89 64 29 141 15.2 2.00 1.90 [60] Femur 15–89 64 30 134 15.0 1.80 1.80 [60] Femur 20–89 33 178 132 16.8 2.83 – [61] ref. N is the number of bones tested and n is the number of samples obtained from the tested bones; σUTS is ultimate tensile strength; E is elastic modulus; emax is ultimate tensile strain, and ρ is density; means male; indicates female; 41.5 and 71 are the average age value from 36 to 75 years of age, 41.5 is a younger group, 71 is an older group. 1801215 (2 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Table 2. Compressive properties of human cortical bones.[12] σUTS Age N n [MPa] E [GPa] H [MPa] Tibia osteons (longitudinal) 57/61 2 2 – 22.5 614 [62] Tibia interstitial Lamellae (longitudinal) 57/61 2 2 – 25.8 736 [62] Femur 20–89 19 95 194 17.6 – [61] Tibia 30–89 11 38 195 28.0 – [61] Tissue source ref. N is the number of bones tested and n is the number of samples obtained from them; σUTS is ultimate tensile strength; E is elastic modulus; and H is hardness. body. Lastly, high biocompatibility (such as osseointegration and antibacterial) is the most significant consideration for using implants in orthopaedic applications, which primarily means the formation of a reliable bonding between the implant and the adjacent bone without an intervening fibrous tissue and infection.[17,18] A successful osseointegration is considered as being no relative motion between the implant and the adjacent bone during functional loading.[19] Hence, it is highly essential that biomedical implants are expected to have an appropriate surface to integrate well with the adjacent bone. As such, surface topography, surface chemical compositions as well as surface coating are vital factors for development of implants with good biocompatibility. Based on the above consideration, metals and alloys have been used as load-bearing implants for a long period.[2,20–22] Until now, 316L stainless steel, Co–Cr-based alloys, Ti, and its alloys are most employed as metallic implant materials.[4,23–26] The use of stainless steels as implants began in the 1920s.[27] The stainless steels contain at least 10.5 wt% Cr to prevent the formation of rust in unpolluted atmospheres.[28] Among stainless steels, 316L stainless steel (18Cr–14Ni–2.5Mo, in wt %, the same hereafter unless indicated) are most commonly used for temporary implants and in some cases as a biomaterial for permanent implants.[29] The “L” in 316L stainless steel indicates low carbon, which can prevent the formation of chromium carbides and enhance the resistance to pitting and crevice corrosion.[30] However, stress corrosion cracking, which cannot be prevented in 316L stainless steel, can be triggered by the combined effect of tensile stress and a Cl-rich environment (i.e., human body fluid), thereby resulting in an unexpected sudden failure of the implant under the stresses much lower than the strength of the alloy.[31] By comparison, Co–Cr-based alloys have a better corrosion resistance in the Cl rich environment than 316L stainless steel. The high Cr contents in Co–Cr-based alloys results in the formation of a passive oxide (Cr2O3) film spontaneously on their surfaces in the human body environment.[32] Meanwhile, the mechanical properties of Co– Cr-based alloys meet the demand criteria of implants.[32] These alloys have often been used for sliding parts of artificial joints.[2] Unfortunately, due to corrosion and wear, some unfriendly elements such as Ni, Co, and Cr are found to be released from the 316L stainless steel and Co–Cr-based alloys in the human body.[33] It has been reported that the released Ni even caused some skin related diseases such as dermatitis, and the presence of Co showed carcinogenicity in numerous animal studies and led to the neurological symptoms in some patients after some years of implantation.[34,35] The released Cr would damage the kidney, liver and blood cells via oxidation reactions.[36] As such, 316L stainless steels and Co–Cr-based alloys have potential hazards when they are employed as biomedical implants. Furthermore, both 316L stainless steels and Co–Cr-based alloys exhibit much higher elastic moduli than bones in the human body, which may lead to the stress shielding effect and loosening of the implant after a long term of implantation.[13] Therefore, these two alloys may not be the best choice for orthopaedic implants. Ti has received extensive attention since the 1950s due to its high specific strength (e.g. the ratio of strength to density), excellent corrosion resistance, and superior biocompatibility.[16,37] During the development of Ti, most Ti products, such as commercially pure Ti (CP–Ti),[13,38,39] Ti alloys,[40–44] and Ti-based composites,[45–54] have been designed for the industries of aerospace, military, automobiles, Table 3. Compressive properties of human cancellous bones.[12] Age N n σUTS [MPa] E [GPa] emax [%] ρ [g cm3] Lumbar vertebra (male) 14–89 60 32 4.60 0.06 6.70 0.20 [63] Lumbar vertebra (female) 14–89 60 32 2.70 0.04 6.10 0.20 [63] Tibia head (male) 14–89 60 32 3.90 0.03 8.30 0.22 [63] Tibia head (female) 14–89 60 32 2.20 0.02 6.90 0.22 [63] Tibia 16–83 31 395/183 8.80 0.64 2.20 0.45 [64] Vertebra (vertical) 15–87 42 84 2.5 0.07 7.40 – [64] Vertebra (horizontal) 15–87 42 84 0.9 0.02 8.50 – [64] Proximal tibia 59–82 9 121 5.33 0.45 – 0.29 [12] Femur 58–83 10 299 7.36 0.39 – 0.50 [12] Lumbar spine 15–87 42 40 2.45 0.07 – 0.24 [12] Lumbar spine 71–84 3 231 1.55 0.02 – 0.19 [12] Tissue source ref. N is the number of bones tested and n is the number of samples obtained from them; 395 samples for elastic modulus, 183 samples for ultimate strength and ultimate strain; σUTS is ultimate tensile strength; E is elastic modulus; emax is ultimate tensile strain; and ρ is density. Adv. Eng. Mater. 2019, 21, 1801215 1801215 (3 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com medicine, jewelry, and mobile phones. Most importantly, due to the low density, high strength, high corrosion resistance, complete inertness to body environment, superior biocompatibility, low elastic modulus, high capacity to join with bone and other tissues, Ti and its alloys are regarded as the highly desirable choice for orthopaedic implants.[1,54,55] Figure 2 shows the elastic moduli of some biomedical materials and bone. Apparently, by comparing the data in Table 1–3, the elastic moduli of Ti alloys are significantly closer to those of bones. The density of Ti alloys (4.4–4.5 g cm3) is significantly less than those of Co–Cr-based alloys (8.3–9.2 g cm3) and 316L stainless steel (7.9 g cm3).[56] However, the strength of the Ti alloys can be as high as 316L stainless steel.[13] Therefore, Ti alloys are superior to other metallic implant biomaterials by comparing their specific strength (i.e. the ratio between yield strength and density). In the human body environment, a passive film (mainly consisted of TiO2) can spontaneously form on the surface of Ti alloy implant to protect the underlying substrate from further corrosion.[57] Once the oxide film of the Ti alloy implant is broken and exposes the substrate to the corrosive environment, a new passive film would be rebuilt rapidly.[58] Therefore, Ti alloys are more tolerant than 316L stainless steels and Co–Cr-based alloys. As aforementioned, Ti is non-toxic for the human body and has high biocompatibility.[36] Combined these advantages, Ti and its alloys provide a better solution to the problems of biomedical implants in the human body. In this review, a brief introduction to biomedical Ti alloys is first presented. Afterward, the development of biomedical Ti alloys is reviewed in order to understand different types of Ti alloys (i.e. α, near-α, (α þ β), β and shape-memory alloys) and their corresponding microstructures and mechanical properties. Third, new preparation methods of Ti and its alloys, such as additive manufacturing, porous powder metallurgy, and severe plastic deformation methods for biomedical applications, are reviewed. Fourth, the surface modification of Ti alloys for biomedical applications is introduced to further improve their biological activity, wear resistance, and corrosion resistance. Finally, the biocompatibility of Ti and Ti alloys is concluded. 2. Alloy Development and Mechanical Behavior Basically, pure Ti has a hexagonal close-packed (hcp) structure at room temperature, called α phase.[65] A phase transformation from hcp structure to body-centered cubic (bcc) structure would take place for pure Ti at 883 C (allotropic temperature), namely β phase.[66] Many previous investigations show that the phase transformation temperature of pure Ti can be strongly influenced by alloying elements.[67] In fact, alloying elements are usually classified into α-stabilizers (such as Al, C, O) and β-stabilizers (such as Mo, Ta, Nb). α-stabilizers increase the temperature of β/α transus while β-stabilizers reduce the β/α transus temperature.[68] Generally, β-stabilizers are transition elements in the Periodic Table. The strength of added βstabilizing transition elements depends on additive amounts. By tailoring the type and amount of alloying elements, β phase can be retained at room temperature. As such, conventional Ti alloys can be primarily classified into four groups of α-, near-α, (α þ β)-, and β-type alloys. Meanwhile, Ti-based shape-memory alloys is another group of Ti alloy with superelasticity and shape memory effect. 2.1. α-Type and Near-α Ti Alloys α-type Ti alloys, which only have α phase, consist of various grades of CP–Ti and Ti alloys.[69] Generally, CP–Ti has four grades which contain 0.20–0.50 wt% Fe and 0.18–0.40 wt% O.[70] Near-α Ti alloys primarily consist of α phase and a minor amount of β phase (less than 5 vol%), which are resulted from the addition of a very small amount of β-stabilizers (1–2 wt%) compared with α-type Ti alloys.[69] Both α-type Ti alloys and near-α Ti alloys have similar properties, such as excellent corrosion resistance, good weldability, and high creep resistance. Hence they are suitable for high-temperature applications.[71] However, their strength is significantly low at room temperature and usually cannot be improved by heat treatment since hcp structure is much stable.[71] As seen from Table 4, four grades of CP–Ti have the yield strength and ultimate tensile strength in the range of 170–480 MPa and 240–550 MPa, respectively.[28] Due to low mechanical strength at room temperature, α-type Ti alloys have not been used as implants.[36] Nonetheless, CP–Ti is the first generation of biomedical materials developed in 1950–1990 and is widely used biomaterial for dental and medical applications since they can promote the rapid osteointegration,[1] because the passive film of TiO2 on the surface of CP–Ti contains much OH ions which can react with the mineral constituents on the surface of bones (e.g., Ca2þ and (PO4)3–).[56] Unfortunately, low meFigure 2. Elastic moduli of various biomedical alloys and bone. (Reproduced with chanical strength and low fatigue strength permission from ref. [1]. Copyright (1998), Elsevier). prohibit α-type Ti alloys to be used in load-bearing Adv. Eng. Mater. 2019, 21, 1801215 1801215 (4 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Table 4. Mechanical properties of Ti and Ti alloys properties developed for orthopaedic implant application.[5,13,28,36,72–79] Materials σ0.2 [MPa] σUTS [MPa] E [GPa] emax [%] α-type CP–Ti grade 1 170 240 105 24 CP–Ti grade 2 275 345 105 20 CP–Ti grade 3 380 445 105 18 CP–Ti grade 4 480 550 105 15 (α þ β)-type Ti–6Al–4V (annealed) 825–869 895-930 110–114 6–10 Ti–6Al–4V ELI (mill annealed) 795–875 960-965 101–110 10–15 10 Ti–6Al–7Nb 795 860 105 Ti–5Al–2.5Fe 820 900 110 6 Ti–3Al–2.5V 585 690 100 15 β-type Ti–13Nb–13Zr (aged) 836–908 973–1037 79–84 42–44 1000–1060 1060–1100 74–85 18-22 Ti–15Mo (annealed) 544 874 78 21 Ti–15Mo–5Zr–3Al (ST) 838 852 80 25 1000–1060 1060–1100 – 18-22 945–987 979–999 83 16-18 Ti–12Mo–6Zr–2Fe (annealed) Ti–15Mo–5Zr–3Al (aged) Ti–15Mo–2.8Nb–0.2Si–0.260 (annealed) Ti–16Nb–10Hf 736 851 81 10 Ti–35.5Nb–7.3Zr–5.7Ta 793 827 55-66 20 Ti–29Nb–13Ta–4.6Zr (aged) 864 911 80 13.2 Ti–24Nb–4Zr–8Sn (Hot rolled) 700 830 46 15.0 Ti–24Nb–4Zr–8Sn (Hot forged) 570 755 55 13.0 Ti–9Mn 1023 1048 94 19.0 Ti–6Mn–4Mo 1090 1105 89 15.0 Ti–10Fe–10Ta–4Zr 960 1092 – 6.0 – 760 65 18.5 670–1150 835–1180 32 6.5–12.9 Ti–12Cr Ti–36Nb–2Ta–3Zr–0.3O Ti–24Nb–0.5O 665 810 54 22 Ti–24Nb–0.5N 665 665 43 13 Ti–23Nb–0.7Ta–2Zr 280 400 55 33 Ti–23Nb–0.7Ta–2Zr–1.2O 830 880 60 14 σ0.2, σUTS, E, emax are yield strength, ultimate tensile strength, elastic modulus, and ultimate strain, respectively. ST means solution treated. applications.[56] Similarly, near-α Ti alloys have not been used for biomedical applications until now. For non-load bearing but corrosion resistant applications, CP–Ti is better than near-α Ti alloys.[28,36] 2.2. (α þ β)-Type Ti Alloys (α þ β)-Type Ti alloys has a higher content of β-stabilizers than near-α Ti alloys. Correspondingly, (α þ β)-type Ti alloys possess higher fraction of β phase (about 5–30 vol%).[1,80] (α þ β)-type Ti alloys exhibit good fabricability, high strength at room temperature and moderate strength at high temperature.[81] In comparison to α-type Ti alloys, (α þ β)-type Ti alloys are heat treatable,[82] and hence, their mechanical properties can be optimized by heat treatment. The volume fractions and natures Adv. Eng. Mater. 2019, 21, 1801215 of α and β phases may vary according to the alloy chemistry, heat treatment temperature, and cooling rate.[83–86] Previous works show that the strength of (α þ β)-type Ti alloys can be improved by 30–50% via appropriate solution treatment and aging while their elastic moduli maintain at a similar level.[36] Therefore, as shown in Table 4, (α þ β)-type Ti alloys exhibit higher yield strength and ultimate tensile strength than α-type Ti alloys. Moreover, (α þ β)-type Ti alloys still have excellent corrosion resistance and capability of fast osteointegration in the human body environment by rapidly forming TiO2/OH film on the surface of bones.[69] Among (α þ β)-type Ti alloys, Ti–6Al–4V (Ti64) is the most widely used biomedical alloy, accounting for 50% of the total Ti production besides CP–Ti.[69] This alloy is commonly used in the annealed condition and for orthopaedic and traumatology implants.[82] Although Ti–6Al–4V is originally developed for 1801215 (5 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com aerospace purposes its attractive combined properties of strength, fracture toughness accompany with excellent corrosion resistance lead to the employment in biomedical applications.[1,87] Ti–6Al–4V has been further improved by decreasing the contents of interstitial impurities (such as O, N, C, and H), which is, well known as Ti–6Al–4V ELI and has been widely used for bone fixation plates and stem of artificial hip joints.[88] As Ti–6Al–4V contains toxic V, Ti–6Al–7Nb, and Ti–5Al–2.5Fe have been developed to replace Ti–6Al–4V.[88,89] Ti–6Al–7Nb possesses better wear resistance than Ti–6Al–4V and has been applied in medical devices including fracture fixation plates, femoral hip stems, fasteners, wires, and screws.[90] Ti–5Al–2.5Fe has been employed in the fabrication of hip prostheses and hip prosthesis heads as it is metallurgically similar to Ti–6Al–4V.[91] Although both Ti–6Al–7Nb and Ti–5Al– 2.5Fe can eliminate toxic V from Ti–6Al–4V, Al still exists in these alloys, which may still lead to a certain disease (such as Alzheimer).[12] Therefore, (α þ β)-type Ti alloys are not the optimal choice for biomedical implants. CP–Ti, Ti–6Al–4V, Ti– 6Al–4V ELI, Ti–6Al–7Nb, and Ti–5Al–2.5Fe are the first generation of biomedical materials that were developed during 1950–1990s.[1] The mechanical properties of these alloys, as well as Ti–3Al–2.5V, are listed in Table 4. Apparently, the moduli of (α þ β)-type Ti alloys are still significantly higher than those of bones (Table 1–3), which may cause bone resorption and implant loosening. Therefore, these reasons have motivated the development of β-type Ti alloys which contain non-toxic βstabilizers (such as Mo, Ta, Zr) and have lower elastic modulus and enhanced biocompatibility.[21,92] 2.3. β-Type Ti Alloys Compared with (α þ β)-type Ti alloys, β-type Ti alloys contain higher amounts of β-stabilizers (such as Mo, Ta, and Zr) and lower amounts of α-stabilizers without formation of any intermetallic phases.[71,93] β-Type Ti Alloys are heat treatable and can be heated at solution treated condition followed by aging at the temperatures between 450 and 650 C in order to enhance their strength. Such a phenomenon is attributed to the dispersion strengthening due to the partial transformation of β to α phase.[11] In fact, the toughness, plasticity, heat treatment capability, and age hardenability can be improved by increasing the portion of β phase in the β-type Ti alloys, resulting in decrease in elastic modulus.[71] Table 4 lists the mechanical properties of some β-type Ti alloys. According to Table 4, β-type Ti alloys have lower elastic moduli compared with the other types of Ti alloys, which can facilitate to reduce the stress shield effect. Meanwhile, β-type Ti alloys have comparable strength and better biocompatibility and are expected to have higher corrosion resistance in the human body compared with (α þ β)-typesen Ti alloys, due to the abce of micro-galvanic effect between different phases.[11,94,95] Therefore, β-type Ti alloys hold the promise of accommodating challenges in being used as biomedical materials. In the last two decades, many new β-type Ti alloys[96] and gum metals (a kind of β-type Ti alloys with specific alloy compositions appear to be deformed via a dislocation-free mechanism)[97] with comparable or optimized properties have been developed for Adv. Eng. Mater. 2019, 21, 1801215 biomedical applications. The properties of such alloys are also listed in Table 4. Most of these β-type Ti alloys have a higher density than other types of Ti alloys due to the higher content of higher density alloying elements (such as Mo, Ta, Nb, and Zr).[97] These alloying elements are rare, thereby leading to high cost of raw materials. The high melting points of these alloying elements also cause the segregation and difficulty in preparation of these alloys.[98] Consequently, recent developments have focused on developing new low-cost β-type Ti alloys which consist of low-cost alloying elements such as Fe, Mn, Sn, and Cr, and possess favorable mechanical properties for biomedical applications.[73,99] It should be noted that attempts for designing more low-cost βtype Ti alloys are never ceased, and therefore the future biomedical implant applications may be achieved by these low-cost β-type Ti alloys which also show favorable properties.[3] Usually, β-type Ti alloys are designed by considering the stability of β phase of the titanium alloys. The stability of β phase in Ti alloys can be predicted based on the molybdenum equivalency (Moeq) and DV-Xα cluster method.[66] The equivalent Mo content used in multi-component Ti alloys can be expressed by the following equation[66]: ½Ta ½Nb ½W ½V þ þ þ þ 1:25½Cr 5 3:6 2:5 1:5 þ 1:25½Ni þ 1:7½Mn þ 1:7½Co þ 2:5½Fe Moeq wt% ¼ ½Mo þ ð1Þ where [x] indicates the content of the element [x] in wt%. In the DV-Xα cluster method, two parameters, i.e. bond order (Bo) and metal d-orbital energy level (Md), are defined. The former is the measurement of the bond strength between Ti and an alloying element. The latter is related to the electronegativity and the metallic radius of the alloying elements.[11,100] For a given alloy, the average values of bond order (Bo) and d-orbital energy level (Md) are given as follows[101,102]: Bo ¼ X X i ðBoÞi X Md ¼ X i ðMdÞi ð2Þ ð3Þ where X i is the atomic fraction of element i in the alloy, (Bo)i and (Md)i are the values of bond order and metal d-orbital energy level for element i, respectively.[101,102] In this method, phase stability map can be constructed using Bo and Md, as shown in Figure 3.[11] The location of designed alloy is varied depending on its chemical compositions. Some new β-type alloys, such as in the Ti–Fe–Ta, Ti–Fe–Nb, Ti– Nb–Fe, Ti–Zr–Fe–Cr, and Ti–Nb–Fe–Cr alloy systems, have been developed according to the aforementioned methods.[65–67,103–107] Such low-cost β-type alloys exhibit comparable mechanical properties to the conventional β-type alloys (Table 5). In these developed new β-type alloys, the stability of β phase can be predicted by the value of Moeq. It is observed that the value of Moeq decreases with increasing the fraction of β-stabilizers (e.g., Nb and Ta) and the resultant strength decreases. However, the strength of the Ti–27Nb–7Fe–xCr does not continuously decrease after the addition of Cr (β-stabilizer) is greater than 1801215 (6 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 3. Phase stability map based on Bo and Md parameters. Modulus (in GPa) of each Ti alloy is shown in the bracket. (Reproduced with permission from ref. [11]. Copyright (1998), Elsevier). 4% but it maintains at a stable level in spite of the increase in Moeq.[106] As shown in Figure 4a–d, the fraction of β phase in the Ti–11Nb–xFe alloys designed by the current group increases with increasing the content of Fe (β-stabilizer).[65] Correspondingly, the value of Moeq increases from 25 to 35. Impressively, both the strength and elongation of the Ti–11Nb–xFe increase simultaneously (Figure 4e). Such an enhancement of strength is ascribed to the solid solution strengthening of Nb and Fe in β phase. As such, Ti–11Nb–3.5Fe containing more β phase, which exhibits large ductility and high strength, has an enhanced yield Table 5. β stability indicators and mechanical properties of some designed Ti alloys. β stability indicators Bo Md Moeq H [GPa] E [GPa] σ0.2 [MPa] emax [%] ref. Ti–8Fe 2.7803 2.3444 20.0 5.75 127 1562 17.1 [67] Ti–8Fe–5Ta 2.7849 2.3417 21.0 5.00 119 1062 37.3 [67] Ti–8Fe–8Ta 2.7878 2.3399 21.6 4.38 107 1030 38.1 [67] Ti–9Fe–9Ta 2.7875 2.3253 24.3 3.75 99 1010 39.5 [67] Ti–10Fe–10Ta 2.7872 2.3105 27.0 3.38 92 1000 40.0 [67] Ti–7Fe 2.7810 2.3570 17.5 5.92 130 1847 8.0 [66] Ti–1Nb–7Fe 2.7830 2.3560 17.8 5.58 124 1785 10.0 [66] Ti–4Nb–7Fe 2.7871 2.3550 18.6 5.13 115 1539 24.3 [66] Ti–6Nb–7Fe 2.7911 2.3530 19.2 4.18 97 1144 32.1 [66] Ti–9Nb–7Fe 2.7960 2.3520 20.0 3.72 89 1010 40.5 [66] Ti–11Nb–7Fe 2.8000 2.3500 20.6 3.57 86 985 41.5 [66] Ti–27Nb–7Fe 2.8303 2.3400 25.0 6.25 125 775 40.2 [106] Ti–27Nb–7Fe–2Cr 2.8301 2.3190 27.5 3.80 108 1100 30.0 [106] Ti–27Nb–7Fe–4Cr 2.8300 2.2976 30.0 3.75 88 950 34.1 [106] Ti–27Nb–7Fe–6Cr 2.8298 2.2768 32.5 3.25 81 925 36.2 [106] Ti–27Nb–7Fe–8Cr 2.8296 2.2555 35.0 3.50 77 930 36.7 [106] Alloy nominal Compositions [wt%] Bo is average bond order; Md is average d-orbital energy level Moeq is molybdenum equivalency; H is hardness; E is elastic modulus; σ0.2 is yield strength; and emax is ultimate compressive strain. Adv. Eng. Mater. 2019, 21, 1801215 1801215 (7 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 4. SEM micrographs of Ti–11Nb–xFe alloys: a) x ¼ 0.5, b) x ¼ 3.5, c) x ¼ 6, and d) x ¼ 9; e) compressive stress–strain curves of the Ti–11Nb–xFe alloys at room temperature and f) elastic energy versus modulus plot comparing the Ti–11Nb–xFe alloys and some other Ti alloys. (Reproduced with permission from ref. [65]. Copyright (2016), Elsevier). strength and plastic deformation as compared to Ti–11Nb– 0.5Fe. Figure 4f shows the elastic energy versus modulus comparing the Ti–11Nb–xFe alloys with some other Ti alloys. Apparently, Ti–11Nb–6Fe and Ti–11Nb–9Fe have higher elastic energies than CP–Ti, Ti–6Al–4V, Ti–15Mo, etc, indicating a better bearing capacity in heavy loading compared with other Ti alloys. Such results demonstrate that the phase constituents of the designed β-type alloys can be tailored by adjusting the alloy compositions, whereby the resultant properties can also be optimized. Furthermore, Ti–Zr–Fe–Cr and Ti–Nb–Fe–Cr series of β-type alloys with C15 laves phase precipitates exhibit high strength (yield strength: 785 to 1285 MPa) and large plasticity (plastic strain: 8.0 to 41.5%).[105,106] It was found that the C15 laves phase precipitates possess larger plasticity than the other laves phase precipitates (such as C14) in the Ti alloys. Although initiation and propagation of cracks were preferred in the C15 laves phase precipitate regions, C15 laves phase precipitates could dissipate the deformation energy to some extent in the Adv. Eng. Mater. 2019, 21, 1801215 form of heat during crack propagation. Therefore, an appropriate amount of C15 laves phase precipitates could improve the strength of the alloys while maintaining large plasticity. The high strength and large plasticity may ensure the load-bearing capability of developed new alloys in the human body. It has been reported that the deformation of solution-treated β-type Ti alloys is governed by stress-induced martensite (SIM), mechanical twinning, and dislocation slip.[102] As proposed by Morinaga,[108] the DV-Xα cluster method also could be used to reveal the relationship between the deformation behavior and β phase stability for β-type Ti alloys. Very recently, Wang et al.[109] pointed out that there was still problematic in the use of the Bo Md diagram. For instance, it was found that the Ti–10V– 3Fe–3Al (wt%) lays within the twinning region in Bo Md diagram; however, in reality, the stress-induced α” martensite was observed in the microstructure after room temperature deformation.[102] Therefore, Wang et al.[87] proposed a semiempirical approach to predict the deformation behavior of β-type 1801215 (8 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 5. The e=a-Δr diagram in which the Twinning/SIM and Slip regions are distinguished according to the reviews of published works. The use of empty and full bullets represents the Slip- and Twinning/SIM-dominated deformation mechanisms, respectively. SIM means stress-induced martensite. (Reproduced with permission from ref. [109]. Copyright (2018), Elsevier). Ti alloys by using the average electron-to-atom ratio (e=a) and atomic radius difference (Δr) as a supplement to the Bo Md diagram, and thereby an e=a Δr map was suggested, as shown in Figure 5. The main features of the e=a Δr map indicate that: 1) the slip region has a higher value of e=a than the twinning/SIM region, which mainly takes place when e=a < 4.2 and Δr > –2.5) the critical values for e=a of the twinning/SIM region have a peak when Δr is close to 0, and then decline as the Δr increases. Therefore, in addition to the β phase stability, it is proved that e=a and Δr also influence the deformation behavior of β-type Ti alloys by controlling the difficulty of lattice shear. However, the current alloy design methods mentioned in this section are primitive at best. In order to develop more new titanium alloys to fully meet the demand of the biomedical applications, new methods with better performance prediction are required. 2.4. Ti-Based Shape Memory Alloys Ti-based shape-memory alloys, such as TiNi, TiNiAg, TiZr, and TiNbSn, are also frequently used as biomedical materials due to their unusual mechanical properties.[110–113] The nearequiatomic TiNi alloy is initially found to have superelasticity and shape-memory effects involving high strength, high recover strain, and a relatively low elastic modulus, which are based on a reversible martensitic transformation.[114] Such advantages enable TiNi alloy to have multifunctional applications. Even so, biomedical and aerial applications remain the main object for TiNi and other Ti-based shape-memory alloys. For biomedical purposes, TiNi alloy exhibits comparable biocompatibility to stainless steel and other Ti implants.[115] TiNi alloy also shows slightly better corrosion resistance than Co-Cr-based alloys in artificial saliva.[116] Thierry et al.[115] compared the thrombogenicity of TiNi vs. stainless steel stents in an ex vivo shunt porcine Adv. Eng. Mater. 2019, 21, 1801215 model and the results showed that TiNi stents represent only a few of thrombus mainly located at the strut intersections while stainless steel stents clearly exhibit more thrombus. Many other studies also have been conducted to investigate the biological performance of TiNi alloy and have a consensus that TiNi alloy exhibits biocompatible behavior both in vitro and in vivo.[117–119] Meanwhile, the high strength and relatively low stiffness enable TiNi alloy to be many bone implants, including maxillofacial implant, stents, cervical and joint replacements, bone tissue engineering, and spine fracture fixation.[110,120] Especially, the unique shape-recovery behavior facilitates the mechanical stability of TiNi alloy implant within the host tissue. The recoverable strains in superelastic TiNi implant are achievable during a patient’s walking and running activities,[121] which facilitates the growth of bone cells near and/or in TiNi (porous) implants. It is well known that implant strain can stimulate cell growth by in vivo experiments using a guinea-pig model.[122] Compared with TiNi alloy, the other metallic implants always tend to be stiffer and therefore exhibit lower strains. Therefore, TiNi alloy and other Ti-based shape-memory alloys implants have the advantages for osseointegration. The superelasticity and shape-memory effects are very sensitive to chemical compositions of TiNi alloys since different compositions may lead to different phase constituents in TiNi alloys, which finally results in distinctive transformation hysteresis behavior and properties. Fu et al.[123] prepared TiNi films with different Ti/Ni ratios by co-sputtering of a Ti50Ni50 (at%) target and a separated Ti target at the temperature of 723 K. The TiNi film possesses a quite low residual stress at room temperature and a two-step phase transformation among martensite, R phase (i.e., the rod-like eutectic phase) and austenite in the case of the Ti content of 51.3 at%. When the Ti content decreases to 47.3 at% or increases to 53.0 at%, residual stress becomes significantly high since the intrinsic stress is great and the relaxation of stress triggered by the R phase transformation is limited in such contents 1801215 (9 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com TiNi alloys.[123] Therefore, based on this mechanism, many researchers alloyed some other elements such as Nb, Cu, Fe, Zr, and Ag in Ti alloy to manipulate the transformation hysteresis behavior of TiNi alloy,[111,112,124–127] therefore the transformation temperatures, mechanical properties, and fatigue properties in recent decades. Hsieh et al.[125] studied the martensitic transformation in Ti50.5-xNi49.5Zrx and Ti51.5-xNi48.5Zrx alloys (x ¼ 0–25 at%) and found that the transformation peak temperature can rise to 50–450 C depending on the different additions of Zr. Addition of Cu or Fe can slightly increase the range of transformation hysteresis of TiNi alloy.[126,127] By contrast, TiNiNb alloy has a wider transformation hysteresis and therefore better shape memory effect.[128] Meanwhile, the addition of Nb results in formation of more R phase in TiNiNb alloy, which would lead to more superelastic recovery and elastic recovery. The addition of Ag in the TiNi alloy has an antibacterial function and the resultant TiNiAg alloy can also obtain a maximum recovery strain of 6.4% with a total pre-strain of 7%.[111] However, Ni is a basic component in most Ti-based shape-memory alloys, hence the release of Ni ions from the corrosion of implant during service is inevitable. As mentioned above, excessive release of Ni ions may cause an allergic response, leading to severe health problems. Therefore, real applications of Ti-based shape-memory alloys should consider the problem of Ni ions release.[129] To decrease the release of Ni ions from the surface of Ti-based shapememory alloys, surface treatments are commonly used, which is introduced in Section 4. 3. New Preparation Methods of Ti Alloys for Biomedical Applications In the development of Ti alloys, many preparation methods, such as solidification/casting, forging, powder metallurgy, and sintering, have been employed. As these conventional methods have been introduced in detail in many reviews, herein, only some new preparation methods of Ti alloys for biomedical applications are reviewed in this article. 3.1. Selective Laser Melting Biomedical implants often have complex shapes and their sizes are varied according to patients. As a result, the production of biomedical implants using conventional technologies always accompanies with significant time, material, and energy costs. In recent decades, selective laser melting (SLM), as one of additive manufacturing (AM) techniques, has been widely employed to solve the conventional manufacturing problems of Ti-based materials.[130–134] SLM technology is a layer-wise process in accordance with a pre-designed 3D model under a protective atmosphere. Ti alloy parts are produced layer by layer by selectively melting, which consolidates the powder at a high rate by using a computer-controlled laser.[130,135] SLM provides a wide range of advantages compared with the conventional manufacturing methods, such as lower production time, higher material utilization, almost no geometric constrictions, and further post-processing.[136] Due to the fast heating/cooling rate in SLM processing, SLM-produced alloys have distinctive microstructures in comparison with the conventional counterparts.[83,137,138] SLM-produced (α þ β)-type Ti–6Al–4V consists of dominant fine acicular α’ martensite and some prior β grains, while the conventional Ti–6Al–4V alloy (Grade 5) reveals typical equiaxed α þ β microstructure.[136] This distinctive microstructure leads to the comparable or even better mechanical properties of SLM-produced Ti–6Al–4V compared with the conventional counterparts, as summarized in Table 6. SLM investigations with respect to (α þ β)-type Ti alloys have also been employed on Ti–6Al–7Nb, which has a favorable set of mechanical properties, corrosion resistance, and biocompatibility.[139] Similar to Ti–6Al–4V, SLM-produced Ti–6Al–7Nb also has primarily α’ martensitic plates.[140] As there is no powder feedstock β-type Ti alloys available for SLM, little SLM investigation has been conducted on β-type Ti alloys. The current research group has conducted some SLM investigations on β-type Ti–24Nb–4Zr–8Sn.[5,141–143] As shown in Table 6, the tensile properties of Ti–24Nb–4Zr–8Sn samples fabricated by SLM, hot rolling and hot forging are closely comparable. Table 6. Mechanical properties of different types of titanium alloys processed by selective laser melting and conventional methods. H [HV] E [GPa] σ0.2 [MPa] σUTS [MPa] emax [%] ref. Selective laser melting 261 13 106 3 555 757 19.5 [144] Selective laser melting – – 500 650 17 [145] Sheet forming – – 280 345 20 [130] Full annealed – – 432 561 14.7 [39] 409 109 1110 1267 7.28 [146] [1,13] Processing method CP–Ti Ti–6Al–4V Selective laser melting Casting/superplastic forming 346 110 847 976 5.1 220 6 53 1 563 38 665 18 13.8 4.1 [5] Ti–24Nb–4Zr–8Sn Selective laser melting Hot rolling – 46 700 830 15.0 [147] Hot forging – 55 570 755 13.0 [148] H is hardness; E is elastic modulus; σ0.2 is yield strength; σUTS is ultimate tensile strength; and emax is fracture strain. Adv. Eng. Mater. 2019, 21, 1801215 1801215 (10 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com However, because of excessively high oxygen concentration in the powder used, the SLM-produced Ti–24Nb–4Zr–8Sn does not show the advantageous superelastic behavior as the counterparts produced by conventional technologies.[5] Based on the above results, it demonstrates that the SLM-produced Ti alloys can meet the mechanical demand for biomedical applications. Considering the application of biomedical implants in the human body, the current research group have investigated the corrosion behavior of SLM-produced Ti–6Al–4V in 3.5 wt% NaCl solution compared with the commercial Grade 5 alloy.[83] Electrochemical measurements showed that the passive current density of SLM-produced Ti–6Al–4V alloy (0.841 0.0275 mA cm2) is two times higher than that of commercial Grade 5 alloy (0.390 0.0125 mA cm2) and the fitted film resistance of commercial Grade 5 alloy (94 7 kV cm2) is also higher than that of SLM-produced Ti–6Al–4V (40 6 kV cm2). These results indicate that SLM-produced Ti–6Al–4V has unfavorable corrosion resistance than the commercial Grade 5 alloy, attributing to the relatively large amount of acicular α’ martensite and less amount of β-Ti phase in the microstructure of SLM-produced Ti–6Al–4V.[83] Although heat treatment was carried out to eliminate the acicular α’ martensite in SLM-produced Ti–6Al– 4V, the corrosion resistance of heat-treated SLM-produced Ti– 6Al–4V was still not improved.[84] By contrast, SLM-produced Ti– TiB biocomposite showed better corrosion resistance than the conventional counterparts in simulated body fluid.[53] All the differences in corrosion behavior between SLM-produced Ti alloys and conventional ones can be ascribed to their different microstructures which result from the processing characteristics. However, there is still lack of investigation on the corrosion behavior of SLM-produced Ti alloys (and other materials, such as Al alloys and stainless steel[87,137,149–152]), therefore more studies are needed to deeply understand the corrosion behavior of SLM-produced alloys. Although SLM is widely used to investigate the processing and mechanical properties of titanium alloys (especially Ti–6Al–4V alloy), the presence of α’ phase in the microstructure of the SLM-produced Ti–6Al–4V has resulted in undesirable service properties of the alloy. The current researches have attempted to avoid the presence of α’ phase in SLM-produced titanium alloys. 3.2. Electron Beam Melting Electron beam melting (EBM), as another popular metal additive manufacturing technique, is considered to be an innovative industrial production technology to produce biomedical Ti alloys.[86,153,154] Similar to SLM, EBM technology also adopts a layer-wise processing method using a high-energy electron beam as a heat source to fabricate alloy parts on a powder bed based on a computer aided designed (CAD) model. EBM can rapidly prototype metal components with complex shapes using minimal processing steps.[153] In comparison to SLM technology, EBM process is conducted in a vacuum environment and can preheat the powder used, thereby producing alloy parts with higher densities.[155] From an industrial point of view, EBM becomes a great alternative method for manufacturing biomedical Ti alloys. During EBM process, the electron beam moves fast and melts the powder to form a molten pool in a vacuum Adv. Eng. Mater. 2019, 21, 1801215 environment, following with solidification of the melt. Therefore, the built layer underneath is influenced by the solidification of the building layer. Due to the low cooling rate and heat accumulation during EBM process, the overall temperature of a built part is maintained at a high level.[153] Such processing characteristics lead to a distinctive microstructure in the EBMproduced alloys compared with SLM-produced counterparts as well as conventionally produced ones.[86,156] To date, EBM has been widely used in the production of Ti–6Al–4V. It has been reported that both EBM-produced Ti–6Al–4V and wrought Ti–6Al–4V reveal a basket-weave microstructure with columnar prior β grains delineated by α grain boundary, regardless of the fair difference in microstructural fineness.[86] The prior β grains in an EBM-produced Ti–6Al–4V have a strong h001i texture component along the build direction.[157] Table 7 lists the mechanical properties of some EBM-produced Ti alloys. As seen from Table 7, ultimate tensile strength (UTS) and elongation of EBM-produced Ti–6Al– 4V have a small fluctuation. This is because the microstructure of the EBM-produced alloy is influenced by its cooling rate which is associated with the sample size in the processing environment.[158] The EBM-produced Ti–6Al–4V has a slightly higher corrosion potential (–0.28 0.03 V vs. SCE) and a slightly lower corrosion current (0.007 0.001 mA cm2) than those of the wrought Ti–6Al–4V (Ecorr: 0.29 0.02 V vs. SCE; Icorr: 0.012 0.001 mA cm2), indicating its slightly better corrosion resistance in phosphate buffered saline.[86] The reason can be attributed to the higher fraction of β phase and significantly refined lamellar α/β phases in the EBM-produced Ti–6Al–4V compared with the wrought Ti–6Al–4V. In comparison to the SLM-produced Ti–6Al–4V, EBM-produced Ti–6Al–4V exhibits a better corrosion behavior. Such a distinction results from the different phase constituents between the EBM-produced Ti– 6Al–4V and the SLM-produced Ti–6Al–4V.[83,136] It is speculated that the corrosion behavior would be significantly similar in monolithic phase Ti alloys irrespective of the manufacturing methods adopted. 3.3. Preparation Methods of Porous Ti Alloys Although the current Ti alloys can solve most of the existed problems of biomedical applications, their elastic moduli are still higher than those of human bones. In order to minimize the stress shielding effect, new Ti alloys with lower moduli are expected. Besides developing the new low-modulus β-type Ti alloys as mentioned above, porous Ti alloy structures also have lower moduli than their bulky counterparts. Therefore, many researchers dedicated to developing porous Ti alloys in recent years. Porous Ti alloys which possess sufficient macro/micropores for bone cell ingrowth and vascularization have adjusted elastic moduli in a considerably wide range.[113,171,172] Although porous metals with higher porosities may improve the regeneration of bone cells, the porous structures have an opposite influence on the mechanical properties. Therefore, it is significant to balance the porosity of porous metal parts for bone ingrowth and mechanical performance. As such, preparation methods of porous Ti alloys with sufficient strength have been investigated extensively.[128,171,173,174] The previous works show 1801215 (11 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Table 7. The mechanical properties of EBM-manufactured titanium materials. Materials σUTS [MPa] σ0.2 [MPa] emax [%] H [GPa] E [GPa] ref. Ti–6Al–4V 950–990 910–940 14–16 – 120 [159] Ti–6Al–4V 910 10 830 5 – 3.21 0.02 118 5 [160] Ti–6Al–4V 1100–1400 – 12–25 4.3 – [161] Ti–6Al–4V 1150-1200 1100–1150 16–25 3.6–3.9 – [162] Ti–6Al–4V 775 735 2.3 3.69 93 [163] Ti–6Al–4V 994–1029 883–938 11.6–13.6 – – [164] Ti–6Al–4V 930 865 12–17 – – [165] Ti–6Al–4V 944.5–964.5 823.4–851.8 13.2–16.3 3.19–3.27 – [166] Ti–6Al–4V – 832–1049 – – – [157] Ti–6Al–4V 990–1180 900–1100 18–23 – – [167] 336 26 253 13 0.27 0.1 – 166 2 [168] γ–TiAl – 1400 – 4.1 – [169] Ti–24Nb–4Zr–8Sn – – – 2.5 – [170] Ti–48Al–2Nb–0.7Cr–0.3Si σUTS is ultimate tensile strength; σ0.2 is yield strength; emax is maximum plastic strain; H is hardness and E is elastic modulus. that pore size, shape, orientation and distribution of fabricated porous Ti alloys can be controlled by different preparation methods, thereby resulting in the distinction in mechanical properties.[171,175,176] Some popular methods for porous Ti alloys are introduced below. 3.3.1. Selective Laser Melting and Electron Beam Melting Both selective laser melting and electron beam melting technologies are capable of producing Ti alloy parts with complex shapes.[141] By pre-designing 3D models and optimizing parameters, porous Ti alloys scaffolds with almost full density can be produced.[27,130,177,178] Due to the distinction in structures and scanning areas compared with the solid Ti alloy parts, porous Ti alloy parts have a smaller melt pool because of the smaller input energy levels needed for obtaining high density. The current research group compared the microstructure and properties of rhombic dodecahedron Ti–24Nb–4Zr– 8Sn scaffolds with a porosity of 75% prepared by EBM and SLM technology, respectively. The spot size of the laser (40 mm) is much smaller than that of electron beam (200 mm), which results in a smaller melt pool of SLM than that of EBM. As a result, the SLM as-produced surface with 50 mm layer thickness is smoother than that of EBM with 70 mm layer thickness, as shown in Figure 6. Correspondingly, the scaffolds with the same structure exhibit somewhat difference in mechanical properties.[155] The SLM-produced Ti–24Nb–4Zr–8Sn parts exhibit slightly lower elastic modulus (0.95 0.05 GPa) and higher compressive strength (50 MPa) than the EBMproduced Ti–24Nb–4Zr–8Sn (elastic modulus: 1.34 0.04 GPa; compressive strength: 45 MPa). This difference can be diminished by heat treatment. Furthermore, Liu et al.[133] investigated the mechanical behavior of SLM-produced porous Ti alloys with 75% porosity using cubic, topology optimized, or rhombic dodecahedron structures and the results revealed that these three structures had different local stress concentrations Adv. Eng. Mater. 2019, 21, 1801215 and stress distributions during deformation, leading to a distinction in energy absorption, as shown in Figure 7. Such a result demonstrates that the mechanical properties of porous Ti alloys can be controlled by tailoring their architecture. Table 8 summarizes the compressive mechanical properties and phase constituents of EBM- or SLM-produced porous Ti alloys. Superelastic properties are found in the EBM-produced Ti– 24Nb–4Zr–8Sn porous parts with a porosity between 67.9% and 91.2%. In comparison to the Ti–6Al–4V porous parts, the Ti–24Nb–4Zr–8Sn porous parts exhibit a greater fatigue strength due to the superelastic properties and the larger plastic zone near the fatigue crack tip. For the same fatigue strength, the modulus of the Ti–6Al–4V porous parts is two times larger than that of the Ti–6Al–4V porous parts.[177] For these reasons, Ti–24Nb–4Zr–8Sn porous parts are more suitable for biomedical implants. 3.3.2. Spark Plasma Sintering Conventional sintering of Ti and its alloys requires a high temperature (1200–1400 C) in a high vacuum environment for a long time (24 to 48 h), which results in harmful changes in the microstructure and mechanical properties of the sintered products.[188] Recently, spark plasma sintering (SPS), which adopts the application of an external current to assist powder consolidation, have attracted much attentions.[189–192] This technique sinters the powder under strong electric field and stress field and low sintering temperature. It also has the advantages of fast heating/cooling rate and the combination of sintering and heat treatment.[193] Therefore, SPS technology can efficiently save energy to sinter the powder used. Current studies on the preparation of porous Ti alloys by SPS mainly focus on the conditions of low temperature and low pressure that can easily sinter Ti and its alloys in powders,[194,195] because high current discharge produces ionization in the plasma can melt the local oxide film on the particles surface and allow the formation of 1801215 (12 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 6. a) SEM micrographs for the surface morphologies of EBM- and SLM-produced rhombic dodecahedron structural Ti–24Nb–4Zr–8Sn scaffolds and b) melting schematic diagram. (Reproduced with permission from ref. [155]. Copyright (2016), Elsevier). junctions between particles. The porous CP–Ti prepared at 700 C for 8 min under 50 MPa by combining SPS with NaCl dissolution method (NaCl can be easily removed by dissolution in water) showed a yield strength from 27 to 94 MPa and elastic modulus from 6.2 to 36.1 GPa and represented the highly interconnected structure and uniform distribution of pores.[196] The typical microstructures of porous CP–Ti with the porosity of 55% but various pore sizes are represented in Figure 8.[196] Porous Ti–5Mn was also prepared by using SPS technique associated with ammonium hydrogen carbonate and by blowing agent TiH2 under a low-pressure condition at different temperatures for 5 min.[197] The porosity of prepared porous Ti–5Mn reduced from 56% to 21% with the increase in sintering temperature from 950 to 1100 C, while their elastic moduli increased from 35.0 to 51.8 GPa.[197] Moreover, SPS can produce field effect between powder particles and effectively prevent the growth of the grains during sintering and therefore it becomes a nanostructured/ultrafine-grained material forming technology.[198] Hence, SPS provides a novel method to fabricate nanostructured/ultrafine-grained Ti and its alloys for biomedical applications. 3.3.3. Space Holder Method Space holder method is one of the powder metallurgy techniques using space holder to sinter the metal powder, Adv. Eng. Mater. 2019, 21, 1801215 which can control the pores including volume fraction, size, shape, and distribution depending on the amount and characteristics of space holder used.[199] Space holders should be strictly chosen since the left space holder may be detrimental to the human body. In general, NH4HCO3, NaCl, starch, Mg powder, polymethyl methacrylate, and urea are used as space holders, which are bio-inert or biocompatible.[199] Such space holders can be evaporated at a relatively low temperature or can be removed by water.[12] Therefore, once the sintering and the spacer holder removal are finished, the porous metal materials are produced. There are many successful examples of the production of porous CP–Ti and Ti alloys. Venezuela et al.[118] used urea as spacer holder to fabricate porous CP–Ti, which was heat treated at 180 C for 2 h in air to eliminate the spacer particles and then sintered at 1200 C for 1 h. The resultant microstructure showed interconnected pores and small isolated pores and had an average pore size of 480 mm and total porosity of 36%. Porous Ti–6Al–4V was ever produced by Esen et al.[200] using 30–70 vol% Mg powder (660 mm in average diameter) as spacer holder. The porosities of final products ranged from 43% to 64% with an average pore size from 485 to 572 mm, resulting in the elastic modulus and yield strength of final products ranging from 1.4 to 14.7 GPa and from 28 to 150 MPa, respectively. β-type Ti alloys can also be synthesized by this method. Wang et al.[171] prepared porous Ti–10Nb–10Zr (wt%) using NH4HCO3 as spacer holder in the size of 500–800 mm. 1801215 (13 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 7. Stress–strain curves for multiple cyclic load-unloading curves and finite element method results for three structures. (Reproduced with permission from refs. [133,155]. Copyright (2018), Elsevier). After adding 20–60 (wt%) NH4HCO3, the porosities of prepared Ti–10Nb–10Zr ranging from 42 to 74% and the porous Ti–10Nb–10Zr alloy with 59% porosity exhibited the highest elastic modulus and yield strength of 5.6 GPa and 137 MPa, respectively. Porous Ti–24Nb–4Zr–8Sn was fabricated using 30 vol% with polymethyl methacrylate and the products showed the porosities in the range of 35–43% with an average pore size of 244–372 μm.[201] The compressive strength and elastic modulus of prepared porous Ti–24Nb– 4Zr–8Sn were in the range of 21–260 MPa and 1.5–6.6 GPa, respectively. All these results match the mechanical properties of bones exhibited in Table 1–3. Therefore, space holder method is a useful method for the production porous Ti and Ti alloys since this method can produce porous parts with controllable pore size, shape, volume fraction, and distribution using an appropriate space holder material which can be removed at a relatively low temperature without excessive contamination. Although this method to produce porous Ti parts is convenient and has a lot of advantages, the accuracy of pore size is not expected. Furthermore, the pore shape in the produced Ti parts is restrained by the shape of the space holder, which also influences the structure of the produced Ti parts. Therefore, unlike the porous structure produced by additive manufacturing technologies, the porous Ti alloys produced by the space holder method have limited porous structure choice. Adv. Eng. Mater. 2019, 21, 1801215 3.3.4. Loose Sintering of Powder Loose sintering of powder is the simplest method for producing porous Ti and Ti alloys based on the partial sintering of powders, and it is a cost-effective and most widely used powder metallurgical technique.[202] Unless necessary, spacer holders are not needed during sintering. Therefore, the possible contamination is minimized. For this method, the porosity increases with increasing the initial powder size or with decreasing the sintering pressure, while the sintering temperature has little effect on the densification of the produced scaffold.[12] Correspondingly, the elastic modulus and yield strength of the scaffold linearly reduce with increasing their porosity. Oh et al.[202] used this method to prepare porous CP–Ti in a vacuum of 1 103 Pa and the result showed that the porosities are varied according to the applied pressure and particle size. The porous CP–Ti with 30 vol% porosity exhibits an elastic modulus of 25 GPa and compressive yield strength of 61 MPa, which is comparable with cortical bone (Table 1). However, due to partial sintering of the powder used, the porosity of produced scaffold is also associated with the degree of particle interconnectivity and particle size. The pore size and shape are determined by the powder size and shape. Therefore, the porosity of the produced scaffold is limited to 50% and its pores are not in spherical shape.[203] Therefore, the Ti and its alloy scaffolds prepared by this method are brittle with low toughness and prone to crack at a relatively low stress. Other powder metallurgical 1801215 (14 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Table 8. Compressive mechanical properties of additive manufactured Ti alloys.[133,153] Materials Unit shape Method Porosity [%] E [GPa] σmax [MPa] ref. Ti–6Al–4V Dodecahedron SLM 75 1.40 42.8 [179] Ti–6Al–4V Rhombic dodecahedron EBM 75 2 40 [180] Ti–6Al–4V Diamond structure EBM 80 Parallel: 1.6 0.3 Parallel: 29.3 0.8 [20] Perpendicular: 0.9 0.1 Perpendicular: 21.0 0.7 Ti–6Al–4V Diamond crystal lattice EBM 60–87 Parallel: 0.4–6.5 Parallel: 16.3–118.8 [181] Ti–6Al–4V Rhombic dodecahedron structure EBM 62–86 Perpendicular: 0.89–6.34 Perpendicular: 12.4–112.8 [182] Ti–6Al–4V Rhombic dodecahedron structure EBM 62.7–83.8 – Perpendicular: 20–90 [183] Ti–6Al–4V Rhombic dodecahedron structure EBM 58–88 Perpendicular: 0.5–6.5 Perpendicular: 10–100 [180] Ti–6Al–4V Honeycomb-like structure EBM 66.3 2.1 Parallel: 2.5 0.5 Parallel: 116 10 [184] Ti–6Al–4V Hatched structure EBM 60 Parallel: 12.9 0.9 Parallel: 148.4 3.5 [185] Perpendicular: 3.9 2.1 Perpendicular: 127.1 29.2 Ti–6Al–4V Cubic structure EBM 49.75–70.32 Perpendicular: 0.57–2.92 Perpendicular: 7.28–163.02 Ti–6Al–4V Cubic structure EBM 58–88 Perpendicular: 0.5–15 Ti–6Al–4V Lattice structures EBM 88.8–94.7 Parallel: 0.025–0.079 Parallel: 2.21–6.01 Perpendicular: 0.05–0.21 Perpendicular: 2.28–8.78 Ti–6Al–4V G7 (Magics software) structure EBM 58–88 Perpendicular: 0.5–5 GPa Perpendicular: 8–80 [180] Ti–6Al–4V Solid cellular foam structure EBM 55–82 Perpendicular: 0.75–9.97 – [187] Ti–6Al–4V Hollow cellular foam structure EBM 55–89 Perpendicular: 0.48–9.77 – [187] Ti–6Al–4V Stochastic foam structure EBM 90.08–91.65 Perpendicular: 0.19–0.49 Perpendicular: 3.8–4.5 [182] G7 (Magics software) structure EBM 70 Perpendicular: 0.7 0.1 Perpendicular: 35 2 [38] Ti–24Nb–4Zr–8Sn Perpendicular: 20-200 [159] [180] [186] Ti–24Nb–4Zr–8Sn Rhombic dodecahedron structure EBM 75 Parallel: 1.34 0.04 Parallel: 46 [155] Ti–24Nb–4Zr–8Sn (annealed) Rhombic dodecahedron structure EBM 67.9–91.2 Parallel: 0.475–1.8 Parallel: 11.6–50 [177] EBM is electron beam melting; SLM is selective laser melting; E is elastic modulus and σmax is ultimate compressive strength. techniques such as microwave sintering,[204] combustion synthesis,[205] metal injection molding,[206] capsule-free hotisostatic pressing,[110] solid-state isothermal foaming,[199,207] freeze (reverse freeze) casting,[208] slip casting,[209] and slurry foaming[207] are also reported to produce porous Ti alloys for potential biomedical applications. Although the porous Ti alloys have significantly great potential applications in biomedicine, there are still a few limitations from a clinical point of view.[210] For example, the pores in the porous Ti alloys may not be interconnected, therefore the ingrowth of the bone cells and osseointegration might be hindered.[210] Meanwhile, a pre-shaped implant may not be used in the situation of an abnormal anatomical structure. Hence, the production of the porous Ti alloys should be better assessed and suited for the patient. To date, the research for some methods mentioned above to produce porous Ti alloys is still at a very early stage. Few works have reported the fatigue and corrosion properties of the porous samples.[155,211,212] Besides strength and elastic modulus, these two properties are also vital for the porous Ti alloys to be used as biomedical materials, which needs further and deeper research in the future. 3.4. Severe Plastic Deformation Compared with coarse-grained materials, ultrafine-grained materials (in submicron or nanometer) often have good physical Adv. Eng. Mater. 2019, 21, 1801215 and chemical properties, such as higher hardness, higher strength, longer fatigue life, and better low-temperature superelasticity.[25,213–222] Some ultrafine-grained materials even exhibit good thermostability, high corrosion resistance, high wear resistance, and excellent biocompatibility.[25,214,223–230] Ultrafinegrained materials, especially nano-grained materials, have fewer atoms in each grain compared with the coarse-grained materials and hence possess more surface atoms, leading to high surface energy.[227] Therefore, the bone cells are prone to attach themselves to the surface of such materials, resulting in high osseointegration. Khang et al.[231] compared the influence of nanometer and submicron surface features on vascular and bone cell adhesion on CP–Ti using flat smooth surfaces and found that the adhesion of bone cells was very high on these surfaces. For instance, the cell density on the surface of nanocrystalline CP–Ti (2000 cell/cm3) was much higher than that of conventional CP–Ti (1400 cell/cm3).[1] Similarly, nanocrystalline Ti–6Al–4V also had a higher cell density (1600 cell/ cm3) than conventional Ti–6Al–4V (950 cell/cm3).[1] Such phenomenon was also found in other metal and ceramic nanomaterials for biomedical applications, such as Co–Cr-based alloys, hydroxyapatite, and TiO2.[232,233] As such, the ultrafinegrained materials are expected to be the next generation of biomedical materials. So far, many different severe plastic deformation (SPD) methods have been proposed, including equal channel angular extrusion (ECAP)[220,221,228,234,235] and accumulative roll bonding (ARB),[236–239] high-pressure torsion 1801215 (15 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 8. Typical microstructures of porous CP–Ti with the porosity of 55% but various pore sizes: a) 125 mm, b) 250 mm, c) 400 mm, and d) 800 mm. (Reproduced with permission from ref. [196]. Copyright (2018), Wiley). (HPT),[240] multi-directional forging,[241] twist extrusion,[242] cyclic-extrusion–compression,[243] friction stir processing (FSP).[244] Of these various methods, ECAP, ARB, and FSP have been applied to Ti alloys for biomedical applications. ECAP produces ultrafine-grained materials by introducing high strains and simple shear using a common extrusion machine.[221] An et al.[235] prepared nanostructured CP–Ti with 200–300 nm in size by ECAP and the in vivo (the femurs of New Zealand rabbits) study showed that the viability, proliferation, and adhesion of cells cultured on the ultrafine-grained CP–Ti are superior to that of conventional CP–Ti with the average grain size of 30 mm. Nie et al.[234] also produced nanostructured CP–Ti with the average grain size of 250 nm and examined the material in vitro and in vivo. The nanostructured CP–Ti showed good bioaffinity and 17% increment in protein adsorption and in vivo experiment in the tibia of Beagles dogs presented no fibrous tissue encapsulation in the gap between the autogenous bone and implant made of nanostructured CP–Ti.[234] ARB is an intense plastic straining process, which is conducted by together rolling overlapped strip.[238] Milner et al.[236] successfully prepared CP–Ti with an average grain size of 100 nm by ARB at 450 C. Cojocar et al.[237] conducted cold ARB on α-type Ti–10Zr–5Nb–5Ta alloy and obtained the nanostructured/ultra-fine grained structure. β-type Ti alloy of Ti–25Nb–3Zr–3Mo–2Sn was also successfully prepared by ARB and showed a significant increase in Adv. Eng. Mater. 2019, 21, 1801215 strength.[238] Kent et al.[239] demonstrated that the grains in nanostructured Ti–25Nb–3Zr–3Mo–2Sn is stable during the prolonged annealing treatment at 400 C and have a limited growth at 500 C. It should be pointed out that ECAP and ARB technique significantly improve the strength of Ti alloys while maintaining a low elastic modulus. Unlike the ECAP and ARB, FSP is an attractive solid-state surface modification technology, which produces a surface layer with ultrafine-grained microstructure. All these surface severe plastic deformation methods can be implemented at room temperature to obtain adherent surface layer. These methods have attracted much attention due to their convenience and reliability for a great potential for biomedical applications. Unfortunately, the limitations of severe plastic deformation are also apparent. Because a large external force is needed during deformation, specific equipment is always needed. Therefore, these technologies are generally associated with high energy cost and equipment cost. Furthermore, the size is limited for the sample fabricated by some of these technologies, such as ECAP, ARB, and HPT, which restricts the wider applications of the severe plastic deformation methods. Based on the statements above, the mechanical properties of Ti alloys prepared by the methods mentioned in this review can be simply summarized in Figure 9. The mechanical properties of cortical bones and cancellous bones are represented by a light gray ellipse and a red star. The Ti alloys are located in the 1801215 (16 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 9. Ultimate strength and modulus of bones and Ti alloys. CON means conventional Ti alloys. Ultimate strength includes ultimate tensile strength and ultimate compressive strength. The mechanical property of cancellous bones is represented by a red star (at very left bottom in the figure). NEW indicates the Ti alloys developed by the DV–Xα cluster method or molybdenum equivalency method, SLM is selective laser melted Ti alloys, EBM is electron beam melted Ti alloys, SPD is the Ti alloys prepared by severe plastic deformation, P is the porous Ti alloys produced by powder metallurgy, selective laser melting or electron beam melting. different positions in this map according to their compositions, microstructures and preparing methods. High strength is a basic demand for load-bearing applications of the implants in the human body. Apparently, all bulky Ti alloys have higher strength than the bones irrespective of preparing methods. However, the elastic moduli of bulky Ti alloys are still much higher than the bones. The Ti alloy implants with an appropriate modulus are one of the most important prerequisites of the successful implantation for long-term service. The ultrafine-grained Ti alloys prepared by severe plastic deformation have higher strength and cell bioaffinity than the conventional counterparts, their moduli are still comparable to the conventional counterparts. Therefore, after solving the questions of strength and biocompatibility, further reducing the moduli of Ti alloys is a critical consideration. As seen in Figure 9, only porous Ti alloys have comparable mechanical properties to the bones. Unlike bulky metallic materials, the mechanical properties of porous metallic materials can be adjusted by their porosities as well as the chemical compositions and microstructures. The porous Ti alloys also have the advantages of bone cell ingrowth and vascularization. Therefore, compared to the development of new low-modulus titanium alloys, porous Ti alloys with refined microstructure may be an important future development for biomedical materials. 4. Surface Modification of Ti Alloys for Biomedical Applications Although Ti and its alloys have been widely used for hard tissue replacement and the cardiovascular applications, there are still Adv. Eng. Mater. 2019, 21, 1801215 some problems for their long-term performance.[245] First, Ti alloys are bio-inert. Although they have excellent biocompatibility, the compositions of Ti-based alloys are significantly different from the surrounding bones. Therefore, no fiber capsules are formed around the biomedical implants, which leads to the mechanical osseointegration between the Ti alloy implants and bones but not strong chemical bone bonding.[245] Second, due to the low resistance to plastic shear, work hardening properties and low adhesion of the surface oxide film, the sub-surface of Ti alloys cannot be protected perfectly. Hence, bare Ti alloys are not capable of resisting to the adhesive and abrasive wear triggered by relative movement between implants and bones, resulting in loosening of implants and final failure.[246] Third, a stable passive film can form on the surface of Ti alloys, thereby having high corrosion resistance in Ti alloys. However, owing to the complexity of the human body environment, the surface passive film may be peeled and dissolved by the combined effect of external force and body fluid.[247] As a result, the releasing substance or wear debris may be toxic or cause inflammation and thrombus in the human body.[247] Such problems are often restricted by the surface properties of the implants. Hence, various surface modification methods have been employed to improve the biofunction, wear, and corrosion resistance of the Ti alloy implants. 4.1. Surface Modifications for Biofunction So far, there are several common methods for preparing the bioceramic bioactive coatings, such as plasma spraying,[248,249] electrochemical deposition,[250] sputtering,[251,252] dip coating,[253] sol-gel,[254] bionic growth method,[255] sintering,[256] micro-arc oxidation.[257] The prepared bioceramic coatings include hydroxyapatite,[249,258] fluorapatite,[254,259,260] β-tricalcium phosphate,[251] β-wollastonite,[248] bioactive glasses,[261] and so on. Among these bioceramic coatings, the first three ones have been extensively investigated. Hydroxyapatite (Ca10(PO4)6(OH)2) is the principal constituent of hard tissues (e.g., bone and tooth) in human body and have excellent biocompatibility and bioactivity, which encourages the growth of new bone tissues on the hydroxyapatite-coated implants and inhibits the release of metal ions from entering the surrounding bone tissues.[249,258] Many research demonstrated that Ca10(PO4)6(OH)2 coated Ti and Ti alloy implants prepared by various methods have an earlier osteoid formation than the uncoated Ti and Ti alloy implants.[249,252,254,258,262] However, the main drawbacks of the Ca10(PO4)6(OH)2 coating are the low bond strength and high residual stress.[263] Therefore, the Ca10(PO4)6(OH)2 coating is prone to peel off from the implants during long-term service in the human body. As such, some researchers introduced transition layer (e.g., ZrO2 and TiO2) between substrate and Ca10(PO4)6(OH)2 coating, resulting in an improvement in bonding strength and the resultant stability.[264,265] Furthermore, fluorapatite (Ca5(PO4)3F) is a stable mineral in the natural world and actually it can be obtained from replacing OH by F in Ca10(PO4)6(OH)2.[260] Since the solubility of Ca5(PO4)3F in body fluid is lower than that of Ca10(PO4)6(OH)2, Ca5(PO4)3F is more stable than Ca10(PO4)6(OH)2 in the human body.[259] Many studies demonstrate that the concentration of F- has an important influence on 1801215 (17 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com the performance of the bioceramic coatings. Tredwin et al.[266] compared the bond strength and interaction of Ca10(PO4)6(OH)2, and fluoridated hydroxyapatite (Ca10(PO4)6F2-xOHx) Ca5(PO4)3F with Ti–6Al–4V produced via the sol-gel method and found that the bond strength increased with increasing the increasing F substitution. Wang et al.[267] also prepared Ca10(PO4)6F2-xOHx coatings with various concentrations of F by the sol-gel dip-coating method and pointed out that the Ca10(PO4)6F2-xOHx coatings of Ca10(PO4)6F0.8–1.1(OH)1.2–0.9 have a stronger stimulating effect on cell proliferation and differentiation activity. The solubility of β-tricalcium phosphate (β-Ca3(PO4)2) is much higher than those of hydroxyapatite and fluorapatite, thereby easily degrading in the human body.[268] The degradation of β-Ca3(PO4)2 may participate in the formation of new bone,[269] thereby accelerating the growth of bone tissue. Additionally, research with respect to use wollastonite (Ca3(Si3O9)) and diopside (MgCaSi2O6) coatings to improve the bioactivity of Ti and Ti alloy implants was also reported.[247,248] For TiNi series shape memory alloys, surface treatments such as surface coating,[270,271] heat treatment,[272] and ion implantation[273] are commonly used in order to reduce the release of Ni ions. TiN and TiO2 coatings are deposited on TiNi alloy by physical vapor deposition and the release of Ni ions, respectively, decreases by the factors of 10 and 14 for TiN and TiO2 coatings compared with uncoated counterparts.[270] Crystalline hydroxyapatite coating on TiNi alloy prepared by chemical treatment can facilitate to reduce the Ni ion release.[271] This method also enhances the bioactivity of TiNi implants. Heat treatment in air promotes the formation of TiO2 film on the surface of TiNi alloy, thereby reducing Ni ion release.[272] Wu et al.[272] have found an optimal temperature of 450 C for the surface treatment of TiNi alloy in air. Lowering the heat treatment temperature (300–450 C) can also reduce Ni ion release of TiNi alloy compared with the untreated counterpart, while the output is less than that treated at 450 C. By contrast, annealing at a higher temperature (550–800 C) has a negative effect.[272] Oxygen plasma immersion ion implantation produces a barrier layer on TiNi alloy to reduce the Ni ions leaching with almost no geometry limitation; therefore this method can be adopted to porous sample in a complicated shape.[273] The treated TiNi alloy shows only one third of Ni ions release compared with the untreated counterpart in stimulated blood fluid for 28 days.[273] Antibacterial property is an important aspect for implants. Bacterial infection on the surface of implant results in the bacterial attachment which may lead the implant to be resistant to the host’s immune system. To avoid this situation, antibacterial implants of Ti alloys are developed based on the idea of anti-adhesive or anti-infective.[274] The anti-adhesive method has a shortcoming for intraosseous implants since the anti-adhesive surface of implant prevents the surface from pollution, thereby influencing osseointegration.[275] The antiinfective method enables the implant to kill bacteria on its periphery based on local drug delivery.[275] Therefore, antiinfective method or combined anti-infective method and antiadhesive strategies are more widely used. Mei et al.[276] used anodization and Ag plasma immersion ion implantation to produce TiO2 nanotubes containing Ag particle on CP–Ti for dental application and both in vitro and in vivo experiment demonstrated sustained antibacterial properties. Combining the Adv. Eng. Mater. 2019, 21, 1801215 osteoconductive coating (such as calcium phosphate) with bactericidal agents (such as anti-microbial Ag particle) can enhance osseointegration and prevent infection of orthopaedic implants simultaneously.[277] Besides the in-organic coatings, biofunctionalized surface modification with biopolymer, biomolecules and protein are usually conducted with the aim of osseointegration and antibacterial function. Physiological bone is a composite material that contains an organic matrix phase with inorganic mineral phase. Collagen I is composed of almost 90% of the organic matrix phase which is known to be easily adhesive to microbial.[277] Therefore, collagen-mimetic peptide coatings are synthesized on implants to afford osteoinductive cues while also reduce bacterial adhesion.[278] The engineered collagen mimetic protein has specific extracellular matrix proteins that can rapidly delivery integrin on osteogenic cells. Meanwhile, this mimic also shows a reduction in adhesion of some types of bacterial compared with human collagen I (100–1000 times) and uncoated Ti alloy implant (10–100 times). Other collagen coatings, which release absorbable antibiotics and enhance osteoblast functions, have also been reported.[279,280] Incorporating growth factors, such as bone morphogenetic protein, into biomolecular or biopolymer coatings are also a widely used method to improve the adhesion of osteogenic cells.[281] It is reported that bone morphogenetic protein has been grafted to an anti-infective chitosan layer to produce a coating with both osseointegration and antibacterial functions.[282] Bone morphogenetic protein in the coatings can significantly enhance both Ca deposition and alkaline phosphatase activity compared with coatings without bone morphogenetic protein.[282] A newer method for osseointegration is the recruitment of osteogenic cells by releasing signaling factors, which can induce osteogenic cells to find the osteoinductive coating. Cytokine stromal cellderived factor 1α is one of the most well investigated recruitment cues, which can improve the efficacy of bone morphogenetic protein.[283] Moreover, cytokine stromal cell-derived factor 1α may reduce inflection since it is a chemoattractant for neutrophils.[284] Biopolymer chitosan coatings have been commonly investigated in both antibacterial application and bone tissue engineering since it can combine with osteogenic agents to produce hybrid materials for biomedical applications. It has been reported that the chitosan coating combined with cell-adhesive arginine-glycine-aspartic acid is capable of reducing S. epidermidis and S. aureus (two types of bacterial) adhesion by 85% and 67%, respectively, and increases the bone cells growth.[285] Chitosan has also been used as a drug-carrier coating combined with other antimicrobial agents, such as adsorbed vancomycin, to enhance its antibacterial capability.[286] As stated above, whatever in-organic or organic coatings used, an ideal orthopaedic implant coating is desired to be multifunctional, which both increases the ability of osteointegration and antibacterial. Therefore, combining different technologies to produce such ideal coatings is expected. 4.2. Surface Modifications for Improving Wear Resistance In general, high wear resistance can prevent the loosening of biomedical implants. Although Ti alloy has high specific 1801215 (18 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com strength and high corrosion resistance, their wear resistance is not desirable.[1] Surface modification techniques including physical deposition methods (such as ion implantation,[287] laser cladding,[288,289] physical vapor deposition,[290] and plasma spray coating[291]), thermochemical surface treatments (such as nitriding,[292] carburization,[293] oxygen diffusion hardening,[294,295] and boriding[296]), and surface severe plastic deformation (such as friction stir processing[244,297–299] and surface mechanical attrition treatment[300,301]) have been employed to improve the surface properties of Ti alloys. TiN[290] and diamond-like carbon (DLC)[302] coatings are commonly used in both physical deposition methods and thermochemical surface treatments. For instance, TiN film and its ramifications (e.g., TiON and TiAlN) have high hardness, good wear resistance, and chemical inertness, which can be prepared by both physical deposition methods and thermochemical surface treatments.[287,290,293] Subramanian et al.[290] compared TiN, TiON, and TiAlN as surface coatings on CP–Ti prepared by direct current reactive magnetron sputtering method and indicated all coatings show good cytocompatibility with L-929 mouse fibroblast cell lines. Choudhury et al.[303] investigated the durability of functional DLC coated Ti–6Al–4V for application in artificial hip joints and the results showed a significant wear reduction to the DLC coated Ti–6Al–4V compared with that of non-coated counterparts. Meanwhile, it is reported that DLC films-coated Ti alloys, as well as other materials, do not cause any cytotoxicity and inflammation during long-term implantation.[304,305] Oxygen diffusion hardening (ODH)[294] is another technology which has been investigated since it can drastically improve the abrasive wear of Ti alloys. The hardening effect of this technology results from the adherent surface modification by oxygen diffusion, which does not delaminate or spall like the deposited coatings.[306] Although physical deposition methods and thermochemical surface treatments can improve the wear resistance of Ti alloy implants to some extent, some problems still exist. The physical deposition methods are prone to delaminate between the coating and substrate under the condition of repeated loading. The thermochemical surface treatments are usually conducted at high temperatures, leading to a twist of the substrate. Therefore, surface severe plastic deformation methods are well developed in recent years.[244,297,300,307,308] Surface mechanical attrition treatment is a newly emerging method to achieve a nanostructured surface layer on metallic materials by peening with a large number of high-frequency impacts over a short time.[301] A gradient nanostructured surface layer of CP–Ti can be formed after surface mechanical attrition treatment.[309] The grain size is extremely small in the top-surface of CP–Ti and gradually increases with increasing depth. Zhu et al.[310] successfully prepared a nanostructured surface with the thickness of 50 um on CP–Ti by shot blasting, demonstrating the feasibility of this method. Subsequently, Huang et al.[311] examined the nanomechanical properties of nanostructured CP–Ti treated by surface mechanical attrition treatment and found that surface nanocrystallization leads to a significant increase in hardness and scratch resistance. The surface nanostructured layer also reveals a greatly better friction property than that of the CP–Ti matrix. Wen et al.[312] conducted surface mechanical attrition treatment on Ti–6Al–4V, resulting in a significant increase in Adv. Eng. Mater. 2019, 21, 1801215 strength with acceptable ductility of the treated Ti–6Al–4V compared with untreated counterpart, which indicates a potential biomedical application. The in vivo and in vitro experiments also elucidated the Ti alloy implants with severe plastic deformed surface have good compatibility with cells.[313,314] Friction stir processing is another attractive solidstate surface modification technology and focuses on the localizing thermomechanical processing, which uses a professional fiction stir welding machine with a friction stir processing tool of tungsten steel to severely deform the surface of the alloy by fiction stirring at a relatively high rotation speed.[244,315,316] Wang et al.[244] conducted fiction stir processing on the Ti–35Nb– 2Ta–3Zr and promoted the formation of α” martensite and ω phase on its surface microstructure, resulting in surface hardening. As it is known that, some beneficial elements or compounds (e.g., Zn, Ag, and TiO2) are not contained in the commonly used Ti alloy. Fortunately, using friction stirring processing (FSP) can prepare surface composite layer. As shown in Figure 10a–c, grooves in Ti–6Al–4V filled with TiO2 nanoparticles are processed before FSP. The amount of TiO2 nanoparticles can be controlled by the depth of the grooves. During processing, not only the microstructure is refined but also the TiO2 nanoparticles are uniformly distributed in the surface layer of Ti–6Al–4V. After FSP, the TiO2 nano-powder is mixed within the surface layer with refined microstructure.[316,317] In vitro experiments (Figure 10d, e) show that cell viability and proliferation are substantially improved by FSP at an appropriate parameter.[316,317] Zn participates in the synthesis of various enzymes in the human body, maintaining cell physiological function and promoting bone growth and maintain cell physiological function.[318] Nanostructured Ag particles have wide applications in biomedical fields due to its antimicrobial properties.[319] Lv et al.[307] and Xie et al.[308] prepared functional Ti–6Al–4V /Zn and Ti6–Al–4V/Ag composite layers by this method. Above all, the Ti alloys prepared by these three methods are expected to have the surface roughness in the nanometer range. Because our bone cells consist of inorganic minerals with a grain size of 20–80 nm long and 2– 3 nm in diameter, the specific reaction can take place between the surrounding tissue and the implant made of ultrafinegrained materials, leading to an enhancement of bioaffinity.[1] Therefore, ultrafine-grained Ti and Ti alloys produced by severe plastic deformation exhibit better biocompatibility. Combining with the intrinsic properties of Ti and Ti alloys, they are expected to have a great potential for biomedical applications. 4.3. Surface Modifications for Improving Corrosion Resistance Further improving the corrosion resistance of Ti alloys can enhance their stability during the long-term service in the human body. There are a lot of methods to improve the corrosion resistance of Ti alloys, such as passivation,[320,321] laser surface modification,[288,322,323] sol-gel,[254] ion implantation,[287] physical vapor deposition,[290] electroplating,[324] and micro-arc oxidation.[325] It should be noted that some methods are the same as the ones used for improving the bioactivity and/or wear resistance of Ti alloys. As surface modifications for Ti alloys 1801215 (19 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Figure 10. Friction stir processing system and the Ti–6Al–4V plates: a) the surface of Ti–6Al–4V plate with the grooves, b) grooves with different depths for filling TiO2 nanoparticles, and c) friction stir processing for preparing composite layer on the surface of Ti–6Al–4V, d) the cell viability of Ti–6Al–4V with and without friction stir processing in the cytotoxicity test, and e) cell proliferation results. Black star means p < 0.05 versus Ti–6Al–4V. Red star represents p < 0.05 versus other two friction stir processing groups. (Reproduced with permission from ref. [267]. Copyright (2017), Elsevier). should consider their properties of bioactivity, wear resistance, and corrosion resistance simultaneously due to the complex environment in the human body, improving one of these three properties cannot be conducted at the cost of the other two properties. Passivation and laser surface modification are usually used for enhancing the corrosion resistance of Ti alloys as well as other surface properties. Bloyce et al.[326] improved both corrosion and wear resistance of CP–Ti, Ti–0.05Pd, Ti–6Al–4V using palladium-treated thermal oxidation (one of the passivation methods) in air. Güleryüz et al.[327] investigated the optimum oxidation conditions of Ti–6Al–4V to further evaluate its corrosion-wear performance and found that the treated Ti– 6Al–4V exhibit excellent corrosion resistance after oxidation at 600 C for 60 h and 25 times higher wear resistance compared with the untreated counterpart. Kumar et al.[321] compared two passivation methods of thermal oxidation and anodizing (another passivation method) and indicated the corrosion performance and wear resistance of thermally oxidized CP–Ti, are better than that of the anodized one. Laser surface modification can be performed by various treatments in a wide range because of the high coherence and directionality of laser.[322] Laser surface remelting of CP–Ti and Ti alloys can induce a layer of fine martensite and a film of Ti oxides (such as Adv. Eng. Mater. 2019, 21, 1801215 TiO, TiO2, and Ti2O3), leading to an increase in both corrosion resistance and wear resistance.[322,328] due to the rapid solidification and the formation of oxides. Laser surface alloying technique can generate an alloyed layer of 0.5–1.0 mm thickness by the reactions between the pre-placed powders/gases and the melting substrate.[322] For example, the TiN layer aforementioned can also be prepared by this method in a dilute nitrogen environment, which has excellent high-temperature and corrosion resistance, and a high degree of hardness.[329] Ti–6Al–4V with an alloyed surface layer containing TiB and TiN can be produced by alloying BN powder.[330] The prepared surface layer has a micro-hardness of about 1700 HV associated with good wear and corrosion resistance.[330] Laser cladding is also a commonly used technique that uses a defocused laser beam to melt cladding materials onto the surface of Ti alloys.[322] Cladding materials are melted to form a metallurgical bond with the underlying Ti alloys. As TiN layer is demonstrated to have better corrosion resistance than Ti alloy,[331] Balla et al.[332] prepared TiN reinforced Ti–6Al–4V composite coating by laser cladding and the results showed that the coatings containing 40 wt% TiN exhibit the highest wear resistance and good biocompatibility in vitro. Other materials are also used as cladding materials to improve the corrosion resistance of Ti 1801215 (20 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com alloys.[333] However, considering the biomedical applications of Ti alloys, the selection of cladding materials must be prudent. The passive film (or layers) with high wear resistance prepared by various methods are also expected to have good corrosion resistance.[333–335] Therefore, it can be concluded that suitable surface modification technologies employed to enhance the corrosion resistance of Ti alloys should not be compromising their other advantageous properties. coatings or surface treatment technologies.[341] Therefore, multifunctional coatings prepared by combining different technologies are likely to receive more attentions. As stated above, β-type Ti alloys with multifunctional coatings are expected to be a significant development for biomedical Ti alloys in order to achieve better biomedical applications. 6. Conclusions 5. Biocompatibility of Ti and Ti Alloys To meet the requirements of mechanical properties, corrosion resistance and wear resistance are merely the prerequisites for Ti and Ti alloys to be implant materials. Ideal implants can serve in the human body for a long-term without revision surgery. After being implanted in the human body, implants induce a lot of reactions in the biological environment with body fluid, proteins and cells, determining the ultimate success of implantation. Therefore, biocompatibility tests are mandatory biomedical Ti and Ti alloy implants. As well known, Ti and Ti alloys are inert materials in the human body environment, which can spontaneously form a stable passive film of TiO2 on the surface of the implants. Even if the passive film is damaged, it is rebuilt rapidly. This is an important reason that Ti and Ti alloys possess good biocompatibility. However, although Ti and Ti alloys have these advantages, the concerns on the biocompatibility of Ti and Ti alloys have been widely discussed and studied since the 1970s.[336] For example, due to their inert characteristics, fibrous tissue capsules are prone to form on the surfaces of implants, while foreign body response may take place. For some Ti alloys, several elements are toxic in elemental states, such as V in Ti– 6Al–4V alloy and Ni in TiNi series shape-memory alloys, hence the release of these elements are harmful. Furthermore, some properties like osseointegration and antibacterial function are desired. Considering that specific interactions take place between the surfaces of implants and the surrounded tissues, the surface compositions and surface topography of implants are therefore expected to be vital in contacting bone tissue. Table 9 summarizes some typical results for the biocompatibility of Ti and Ti alloys. It is found that the biocompatibility of Ti and Ti alloys can be mainly improved in two aspects. One is to reduce the toxic elements used. Cell viability and growth rate for β-type Ti alloys with nontoxic elements are much higher than those for Ti–6Al–4V.[337,338] Hence, β-type Ti alloys are developed rapidly in recent years. Up to date, the investigation of β-type Ti alloys is still at a very early stage. Further studies about β-type Ti alloys are urgently required. Another is the surface modification which can improve the capability of osseointegration and antibacterial function for Ti and Ti alloys. Although a large number of studies show a lot of methods to enhance the osseointegration and/or antibacterial function including organic and inorganic coatings, specific coating often has a single function. For instance, Collagen I coated and uncoated CP–Ti have no significant difference in the growth rate of osteoblast-like cells.[339] By contrast, human mesenchymal cells grow faster on the Collagen I coated CP–Ti than those on the uncoated counterpart.[340] These findings indicate the coatings have a distinctive response to different types of cells. Similar results are also found in other Adv. Eng. Mater. 2019, 21, 1801215 This article briefly reviews a number of recent developments of Ti alloys for biomedical applications, including their processing, microstructures, and properties. First, biomedical materials are succinctly introduced in the view of the requirements of artificial implants. Afterward, a detailed comparison among 316L stainless steel, Co–Cr-based alloys, and Ti alloys is carried out in according to their properties for using as implant materials. Due to the excellent combined properties of low elastic modulus, high specific strength, excellent corrosion resistance, complete inertness to body environment and superior biocompatibility, Ti and its alloys are a better choice for manufacturing biomedical implants. As reviewed, α-type and near-α Ti alloys were developed as the first generation of biomedical materials. However, low strength at room temperature and poor wear resistance limit them to be used as implant materials. (α þ β)-type Ti alloys exhibit better mechanical properties and have been used for orthopaedic implants. Unfortunately, the moduli of (α þ β)-type Ti alloys are significantly higher than those of bones and contains harmful elements (e.g. Al and V). Therefore, lower elastic modulus β-type Ti alloys with non-toxic β-stabilizers (e.g. Nb, Zr, Ta, and Sn) and enhanced biocompatibility were developed in the last two decades for biomedical applications. Ti-based shape memory alloys are also frequently used as biomedical materials due to their superelasticity and shape-memory effects. Up to date, some new technologies are emerged to produce Ti alloys for biomedical applications. Additive manufacturing (AM) techniques, especially selective laser melting and electron beam melting, have been frequently applied to produces Ti alloy parts with the combined advantages of low production time, high material utilization, almost no geometric constrictions and further post-processing. Besides the AM techniques, porous powder sintering technologies including spark plasma sintering, space holder method and loose sintering of powder have been widely employed, which produce Ti alloy scaffolds with comparable mechanical properties to the bones in the human body. Severe plastic deformation methods have also been applied to Ti alloys for biomedical applications due to better combined properties of strength, corrosion resistance and bioaffinity to bone cells of ultrafine-grained materials. However, low biological activity to surrounding tissue, poor wear resistance, and inadequate corrosion resistance are still the questions for Ti alloys during long-term service. Various surface modification methods are conducted on Ti alloys to solve these problems. For improving biological activity, the biofunctionalized surface modification including inorganic and organic coatings are commonly used methods. Wear and corrosion resistance of Ti alloys can be improved simultaneously through various ways such as physical deposition methods, thermochemical surface 1801215 (21 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Table 9. Summary of the biocompatibility for Ti and Ti alloys. Materials Processing CP–Ti Titanium-based dental or prosthetic implants Evaluation Comment ref. Lymphocyte migration inhibition All blood samples from patients showed no immune reactions after 3–12 months implantation [342] Memory lymphocyte immune-stimulation assay Patients had various symptoms after implantation; 54 patients (total 56) were negative in patch testing [343] Ti–6Al–4V Wrought Proliferation assay No proliferative response [344] Ti–6Al–4V Hot rolled Dissolution test in Hank’s solution Measurable dissolution was found in a solution containing ethylenediamine tetra-acetate or Na-citrate [345] Ti–15Zr–4Nb– 4Ta Aged Rat tibia implantation for 6–48 weeks Relative growth of cells for Ti–15Zr–4Nb–4Ta was much higher than that for Ti–6Al–4V [337] Ti–29Nb–13Ta– 4.6Zr Wrought Cell viability test Cell viability for Ti–29Nb–13Ta–4.6Zr was much better than that for Ti–6Al–4V [338] Nanocrystalline CP–Ti Equal channel angular extrusion Bonelike apatite immersion; osteoblast cell lines test Good bioaffinity and 17% increment in protein adsorption [234] Nanocrystalline CP–Ti Equal channel angular extrusion Beagles dog tibia implantation No fibrous tissue encapsulation in the gap between the autogenous bone and implant [234] Osteoblast adhesion test Enhanced osteoblast adhesion [232] Nanocrystalline Ti–6Al–4V CP–Ti Laser engineered net shaping Human fetal osteoblasts Cells well spread on the porous sample [346] CP–Ti Fused deposition modeling L929 mouse fibroblast Excellent bone cell attachment and proliferation [347] Ti–6Al–4V Electron beam melting Human fetal osteoblasts Decreased cell proliferation in greatly rough surface [348] Ti–6Al–4V Electron beam melting Human adipose-derived adult stem cells Increased cell proliferation in porous samples [349] Ti–6Al–4V Electron beam melting Rabbit femur and tibia Displaying similar biological short-term behavior with conventional counterpart [350] Ti–6Al–4V scaffold Selective laser melting Human periosteum-derived cell culture Enhanced cell seeding while maintaining nutrient transport [351] Ti–6Al–4V Metal injection molding MC-3T3-E1 pre-osteoblasts culture Cells are proliferated, adhesive and differentiated. [352] CP–Ti Acid processed in HF-HNO3 Osteoblast-like cells (MG63) culture No change in cell proliferation rate. [353] CP–Ti Coarse grit-blasted and acid-etched in HCl-H2SO4 Osteoblast-like cells (MG63) culture Lower enzyme activity in isolated. [353] CP–Ti Radio frequency plasma sprayed Sr-hydroxyapatite Osteogenic cell response test Osteoid formation at 7th week and 12th week for on Srhydroxyapatite coated implants and hydroxyapatite after implantation coated implants [262] CP–Ti Coated with α- or β-tricalcium phosphate Rabbit cranial bones α-tricalcium phosphate coating degraded faster; both [354] coatings were absorbed by osteoclast-like cells Ti–6Al–4V Coated with fluoridated hydroxyapatite Osteoblast-like cell number test Similar presence of cells on the surface with different roughness; fluoridated hydroxyapatite supported osteoblast adhesion and growth [355] Ti–6Al–4V Coated with fluoridated hydroxyapatite Osteoblastic cell response test Similar cell morphologies and good cell viability in all tested samples. Coatings with 0.8–1.1 mol/L Fconcentrations had a stronger stimulating effect on cell proliferation and differentiation activities [267] Porous CP–Ti cylinder Chemical and heat treatment Rabbit medial femoral condyle Improved bioactivity without compromising mechanical properties [356] Porous CP–Ti cylinder Hydroxyapatite coating by sol-gel method Murine macrophage RAW 264.7 test Better osteogenic effect than uncoated sample [357] Porous CP–Ti scaffold Alkali-acid and alkali-heat coatings Canine femur Improved the osseointegration [358] Porous CP–Ti Microarc oxidation and electrochemical Bone marrow stem cell culture Enhanced cell proliferation; improved levels of osteorix, [359] (Continued) Adv. Eng. Mater. 2019, 21, 1801215 1801215 (22 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com Table 9. (Continued) Materials scaffold Processing Evaluation reduction in alkaline solution Comment ref. alkaline phosphates, osteopontin and osteocalcin Microarc oxidation and electrochemical reduction in alkaline solution Canine femur Enhanced bone cell regeneration and increased bone-implant contact ratio [359] Porous Ti–6Al– 4V scaffold Polydopamine-assisted hydroxyapatite coating MC3T3-E1 cells proliferation test Increased cell attachment and proliferation; higher expression of alkaline phosphatase, osteopontin, osteocalcin and collagen [360] Porous Ti–6Al– 4V scaffold Polydopamine-assisted hydroxyapatite coating Rabbit femoral condylar Improved osteointegration and bone regeneration [360] Ti–6Al–4V Friction stir processing surface with TiO2 composite layer Cell viability and proliferation tests Improved cell viability and proliferation [316] Ti–6Al–4V Friction stir processing surface with Ag composite layer Antimicrobial tests Improved antimicrobial properties [308] Tantalum plasma immersion ion implantation Cytotoxicity and cell proliferation test Ni release was inhibited up to 60%; muscle cells and osteoblasts were proliferated [361] Cell incubation Cells shows 1.33 to 2-fold increase; After 28-day implantation, all pores were completely filled with cells [362] Porous CP–Ti scaffold TiNi Porous TiNi CP–Ti Coated with collagen I Mesenchymal cell culture Faster growth rate than uncoated sample [340] CP–Ti Coated with collagen I Osteoblast-like cell culture No significantly differences with uncoated sample [339] CP–Ti Covalently linked with short biomimetic peptides Sprague-Dawley rat osteoblast cell culture Increased osteoblast cell adhesion [363] CP–Ti Immobilized with synthetic peptide Dog mandible Higher growth rate of osteoblast-like MC3T3 cells [364] Coated with collagen I Mesenchymal cell culture Increased cell adhesion, proliferation and differentiation regarding with both the amount and stability of collagen I [365] Coated with biodegradable poly (D, L-lactic acid) integrated with gentamicin and teicoplanin Antibacterial test (Staphylococcus epidermidis) Significantly reduction in bacterial adhesion [366] Ultraviolet light irradiation Osteoblastic cell culture and antibacterial test (Staphylococcus epidermidis and Staphylococcus aureus) Cell adhesion was not altered; bacterial adhesion was reduced [367] Coated with hyaluronic acid/chitosan polyelectrolyte multilayers, coupled with surface-immobilized cell-adhesive arginine-glycine-aspartic acid Osteoblast adhesion and antibacterial test Enhanced beneficial host cell responses; reduced pathogenic microbial adhesion [368] Ti–6Al–4V CP–Ti Ti–6Al–4V CP–Ti treatments and surface severe plastic deformation (e.g. friction stir processing). An adherent or coherent layer with high wear resistance and corrosion resistance are often prepared to enhance the surface properties of Ti alloys. Although Ti and its alloys have been widely used as biomedical materials, deeper investigations are still requested to increase their reliability in the human body during long-term service. Based on the review above, a Ti alloy with non-toxic elements, satisfied mechanical properties, and excellent biocompatibility is more expected. Therefore, porous β-Ti alloys with refined microstructures coated by multifunctional materials may be the next generation of biomedical implants. Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers. The authors are grateful to C.D. Rabadia, S.E. Haghighi, Y.J. Liu, S.F. Jawed, H.B. Lu, L.Q. Wang, G.H. Cao, and T.B. Sercombe for the collaboration. Conflict of Interest The authors declare no conflict of interest. Keywords additive manufacturing, processing, properties, surface modification, titanium alloys Acknowledgements This research was partially supported by the Australian Research Council Discovery Projects (DP110101653, DP130103592), National Natural Science Foundation (51601075), China Postdoctoral Science Foundation (2017M611751), and Jiangsu University of Science and Technology Adv. Eng. Mater. 2019, 21, 1801215 1801215 (23 of 29) Received: November 15, 2018 Revised: February 6, 2019 Published online: March 20, 2019 © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com [1] M. Geetha, A. K. Singh, R. Asokamani, A. K. Gogia, Prog. Mater. Sci. 2009, 54, 397. [2] M. Long, H. J. Rack, Biomaterials 1998, 19, 1621. [3] M. Abdel-Hady Gepreel, M. Niinomi, J. Mech. Behav. Biomed. Mater. 2013, 20, 407. [4] M. Navarro, A. Michiardi, O. Casta~ no, J. A. Planell, J. R. Soc. Interface 2008, 5, 1137. [5] L. C. Zhang, D. Klemm, J. Eckert, Y. L. Hao, T. B. Sercombe, Scr. Mater. 2011, 65, 21. [6] J. E. Cohen, Science 2003, 302, 1172. [7] S. Kurtz, K. Ong, E. Lau, F. Mowat, M. Halpern, J. Bone Joint Surg. 2007, 89, 780. [8] S. M. Kurtz, K. L. Ong, J. Schmier, F. Mowat, K. Saleh, E. Dybvik, J. Kärrholm, G. Garellick, L. I. Havelin, O. Furnes, H. Malchau, E. Lau, J. Bone Joint Surg. 2007, 89, 144. [9] E. Nes, K. Marthinsen, Y. Brechet, Scr. Mater. 2002, 47, 607. [10] A. Biesiekierski, J. Wang, M. A. Gepreel, C. Wen, Acta Biomater. 2012, 8, 1661. [11] D. Kuroda, M. Niinomi, M. Morinaga, Y. Kato, T. Yashiro, Mater. Sci. Eng. A 1998, 243, 244. [12] Y. Li, C. Yang, H. Zhao, S. Qu, X. Li, Y. Li, Materials 2014, 7, 1709. [13] M. Niinomi, Mater. Sci. Eng. A 1998, 243, 231. [14] J. L. Katz, Nature 1980, 283, 106. [15] D. R. Sumner, T. M. Turner, R. Igloria, R. M. Urban, J. O. Galante, J. Biomech. 1998, 31, 909. [16] A. Sargeant, T. Goswami, Mater. Des. 2006, 27, 287. [17] H. L. Freese, Encycl. Mater. Sci. Technol. 2001, 10, 1. [18] M. Viceconti, R. Muccini, M. Bernakiewicz, M. Baleani, L. Cristofolini, J. Biomech. 2000, 33, 1611. [19] A. F. Mavrogenis, R. Dimitriou, J. Parvizi, G. C. Babis, J. Musculoskelet. Neuronal Interact. 2009, 9, 61. [20] X. Wang, S. Xu, S. Zhou, W. Xu, M. Leary, P. Choong, M. Qian, M. Brandt, Y. M. Xie, Biomaterials 2016, 83, 127. [21] M. Niinomi, Sci. Technol. Adv. Mater. 2003, 4, 445. [22] Z. X. Wang, G. Q. Chen, L.-Y. Chen, L. Xu, S. Lu, Metals 2018, 8, 724. [23] W. Khan, M. Kapoor, N. Kumar, Acta Biomater. 2007, 3, 541. [24] W. Khan, T. Marew, N. Kumar, Biomed. Mater. 2006, 1, 235. [25] L. H. Liu, C. Yang, F. Wang, S. G. Qu, X. Q. Li, W. W. Zhang, Y. Y. Li, L. C. Zhang, Mater. Des. 2015, 79, 1. [26] Y. S. Zhang, X. Wang, W. Zhang, W. T. Huo, J. J. Hu, L. C. Zhang, Mater. Sci. Eng. A 2017, 696, 360. [27] S. Zhao, S. J. Li, S. G. Wang, W. T. Hou, Y. Li, L. C. Zhang, Y. L. Hao, R. Yang, R. D. K. K. Misra, L. E. Murr, Acta Mater. 2018, 150, 1. [28] J. Davis, Handbook of Materials for Medical Devices, ASM International, Materials Park, OH 2003. [29] E. Karamian, M. R. K. Motamedi, A. Khandan, P. Soltani, S. Maghsoudi, Prog. Nat. Sci. Mater. Int. 2014, 24, 150. [30] M. Saravanan, A. Devaraju, N. Venkateshwaran, A. Krishnakumari, J. Saarvesh, Mater. Today Proc. 2018, 5, 14392. [31] K. J. Bundy, M. Marek, R. F. Hochman, J. Biomed. Mater. Res. 1983, 17, 467. [32] Y. S. Al Jabbari, J. Adv. Prosthodont. 2014, 6, 138. [33] Y. Okazaki, E. Gotoh, Biomaterials 2005, 26, 11. [34] D. B. McGregor, R. A. Baan, C. Partensky, J. M. Rice, J. D. Wilbourn, Eur. J. Cancer 2000, 36, 307. [35] E. J. Evans, I. T. Thomas, Biomaterials 1986, 7, 25. [36] Q. Chen, G. A. Thouas, Mater. Sci. Eng. R Rep. 2015, 87, 1. [37] P. Majumdar, S. B. Singh, M. Chakraborty, J. Mech. Behav. Biomed. Mater. 2011, 4, 1132. [38] H. Attar, L. Löber, A. Funk, M. Calin, L. C. Zhang, K. G. Prashanth, S. Scudino, Y. S. Zhang, J. Eckert, Mater. Sci. Eng. A 2015, 625, 350. [39] U. Bathini, T. S. Srivatsan, A. Patnaik, T. Quick, J. Mater. Eng. Perform. 2010, 19, 1172. Adv. Eng. Mater. 2019, 21, 1801215 [40] T. F. Ma, X. Zhou, Y. Du, L. Li, L. C. Zhang, Y. S. Zhang, P. X. Zhang, J. Alloys Compd. 2019, 775, 316. [41] L. H. Liu, C. Yang, L. M. Kang, Y. Long, Z. Y. Xiao, P. J. Li, L. C. Zhang, Mater. Sci. Eng. A 2016, 650, 171. [42] H. B. Lu, C. K. Poh, L. C. Zhang, Z. P. Guo, X. B. Yu, H. K. Liu, J. Alloys Compd. 2009, 481, 152. [43] Y. S. Zhang, W. Zhang, W. T. Huo, J. J. Hu, L. C. Zhang, Vacuum 2017, 139, 44. [44] L. H. Liu, C. Yang, L. M. Kang, S. G. Qu, X. Q. Li, W. W. Zhang, W. P. Chen, Y. Y. Li, P. J. Li, L. C. Zhang, Sci. Rep. 2016, 6, 23467. [45] P. Yu, L. C. Zhang, W. Y. Zhang, J. Das, K. B. Kim, J. Eckert, Mater. Sci. Eng. A 2007, 444, 206. [46] L. C. Zhang, J. Xu, E. Ma, Mater. Sci. Eng. A 2006, 434, 280. [47] L. C. Zhang, J. Xu, J. Eckert, J. Appl. Phys. 2006, 100, 033514. [48] L. C. Zhang, Z. Q. Shen, J. Xu, J. Non-Cryst. Solids 2005, 351, 2277. [49] L. C. Zhang, Z. Q. Shen, J. Xu, Mater. Sci. Eng. A 2005, 394, 204. [50] L. C. Zhang, J. Xu, J. Non-Cryst. Solids 2004, 347, 166. [51] L. C. Zhang, Z. Q. Shen, J. Xu, J. Mater. Res. 2003, 18, 2141. [52] L. C. Zhang, J. Xu, E. Ma, J. Mater. Res. 2002, 17, 1743. [53] Y. Chen, J. Zhang, N. Dai, P. Qin, H. Attar, L. C. Zhang, Electrochim. Acta 2017, 232, 89. [54] L. C. Zhang, K. B. Kim, P. Yu, W. Y. Zhang, U. Kunz, J. Eckert, J. Alloys Compd. 2007, 428, 157. [55] M. Calin, L. C. Zhang, J. Eckert, Scr. Mater. 2007, 57, 1101. [56] S. Mitragotri, J. Lahann, Nat. Mater. 2009, 8, 15. [57] R. A. Antunes, M. C. L. De Oliveira, Acta Biomater. 2012, 8, 937. [58] R. Yazdi, H. M. Ghasemi, C. Wang, A. Neville, Corros. Sci. 2017, 128, 23. [59] F. G. Evans, Anat. Rec. 1976, 185, 1. [60] O. Lindahl, A. G. H. Lindgren, Acta Orthop. Scand. 1967, 38, 141. [61] A. Burstein, D. Reilly, M. Martens, J. Bone Joint Surg. 1976, 58, 82. [62] J.-Y. Rho, T. Y. Tsui, G. M. Pharr, Biomaterials 1997, 18, 1325. [63] O. Lindahl, Acta Orthop. Scand. 1976, 47, 11. [64] M. Ding, M. Dalstra, C. C. Danielsen, J. Kabel, I. Hvid, F. Linde, J. Bone Joint Surg. 1997, 79, 995. [65] S. Ehtemam-Haghighi, Y. Liu, G. Cao, L. C. Zhang, Mater. Des. 2016, 97, 279. [66] S. Ehtemam-Haghighi, Y. Liu, G. Cao, L. C. Zhang, Mater. Sci. Eng. C 2016, 60, 503. [67] S. E. Haghighi, H. B. Lu, G. Y. Jian, G. H. Cao, D. Habibi, L. C. Zhang, Mater. Des. 2015, 76, 47. [68] V. E. Yeganeh, P. Li, Mater. Des. 2017, 124, 78. [69] A. T. Sidambe, Materials 2014, 7, 8168. [70] C. N. Elias, D. J. Fernandes, C. R. S. Resende, J. Roestel, Dent. Mater. 2015, 31, e1. [71] Y. Oshida, Bioscience and Bioengineering of Titanium Materials, Elsevier, Oxford 2007. [72] A. Biesiekierski, J. Lin, Y. Li, D. Ping, Y. Yamabe-Mitarai, C. Wen, Acta Biomater. 2016, 32, 336. [73] P. F. Santos, M. Niinomi, H. Liu, K. Cho, M. Nakai, A. Trenggono, S. Champagne, H. Hermawan, T. Narushima, Mater. Des. 2016, 110, 414. [74] K. Ushida, K. Tsuge, T. Akahori, T. Hattori, M. Niinomi, K. Ishikura, M. A. Gepreel, J. Jpn. Inst. Met. 2015, 45, 5. [75] D. Kuroda, H. Kawasaki, S. Hiromoto, T. Hanawa, Mater. Trans. 2005, 46, 1532. [76] Y. Kusano, T. Inamura, H. Kanetaka, S. Miyazaki, H. Hosoda, Mater. Sci. Forum. 2010, 654–656, 2118. [77] R. J. Talling, R. J. Dashwood, M. Jackson, S. Kuramoto, D. Dye, Scr. Mater. 2008, 59, 669. [78] A. Ramarolahy, P. Castany, F. Prima, P. Laheurte, I. Peron, T. Gloriant, J. Mech. Behav. Biomed. Mater. 2012, 9, 83. [79] M. Besse, P. Castany, T. Gloriant, Acta Mater. 2011, 59, 5982. 1801215 (24 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com [80] X. Lei, L. Dong, Z. Zhang, Y. Liu, Y. Hao, R. Yang, L. C. Zhang, Metals 2017, 7, 131. [81] A. R. McAndrew, P. A. Colegrove, C. Bühr, B. C. D. Flipo, A. Vairis, Prog. Mater. Sci. 2018, 92, 225. [82] J. Cohen, Am. J. Surg. 1967, 114, 31. [83] N. Dai, L. C. Zhang, J. Zhang, Q. Chen, M. Wu, Corros. Sci. 2016, 102, 484. [84] N. Dai, J. Zhang, Y. Chen, L. C. Zhang, J. Electrochem. Soc. 2017, 164, C428. [85] M. Geetha, A. K. Singh, A. K. Gogia, R. Asokamani, J. Alloys Compd. 2004, 384, 131. [86] Y. Bai, X. Gai, S. Li, L.-C. Zhang, Y. Liu, Y. Hao, X. Zhang, R. Yang, Y. Gao, Corros. Sci. 2017, 123, 289. [87] N. Dai, L. C. Zhang, J. Zhang, X. Zhang, Q. Ni, Y. Chen, M. Wu, C. Yang, Corros. Sci. 2016, 111, 703. [88] S. Tamilselvi, V. Raman, N. Rajendran, Electrochim. Acta 2006, 52, 839. [89] A. Choubey, R. Balasubramaniam, B. Basu, J. Alloys Compd. 2004, 381, 288. [90] D. Iijima, T. Yoneyama, H. Doi, H. Hamanaka, N. Kurosaki, Biomaterials 2003, 24, 1519. [91] K. Bordji, J. Y. Jouzeau, D. Mainard, E. Payan, P. Netter, K. T. Rie, T. Stucky, M. Hage-Ali, Biomaterials 1996, 17, 929. [92] K. Wang, Mater. Sci. Eng. A 1996, 213, 134. [93] Y. S. Zhang, J. J. Hu, W. Zhang, S. Yu, Z. T. Yu, Y. Q. Zhao, L. C. Zhang, Mater. Charact. 2019, 147, 127. [94] J.-R. Chen, W.-T. Tsai, Electrochim. Acta 2011, 56, 1746. [95] A. Carman, L. C. Zhang, O. M. Ivasishin, D. G. Savvakin, M. V. Matviychuk, E. V. Pereloma, Mater. Sci. Eng. A 2011, 528, 1686. [96] M. Niinomi, M. Nakai, J. Hieda, Acta Biomater. 2012, 8, 3888. [97] Y. Yang, G. P. Li, H. Wang, S. Q. Wu, L. C. Zhang, Y. L. Li, K. Yang, Scr. Mater. 2012, 66, 211. [98] K. Narita, M. Niinomi, M. Nakai, J. Hieda, K. Oribe, J. Mech. Behav. Biomed. Mater. 2012, 9, 207. [99] M. Nakai, M. Niinomi, X. Zhao, X. Zhao, Mater. Lett. 2011, 65, 688. [100] M. Abdel-Hady, K. Hinoshita, M. Morinaga, Scr. Mater. 2006, 55, 477. [101] C. Li, D.-G. Lee, X. Mi, W. Ye, S. Hui, Y. Lee, J. Alloys Compd. 2013, 549, 152. [102] M. Ahmed, D. Wexler, G. Casillas, O. M. Ivasishin, E. V. Pereloma, Acta Mater. 2015, 84, 124. [103] S. Ehtemam-Haghighi, K. G. Prashanth, H. Attar, A. K. Chaubey, G. H. Cao, L. C. Zhang, Mater. Des. 2016, 111, 592. [104] S. Ehtemam-Haghighi, G. Cao, L.-C. Zhang, J. Alloys Compd. 2017, 692, 892. [105] C. D. Rabadia, Y. J. Liu, L. Wang, H. Sun, L. C. Zhang, Mater. Des. 2018, 154, 228. [106] C. D. Rabadia, Y. J. Liu, G. H. Cao, Y. H. Li, C. W. Zhang, T. B. Sercombe, H. Sun, L. C. Zhang, Mater. Sci. Eng. A 2018, 732, 368. [107] C. D. Rabadia, Y. J. Liu, S. F. Jawed, L. Wang, Y. H. Li, X. H. Zhang, T. B. Sercombe, H. Sun, L. C. Zhang, Mater. Des. 2018, 160, 1059. [108] M. Morinaga, N. Yukawa, T. Maya, K. Sone, H. Adachi, in 6th World Conf. Titanium. III, Cannes, France, 1988, 6 [109] C. H. Wang, A. M. Russell, G. H. Cao, Scr. Mater. 2019, 158, 62. [110] A. Bansiddhi, T. D. Sargeant, S. I. Stupp, D. C. Dunand, Acta Biomater. 2008, 4, 773. [111] Y. F. Zheng, B. B. Zhang, B. L. Wang, Y. B. Wang, L. Li, Q. B. Yang, L. S. Cui, Acta Biomater. 2011, 7, 2758. [112] S. Griza, D. H. G. de Souza Sá, W. W. Batista, J. C. G. de Blas, L. C. Pereira, Mater. Des. 2014, 56, 200. [113] C. E. Wen, Y. Yamada, P. D. Hodgson, Mater. Sci. Eng. C 2006, 26, 1439. Adv. Eng. Mater. 2019, 21, 1801215 [114] K. Otsuka, X. Ren, Prog. Mater. Sci. 2005, 50, 511. [115] B. Thierry, Y. Merhi, L. Bilodeau, C. Trepanier, M. Tabrizian, Biomaterials 2002, 23, 2997. [116] M. Es-Souni, H. Fischer-Brandies, M. Es-Souni, J. Orofac. Orthop. Der Kieferorthopädie 2003, 64, 16. [117] S. Rhalmi, M. Odin, M. Assad, M. Tabrizian, C. H. Rivard, L. Yahia, Biomed. Mater. Eng. 1999, 9, 151. [118] A. Venezuela, Mater. Res. 2008, 11, 3. [119] S.-B. Kang, K.-S. Yoon, J.-S. Kim, T.-H. Nam, V. E. Gjunter, Mater. Trans. 2002, 43, 1045. [120] S. A. Shabalovskaya, Biomed. Mater. Eng. 1996, 6, 267. [121] A. Bansiddhi, D. C. Dunand, Intermetallics 2007, 15, 1612. [122] E. De Smet, S. V. N. Jaecques, J. J. Jansen, F. Walboomers, J. Vander Sloten, I. E. Naert, J. Clin. Periodontol. 2007, 34, 618. [123] Y. Fu, H. Du, Mater. Sci. Eng. A 2003, 342, 236. [124] M. Es-Souni, M. Es-Souni, H. Fischer-Brandies, Anal. Bioanal. Chem. 2005, 381, 557. [125] S. F. Hsieh, S. K. Wu, Mater. Charact. 1998, 41, 151. [126] F. J. Gil, J. A. Planell, J. Biomed. Mater. Res. Part A 1999, 48, 682. [127] C. M. Hwang, C. M. Wayman, Scr. Metall. 1983, 17, 1345. [128] L. Wang, C. Wang, L. C. Zhang, L. Chen, W. Lu, D. Zhang, Sci. Rep. 2016, 6, 23905. [129] G. Schuster, R. Reichle, R. R. Bauer, P. M. Schopf, J. Orofac. Orthop. Der Kieferorthopädie 2004, 65, 48. [130] L. C. Zhang, H. Attar, M. Calin, J. Eckert, Mater. Technol. 2016, 31, 66. [131] L. C. Zhang, H. Attar, Adv. Eng. Mater. 2016, 18, 463. [132] H. Attar, M. Bönisch, M. Calin, L. C. Zhang, S. Scudino, J. Eckert, Acta Mater. 2014, 76, 13. [133] Y. J. Liu, S. J. Li, L. C. Zhang, Y. L. Hao, T. B. Sercombe, Scr. Mater. 2018, 153, 99. [134] H. Attar, M. Bönisch, M. Calin, L. C. Zhang, K. Zhuravleva, A. Funk, S. Scudino, C. Yang, J. Eckert, J. Mater. Res. 2014, 29, 1941. [135] Y. J. Liu, Z. Liu, Y. Jiang, G. W. Wang, Y. Yang, L. C. Zhang, J. Alloys Compd. 2018, 735, 1414. [136] L. Thijs, F. Verhaeghe, T. Craeghs, J. Van Humbeeck, J.-P. Kruth, Acta Mater. 2010, 58, 3303. [137] Y. Yang, Y. Chen, J. Zhang, X. Gu, P. Qin, N. Dai, X. Li, J.-P. Kruth, L.-C. Zhang, Mater. Des. 2018, 146, 239. [138] H. Attar, K. G. Prashanth, A. K. Chaubey, M. Calin, L. C. Zhang, S. Scudino, J. Eckert, Mater. Lett. 2015, 142, 38. [139] M. F. L opez, A. Gutierrez, J. A. Jimenez, Electrochim. Acta 2002, 47, 1359. [140] E. Chlebus, B. Kuznicka, T. Kurzynowski, B. Dybała, Mater. Charact. 2011, 62, 488. [141] Y. J. Liu, X. P. Li, L. C. Zhang, T. B. Sercombe, Mater. Sci. Eng. A 2015, 642, 268. [142] L. C. Zhang, T. B. Sercombe, Key Eng. Mater. 2012, 520, 226. [143] P. Qin, Y. Chen, Y.-J. Liu, J. Zhang, L.-Y. Chen, Y. Li, X. Zhang, C. Cao, H. Sun, L.-C. Zhang, ACS Biomater. Sci. Eng. 2019, 5, 1141. [144] H. Attar, M. Calin, L. C. Zhang, S. Scudino, J. Eckert, Mater. Sci. Eng. A 2014, 593, 170. [145] A. Barbas, A.-S. Bonnet, P. Lipinski, R. Pesci, G. Dubois, J. Mech. Behav. Biomed. Mater. 2012, 9, 34. [146] B. Vrancken, L. Thijs, J.-P. Kruth, J. Van Humbeeck, J. Alloys Compd. 2012, 541, 177. [147] S. Q. Zhang, S. J. Li, M. T. Jia, Y. L. Hao, R. Yang, Scr. Mater. 2009, 60, 733. [148] S. J. Li, T. C. Cui, Y. L. Hao, R. Yang, Acta Biomater. 2008, 4, 305. [149] Y. Chen, J. Zhang, X. Gu, N. Dai, P. Qin, L. C. Zhang, J. Alloys Compd. 2018, 747, 648. [150] P. Qin, Y. Liu, T. B. Sercombe, Y. Li, C. Zhang, C. Cao, H. Sun, L.C. Zhang, ACS Biomater. Sci. Eng. 2018, 4, 2633. [151] J. Suryawanshi, T. Baskaran, O. Prakash, S. B. Arya, U. Ramamurty, Mater. 2018, 3, 153. 1801215 (25 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com [152] Y. Lu, S. Guo, Y. Yang, Y. Liu, Y. Zhou, S. Wu, C. Zhao, J. Lin, J. Alloys Compd. 2018, 730, 552. [153] L.-C. Zhang, Y. Liu, S. Li, Y. Hao, Adv. Eng. Mater. 2018, 20, 1700842. [154] W. Yuan, W. Hou, S. Li, Y. Hao, R. Yang, L.-C. Zhang, Y. Zhu, J. Mater. Sci. Technol. 2018, 34, 1127. [155] Y. J. Liu, S. J. Li, H. L. Wang, W. T. Hou, Y. L. Hao, R. Yang, T. B. Sercombe, L. C. Zhang, Acta Mater. 2016, 113, 56. [156] X. Gong, Y. Li, Y. Nie, Z. Huang, F. Liu, L. Huang, L. Jiang, H. Mei, Corros. Sci. 2018. [157] C. De Formanoir, S. Michotte, O. Rigo, L. Germain, S. Godet, Mater. Sci. Eng. A 2016, 652, 105. [158] S. Zhao, S. J. Li, W. T. Hou, Y. L. Hao, R. Yang, R. D. K. Misra, J. Mech. Behav. Biomed. Mater. 2016, 59, 251. [159] J. Parthasarathy, B. Starly, S. Raman, A. Christensen, J. Mech. Behav. Biomed. Mater. 2010, 3, 249. [160] L. Facchini, E. Magalini, P. Robotti, A. Molinari, Rapid Prototyp. J. 2009, 15, 171. [161] S. M. Gaytan, L. E. Murr, F. Medina, E. Martinez, M. I. Lopez, R. B. Wicker, Mater. Technol. 2009, 24, 180. [162] L. E. Murr, E. V. Esquivel, S. A. Quinones, S. M. Gaytan, M. I. Lopez, E. Y. Martinez, F. Medina, D. H. Hernandez, E. Martinez, J. L. Martinez, Mater. Charact. 2009, 60, 96. [163] M. Koike, K. Martinez, L. Guo, G. Chahine, R. Kovacevic, T. Okabe, J. Mater. Process. Technol. 2011, 211, 1400. [164] S. S. Al-Bermani, M. L. Blackmore, W. Zhang, I. Todd, Metall. Mater. Trans. A 2010, 41, 3422. [165] T. Scharowsky, V. Juechter, R. F. Singer, C. Körner, Adv. Eng. Mater. 2015, 17, 1573. [166] X. Tan, Y. Kok, Y. J. Tan, M. Descoins, D. Mangelinck, S. B. Tor, K. F. Leong, C. K. Chua, Acta Mater. 2015, 97, 1. [167] X. Zhao, S. Li, M. Zhang, Y. Liu, T. B. Sercombe, S. Wang, Y. Hao, R. Yang, L. E. Murr, Mater. Des. 2016, 95, 21. [168] G. Baudana, S. Biamino, B. Klöden, A. Kirchner, T. Weißgärber, B. Kieback, M. Pavese, D. Ugues, P. Fino, C. Badini, Intermetallics 2016, 73, 43. [169] L. E. Murr, S. M. Gaytan, A. Ceylan, E. Martinez, J. L. Martinez, D. H. Hernandez, B. I. Machado, D. A. Ramirez, F. Medina, S. Collins, Acta Mater. 2010, 58, 1887. [170] J. Hernandez, S. J. Li, E. Martinez, L. E. Murr, X. M. Pan, K. N. Amato, X. Y. Cheng, F. Yang, C. A. Terrazas, S. M. Gaytan, J. Mater. Sci. Technol. 2013, 29, 1011. [171] X. Wang, Y. Li, J. Xiong, P. D. Hodgson, C. Wen, Acta Biomater. 2009, 5, 3616. [172] C. E. Wen, M. Mabuchi, Y. Yamada, K. Shimojima, Y. Chino, T. Asahina, Scr. Mater. 2001, 45, 1147. [173] X. Zhao, H. Sun, L. Lan, J. Huang, H. Zhang, Y. Wang, Mater. Lett. 2009, 63, 2402. [174] L. Wang, L. Xie, L.-C. Zhang, L. Chen, Z. Ding, Y. Lv, W. Zhang, W. Lu, D. Zhang, Acta Mater. 2018, 143, 214. [175] W. Pompe, H. Worch, M. Epple, W. Friess, M. Gelinsky, P. Greil, U. Hempel, D. Scharnweber, K. Schulte, Mater. Sci. Eng. A 2003, 362, 40. [176] R. Menini, M.-J. Dion, S. K. So, M. Gauthier, L.-P. Lefebvre, J. Electrochem. Soc. 2006, 153, B13. [177] Y. J. Liu, H. L. Wang, S. J. Li, S. G. Wang, W. J. Wang, W. T. Hou, Y. L. Hao, R. Yang, L. C. Zhang, Acta Mater. 2017, 126, 58. [178] Y. Liu, S. Li, W. Hou, S. Wang, Y. Hao, R. Yang, T. B. Sercombe, L. C. Zhang, J. Mater. Sci. Technol. 2016, 32, 505. [179] S. A. Yavari, R. Wauthle, J. van der Stok, A. C. Riemslag, M. Janssen, M. Mulier, J.-P. Kruth, J. Schrooten, H. Weinans, A. A. Zadpoor, Mater. Sci. Eng. C 2013, 33, 4849. [180] S. J. Li, Q. S. Xu, Z. Wang, W. T. Hou, Y. L. Hao, R. Yang, L. E. Murr, Acta Biomater. 2014, 10, 4537. [181] P. Heinl, C. Körner, R. F. Singer, Adv. Eng. Mater. 2008, 10, 882. Adv. Eng. Mater. 2019, 21, 1801215 [182] X. Y. Cheng, S. J. Li, L. E. Murr, Z. B. Zhang, Y. L. Hao, R. Yang, F. Medina, R. B. Wicker, J. Mech. Behav. Biomed. Mater. 2012, 16, 153. [183] S. J. Li, L. E. Murr, X. Y. Cheng, Z. B. Zhang, Y. L. Hao, R. Yang, F. Medina, R. B. Wicker, Acta Mater. 2012, 60, 793. [184] X. Li, C. Wang, W. Zhang, Y. Li, Mater. Lett. 2009, 63, 403. [185] P. Heinl, L. Müller, C. Körner, R. F. Singer, F. A. Müller, Acta Biomater. 2008, 4, 1536. [186] O. Cansizoglu, O. Harrysson, D. Cormier, H. West, T. Mahale, Mater. Sci. Eng. A 2008, 492, 468. [187] L. E. Murr, S. M. Gaytan, F. Medina, E. Martinez, J. L. Martinez, D. H. Hernandez, B. I. Machado, D. A. Ramirez, R. B. Wicker, Mater. Sci. Eng. A 2010, 527, 1861. [188] K. Asaoka, N. Kuwayama, O. Okuno, I. Miura, J. Biomed. Mater. Res. 1985, 19, 699. [189] C. Yang, L. M. Kang, X. X. Li, W. W. Zhang, D. T. Zhang, Z. Q. Fu, Y. Y. Li, L. C. Zhang, E. J. Lavernia, Acta Mater. 2017, 132, 491. [190] C. Yang, D. G. Mo, H. Z. Lu, X. Q. Li, W. W. Zhang, Z. Q. Fu, L. C. Zhang, E. J. Lavernia, Scr. Mater. 2017, 134, 91. [191] C. Yang, M. D. Zhu, X. Luo, L. H. Liu, W. W. Zhang, Y. Long, Z. Y. Xiao, Z. Q. Fu, L. C. Zhang, E. J. Lavernia, Scr. Mater. 2017, 139, 96. [192] C. Yang, H. Guo, D. Mo, S. Qu, X. Li, W. Zhang, L. Zhang, Materials 2014, 7, 7105. [193] Z. A. Munir, U. Anselmi-Tamburini, M. Ohyanagi, J. Mater. Sci. 2006, 41, 763. [194] R. Nicula, F. Lüthen, M. Stir, B. Nebe, E. Burkel, Biomol. Eng. 2007, 24, 564. [195] Y. Sakamoto, K. Asaoka, M. Kon, T. Matsubara, K. Yoshida, Biomed. Mater. Eng. 2006, 16, 83. [196] F. Zhang, E. Otterstein, E. Burkel, Adv. Eng. Mater. 2010, 12, 863. [197] A. Ibrahim, F. Zhang, E. Otterstein, E. Burkel, Mater. Des. 2011, 32, 146. [198] Y. Y. Li, L. M. Zou, C. Yang, Y. H. Li, L. J. Li, Mater. Sci. Eng. A 2013, 560, 857. [199] Z. Esen, SS . Bor, Scr. Mater. 2007, 56, 341. [200] Z. Esen, SS . Bor, Mater. Sci. Eng. A 2011, 528, 3200. [201] X. H. Wang, S. J. Li, M. T. Jia, Y. L. Hao, R. Yang, Z. X. Guo, Chin. J. Nonferr. Met 2010, 20, s967. [202] I.-H. Oh, N. Nomura, N. Masahashi, S. Hanada, Scr. Mater. 2003, 49, 1197. [203] G. Ryan, A. Pandit, D. P. Apatsidis, Biomaterials 2006, 27, 2651. [204] S. Das, A. K. Mukhopadhyay, S. Datta, D. Basu, Bull. Mater. Sci. 2009, 32, 1. [205] M. Kobashi, D. Ichioka, N. Kanetake, Materials 2010, 3, 3939. [206] R. M. German, Materials 2013, 6, 3641. [207] D. C. Dunand, Adv. Eng. Mater. 2004, 6, 369. [208] U. G. K. Wegst, M. Schecter, A. E. Donius, P. M. Hunger, Philos. Trans. R. Soc. London A Math. Phys. Eng. Sci. 2010, 368, 2099. [209] B. Neirinck, T. Mattheys, A. Braem, J. Fransaer, O. Van der Biest, J. Vleugels, Adv. Eng. Mater. 2009, 11, 633. [210] Z. Wang, C. Wang, C. Li, Y. Qin, L. Zhong, B. Chen, Z. Li, H. Liu, F. Chang, J. Wang, J. Alloys Compd. 2017, 717, 271. [211] K. H. W. Seah, R. Thampuran, S. H. Teoh, Corros. Sci. 1998, 40, 547. [212] X. Chen, Q. Fu, Y. Jin, M. Li, R. Yang, X. Cui, M. Gong, Mater. Sci. Eng. C 2017, 70, 1071. [213] B. Yu, F. Qi, Y. Chen, X. Wang, B. Zheng, W. Zhang, Y. Li, L.C. Zhang, ACS Appl. Mater. Interfaces 2017, 9, 30703. [214] L. Y. Chen, P. Shen, L. Zhang, S. Lu, L. Chai, Z. Yang, L. C. Zhang, Corros. Sci. 2018, 136, 221. [215] L. H. Liu, C. Yang, Z. Y. Liu, L. C. Zhang, W. W. Zhang, X. S. Huang, L. J. He, P. J. Li, Mater. Charact. 2017, 124, 260. [216] L.-Y. Chen, T. Xu, S. Lu, Z.-X. Wang, S. Chen, L.-C. Zhang, Surf. Coat. Technol. 2018, 350, 436. 1801215 (26 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com [217] L. C. Zhang, H. B. Lu, M. Carlin, E. V. Pereloma, J. Eckert, J. Phys. Conf. Ser. 2010, 240, 12103. [218] Y. S. Zhang, H. Z. Niu, L. C. Zhang, X. F. Bai, X. M. Zhang, P. X. Zhang, Mater. Lett. 2014, 123, 261. [219] L. C. Zhang, M. Calin, F. Paturaud, J. Eckert, Mater. Sci. Eng. A 2010, 527, 5796. [220] L. Wang, X. Wang, L.-C. Zhang, W. Lu, Mater. Sci. Eng. A 2015, 645, 99. [221] R. Z. Valiev, T. G. Langdon, Prog. Mater. Sci. 2006, 51, 881. [222] L. H. Su, C. Lu, G. Y. Deng, K. Tieu, L. C. Zhang, P. Guagliardo, S. N. Samarin, J. F. Williams, Sci. Adv. Mater. 2014, 6, 1338. [223] W. Zhang, W. T. Huo, J. W. Lu, J. J. Hu, L. C. Zhang, Y. S. Zhang, JOM 2018, 70, 2596. [224] P. Chen, W. B. Liao, L. H. Liu, F. Luo, X. Y. Wu, P. J. Li, C. Yang, M. Yan, Y. Liu, L. C. Zhang, Z. Y. Liu, Sci. Rep. 2018, 8, 801. [225] L. Chen, J. Li, Y. Zhang, L. C. Zhang, W. Lu, L. Wang, L. Zhang, D. Zhang, Corros. Sci. 2015, 100, 332. [226] L. C. Zhang, M. Calin, M. Branzei, L. Schultz, J. Eckert, J. Mater. Res. 2007, 22, 1145. [227] V. K. Truong, R. Lapovok, Y. S. Estrin, S. Rundell, J. Y. Wang, C. J. Fluke, R. J. Crawford, E. P. Ivanova, Biomaterials 2010, 31, 3674. [228] L. C. Zhang, J. Das, H. B. Lu, C. Duhamel, M. Calin, J. Eckert, Scr. Mater. 2007, 57, 101. [229] L. C. Zhang, H. B. Lu, C. Mickel, J. Eckert, Appl. Phys. Lett. 2007, 91, 051906. [230] L. H. Su, C. Lu, L. Z. He, L. C. Zhang, P. Guagliardo, A. K. Tieu, S. N. Samarin, J. F. Williams, H. J. Li, Acta Mater. 2012, 60, 4218. [231] D. Khang, J. Lu, C. Yao, K. M. Haberstroh, T. J. Webster, Biomaterials 2008, 29, 970. [232] T. J. Webster, J. U. Ejiofor, Biomaterials 2004, 25, 4731. [233] T. Webster, Biomaterials 2000, 21, 1803. [234] F. L. Nie, Y. F. Zheng, S. C. Wei, D. S. Wang, Z. T. Yu, G. K. Salimgareeva, A. V. Polyakov, R. Z. Valiev, J. Biomed. Mater. Res. Part A 2013, 101, 1694. [235] B. An, Z. Li, X. Diao, H. Xin, Q. Zhang, X. Jia, Y. Wu, K. Li, Y. Guo, Mater. Sci. Eng. C 2016, 67, 34. [236] J. L. Milner, F. Abu-Farha, C. Bunget, T. Kurfess, V. H. Hammond, Mater. Sci. Eng. A 2013, 561, 109. [237] V.-D. Cojocaru, D. Raducanu, D. M. Gordin, I. Cinca, J. Alloys Compd. 2013, 546, 260. [238] D. Kent, G. Wang, Z. Yu, X. Ma, M. Dargusch, J. Mech. Behav. Biomed. Mater. 2011, 4, 405. [239] D. Kent, W. L. Xiao, G. Wang, Z. Yu, M. S. Dargusch, Mater. Sci. Eng. A 2012, 556, 582. [240] A. P. Zhilyaev, G. V. Nurislamova, B. K. Kim, M. D. Bar o, J. A. Szpunar, T. G. Langdon, Acta Mater. 2003, 51, 753. [241] A. Danno, C. C. Wong, S. Tong, A. Jarfors, K. Nishino, T. Furuta, Mater. Des. 2010, 31, S61. [242] S. A. A. A. Mousavi, A. R. Shahab, M. Mastoori, Mater. Des. 2008, 29, 1316. [243] W. Guo, Q. Wang, B. Ye, X. Li, X. Liu, H. Zhou, Mater. Sci. Eng. A 2012, 556, 267. [244] L. Wang, L. Xie, Y. Lv, L.-C. Zhang, L. Chen, Q. Meng, J. Qu, D. Zhang, W. Lu, Acta Mater. 2017, 131, 499. [245] A. Fukuda, M. Takemoto, T. Saito, S. Fujibayashi, M. Neo, S. Yamaguchi, T. Kizuki, T. Matsushita, M. Niinomi, T. Kokubo, Acta Biomater. 2011, 7, 1379. [246] A. Buford, T. Goswami, Mater. Des. 2004, 25, 385. [247] X. Liu, P. K. Chu, C. Ding, Mater. Sci. Eng. R Rep. 2004, 47, 49. [248] X. Liu, C. Ding, Z. Wang, Biomaterials 2001, 22, 2007. [249] L. Sun, C. C. Berndt, K. A. Gross, A. Kucuk, J. Biomed. Mater. Res. 2002, 58, 570. [250] L. Besra, M. Liu, Prog. Mater. Sci. 2007, 52, 1. [251] Y. Yang, K.-H. Kim, J. L. Ong, Biomaterials 2005, 26, 327. Adv. Eng. Mater. 2019, 21, 1801215 [252] A. R. Boyd, L. Rutledge, L. D. Randolph, B. J. Meenan, Mater. Sci. Eng. C 2015, 46, 290. [253] S. R. Paital, N. B. Dahotre, Mater. Sci. Eng. R Rep. 2009, 66, 1. [254] K. Batebi, B. A. Khazaei, A. Afshar, Surf. Coat. Technol. 2018, 352, 522. [255] J. Ji, X. Ding, M. Xiong, Comput. Struct. 2014, 135, 88. [256] C. S. Kim, P. Ducheyne, Biomaterials 1991, 12, 461. [257] W. Liu, B. Zhou, Q. Li, M. Yao, Corros. Sci. 2005, 47, 1855. [258] A. Kumar, K. Biswas, B. Basu, J. Biomed. Mater. Res. Part A 2015, 103, 791. [259] K. A. Bhadang, K. A. Gross, Biomaterials 2004, 25, 4935. [260] M. Wei, J. H. Evans, T. Bostrom, L. Grøndahl, J. Mater. Sci. Mater. Med. 2003, 14, 311. [261] G. Kaur, O. P. Pandey, K. Singh, D. Homa, B. Scott, G. Pickrell, J. Biomed. Mater. Res. Part A 2014, 102, 254. [262] S. Vahabzadeh, M. Roy, A. Bandyopadhyay, S. Bose, Acta Biomater. 2015, 17, 47. [263] Y.-C. Yang, E. Chang, Surf. Coat. Technol. 2005, 190, 122. [264] Y. Huang, Y. Yan, X. Pang, Ceram. Int. 2013, 39, 245. [265] H.-W. Kim, Y.-H. Koh, L.-H. Li, S. Lee, H.-E. Kim, Biomaterials 2004, 25, 2533. [266] C. J. Tredwin, G. Georgiou, H.-W. Kim, J. C. Knowles, Dent. Mater. 2013, 29, 521. [267] Y. Wang, S. Zhang, X. Zeng, L. L. Ma, W. Weng, W. Yan, M. Qian, Acta Biomater. 2007, 3, 191. [268] R. Fujita, A. Yokoyama, T. Kawasaki, T. Kohgo, J. Oral Maxillofac. Surg. 2003, 61, 1045. [269] J. Yuan, L. Cui, W. J. Zhang, W. Liu, Y. Cao, Biomaterials 2007, 28, 1005. [270] L.-P. Lefebvre, J. Banhart, D. C. Dunand, Adv. Eng. Mater. 2008, 10, 775. [271] H. C. Jiang, L. J. Rong, Surf. Coat. Technol. 2006, 201, 1017. [272] S. Wu, X. Liu, Y. L. Chan, J. P. Y. Ho, C. Y. Chung, P. K. Chu, C. L. Chu, K. W. K. Yeung, W. W. Lu, K. M. C. Cheung, J. Biomed. Mater. Res. Part A 2007, 81, 948. [273] J. P. Y. Ho, S. L. Wu, R. W. Y. Poon, C. Y. Chung, S. C. Tjong, P. K. Chu, K. W. K. Yeung, W. W. Lu, K. M. C. Cheung, K. D. K. Luk, Surf. Coat. Technol. 2007, 201, 4893. [274] J. Gallo, M. Holinka, C. Moucha, Int. J. Mol. Sci. 2014, 15, 13849. [275] K. Pałka, R. Pokrowiecki, Adv. Eng. Mater. 2018, 20, 1700648. [276] S. Mei, H. Wang, W. Wang, L. Tong, H. Pan, C. Ruan, Q. Ma, M. Liu, H. Yang, L. Zhang, Y. Cheng, Y. Zhang, L. Zhao, P. K. Chu, Biomaterials 2014, 35, 4255. [277] J. Raphel, M. Holodniy, S. B. Goodman, S. C. Heilshorn, Biomaterials 2016, 84, 301. [278] J. K. Bronk, B. H. Russell, J. J. Rivera, R. Pasqualini, W. Arap, M. Höök, E. M. Barbu, Acta Biomater. 2014, 10, 3354. [279] F. Zhang, Z. Zhang, X. Zhu, E.-T. Kang, K.-G. Neoh, Biomaterials 2008, 29, 4751. [280] J. Tu, M. Yu, Y. Lu, K. Cheng, W. Weng, J. Lin, H. Wang, P. Du, G. Han, J. Mater. Sci. Mater. Med. 2012, 23, 2413. [281] P. C. Bessa, M. Casal, R. L. Reis, J. Tissue Eng. Regen. Med. 2008, 2, 81. [282] Z. Shi, K. G. Neoh, E. T. Kang, C. K. Poh, W. Wang, Biomacromolecules 2009, 10, 1603. [283] S. Zwingenberger, Z. Yao, A. Jacobi, C. Vater, R. D. Valladares, C. Li, C. Nich, A. J. Rao, J. E. Christman, J. K. Antonios, E. Gibon, A. Schambach, T. Maetzig, S. B. Goodman, M. Stiehler, Tissue Eng. Part A 2013, 20, 131112094536009. [284] H. Nagase, M. Miyamasu, M. Yamaguchi, M. Imanishi, N. Tsuno, K. Matsushima, K. Yamamoto, Y. Morita, K. Hirai, J. Leukoc Biology 2002, 71, 711. [285] Z. Shi, K. G. Neoh, E. T. Kang, C. Poh, W. Wang, J. Biomed. Mater. Res. -Part A 2008, 86, 865. 1801215 (27 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com [286] F. Ordikhani, E. Tamjid, A. Simchi, Mater. Sci. Eng. C 2014, 41, 240. [287] G. Jin, H. Qin, H. Cao, S. Qian, Y. Zhao, X. Peng, X. Zhang, X. Liu, P. K. Chu, Biomaterials 2014, 35, 7699. [288] F. Weng, C. Chen, H. Yu, Mater. Des. 2014, 58, 412. [289] L. Chai, K. Chen, Y. Zhi, K. L. Murty, L. Y. Chen, Z. Yang, J. Alloys Compd. 2018, 748, 163. [290] B. Subramanian, C. V. Muraleedharan, R. Ananthakumar, M. Jayachandran, Surf. Coat. Technol. 2011, 205, 5014. [291] F. Yildiz, A. F. Yetim, A. Alsaran, I. Efeoglu, Wear 2009, 267, 695. [292] X. Zhou, T. F. Ma, L. C. Zhang, Y. S. Zhang, P. X. Zhang, Mater. Charact. 2018, 142, 270. [293] E. Marin, R. Offoiach, M. Regis, S. Fusi, A. Lanzutti, L. Fedrizzi, Mater. Des. 2016, 89, 314. [294] H. Hornberger, C. Randow, C. Fleck, Mater. Sci. Eng. A 2015, 630, 51. [295] Y. S. Zhang, J. J. Hu, Y. Q. Zhao, X. F. Bai, W. T. Huo, W. Zhang, L. C. Zhang, Vacuum 2018, 149, 140. [296] V. Ataibis, S. Taktak, Surf. Coat. Technol. 2015, 279, 65. [297] L. Wang, J. Qu, L. Chen, Q. Meng, L. C. Zhang, J. Qin, D. Zhang, W. Lu, Metall. Mater. Trans. 2015, 46, 4813. [298] Y. Lv, Y. Ding, Y. Han, L.-C. Zhang, L. Wang, W. Lu, Mater. Sci. Eng. A 2017, 685, 439. [299] J. Chen, F. Ma, P. Liu, X. Liu, W. Li, X. Chen, K. Zhang, L.-C. Zhang, Vacuum 2017, 146, 164. [300] M. Wen, G. Liu, J. Gu, W. Guan, J. Lu, Appl. Surf. Sci. 2009, 255, 6097. [301] T. Roland, D. Retraint, K. Lu, J. Lu, Scr. Mater. 2006, 54, 1949. [302] A. Erdemir, C. Donnet, J. Phys. D. Appl. Phys. 2006, 39, R311. [303] D. Choudhury, J. M. Lackner, L. Major, T. Morita, Y. Sawae, A. Bin Mamat, I. Stavness, C. K. Roy, I. Krupka, J. Mech. Behav. Biomed. Mater. 2016, 59, 586. [304] R. K. Roy, K. Lee, J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 72. [305] M. Mohanty, T. V. Anilkumar, P. V. Mohanan, C. V. Muraleedharan, G. S. Bhuvaneshwar, F. Derangere, Y. Sampeur, R. Suryanarayanan, Biomol. Eng. 2002, 19, 125. [306] R. M. Streicher, H. Weber, R. Schön, M. Semlitsch, Biomaterials 1991, 12, 125. [307] Y. Lv, Z. Ding, J. Xue, G. Sha, E. Lu, L. Wang, W. Lu, C. Su, L.-C. Zhang, Scr. Mater. 2018, 157, 142. [308] L. Xie, L. Wang, K. Wang, G. Yin, Y. Fu, D. Zhang, W. Lu, L. Hua, L.-C. Zhang, Materialia 2018, 3, 139. [309] M. Wen, J.-F. Gu, G. Liu, Z.-B. Wang, J. Lu, Appl. Surf. Sci. 2008, 254, 2905. [310] K. Y. Zhu, A. Vassel, F. Brisset, K. Lu, J. Lu, Acta Mater. 2004, 52, 4101. [311] L. Huang, J. Lu, M. Troyon, Surf. Coat. Technol. 2006, 201, 208. [312] M. Wen, G. Liu, J. Gu, W. Guan, J. Lu, Surf. Coat. Technol. 2008, 202, 4728. [313] M. Piattelli, A. Scarano, M. Paolantonio, G. Iezzi, G. Petrone, A. Piattelli, J. Oral Implantol. 2002, 28, 2. [314] X. Liu, P. K. Chu, C. Ding, Mater. Sci. Eng. R Rep. 2010, 70, 275. [315] R. Ran, Y. Liu, L. Wang, E. Lu, L. Xie, W. Lu, K. Wang, L.-C. Zhang, Metall. Mater. Trans. A 2018, 49, 1986. [316] C. Zhang, Z. Ding, L. Xie, L.-C. Zhang, L. Wu, Y. Fu, L. Wang, W. Lu, Appl. Surf. Sci. 2017, 423, 331. [317] Z. Ding, C. Zhang, L. Xie, L.-C. Zhang, L. Wang, W. Lu, Metall. Mater. Trans. A 2016, 47, 5675. [318] Y. Yu, G. Jin, Y. Xue, D. Wang, X. Liu, J. Sun, Acta Biomater. 2017, 49, 590. [319] M. Cheng, Y. Qiao, Q. Wang, G. Jin, H. Qin, Y. Zhao, X. Peng, X. Zhang, X. Liu, ACS Appl. Mater. Interfaces 2015, 7, 13053. [320] A. Bagno, C. Di Bello, J. Mater. Sci. Mater. Med. 2004, 15, 935. Adv. Eng. Mater. 2019, 21, 1801215 [321] S. Kumar, T. S. N. S. Narayanan, S. G. S. Raman, S. K. Seshadri, Mater. Sci. Eng. C 2010, 30, 921. [322] Y. S. Tian, C. Z. Chen, S. T. Li, Q. H. Huo, Appl. Surf. Sci. 2005, 242, 177. [323] K. Chen, L. Zeng, Z. Li, L. Chai, Y. Wang, L.-Y. Chen, H. Yu, J. Alloys Compd. 2019, 784, 1106. [324] S.-H. Chen, W.-L. Tsai, P.-C. Chen, A. Fang, W.-C. Say, J. Electrochem. Soc. 2016, 163, D305. [325] L.-H. Li, Y.-M. Kong, H.-W. Kim, Y.-W. Kim, H.-E. Kim, S.-J. Heo, J.-Y. Koak, Biomaterials 2004, 25, 2867. [326] A. Bloyce, P.-Y. Qi, H. Dong, T. Bell, Surf. Coat. Technol. 1998, 107, 125. [327] H. Güleryüz, H. Cimeno S glu, Biomaterials 2004, 25, 3325. [328] D. Gu, Y.-C. Hagedorn, W. Meiners, G. Meng, R. J. S. Batista, K. Wissenbach, R. Poprawe, Acta Mater. 2012, 60, 3849. [329] M. S. Selamat, T. N. Baker, L. M. Watson, J. Mater. Process. Technol. 2001, 113, 509. [330] J. S. Selvan, K. Subramanian, A. K. Nath, H. Kumar, C. Ramachandra, S. P. Ravindranathan, Mater. Sci. Eng. A 1999, 260, 178. [331] A. Biswas, L. Li, U. K. Chatterjee, I. Manna, S. K. Pabi, J. D. Majumdar, Scr. Mater. 2008, 59, 239. [332] V. K. Balla, A. Bhat, S. Bose, A. Bandyopadhyay, J. Mech. Behav. Biomed. Mater. 2012, 6, 9. [333] C. Blanco-Pinzon, Z. Liu, K. Voisey, F. A. Bonilla, P. Skeldon, G. E. Thompson, J. Piekoszewski, A. G. Chmielewski, Corros. Sci. 2005, 47, 1251. [334] R. W. Y. Poon, K. W. K. Yeung, X. Y. Liu, P. K. Chu, C. Y. Chung, W. W. Lu, K. M. C. Cheung, D. Chan, Biomaterials 2005, 26, 2265. [335] I. Milosev, T. Kosec, H.-H. Strehblow, Electrochim. Acta 2008, 53, 3547. [336] L. S. Castleman, S. M. Motzkin, F. P. Alicandri, V. L. Bonawit, A. A. Johnson, J. Biomed. Mater. Res. 1976, 10, 695. [337] Y. Okazaki, Curr. Opin. Solid State Mater. Sci. 2001, 5, 45. [338] M. Niinomi, Metall. Mater. Trans. A 2002, 33, 477. [339] M. Morra, C. Cassinelli, G. Cascardo, D. Bollati, R. R. Baena, J. Biomed. Mater. Res. Part A 2010, 94, 271. [340] M. Morra, C. Cassinelli, G. Cascardo, D. Bollati, R. R. Baena, J. Biomed. Mater. Res. Part A 2011, 96, 449. [341] Y. Kirmanidou, M. Sidira, M.-E. Drosou, V. Bennani, A. Bakopoulou, A. Tsouknidas, N. Michailidis, K. Michalakis, Biomed Res. Int. 2016, 2016, 2908570. [342] J. J. R. K. Merritt, Clin. Orthop. Relat. Res. 1996, 326, 71. [343] K. Müller, E. Valentine-Thon, Neuroendocrinol. Lett. 2006, 27, 31. [344] N. J. Hallab, K. Mikecz, J. J. Jacobs, J. Biomed. Mater. Res. 2000, 53, 480. [345] N. Bruneel, J. A. Helsen, J. Biomed. Mater. Res. 1988, 22, 203. [346] W. Xue, B. V. Krishna, A. Bandyopadhyay, S. Bose, Acta Biomater. 2007, 3, 1007. [347] F. E. Wiria, J. Y. M. Shyan, P. N. Lim, F. G. C. Wen, J. F. Yeo, T. Cao, Mater. Des. 2010, 31, S101. [348] S. Ponader, E. Vairaktaris, P. Heinl, C. V. Wilmowsky, A. Rottmair, C. Körner, R. F. Singer, S. Holst, K. A. Schlegel, F. W. Neukam, E. Nkenke, J. Biomed. Mater. Res. Part A 2008, 84, 1111. [349] C. M. Haslauer, J. C. Springer, O. L. A. Harrysson, E. G. Loboa, N. A. Monteiro-Riviere, D. J. Marcellin-Little, Med. Eng. Phys. 2010, 32, 645. [350] P. Thomsen, J. Malmström, L. Emanuelsson, M. Rene, A. Snis, J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 35. [351] S. Van Bael, Y. C. Chai, S. Truscello, M. Moesen, G. Kerckhofs, H. Van Oosterwyck, J. P. Kruth, J. Schrooten, Acta Biomater. 2012, 8, 2824. [352] C. Demangel, D. Auzene, M. Vayssade, J. L. Duval, P. Vigneron, M. D. Nagel, J. C. Puippe, Mater. Sci. Eng. C 2012, 32, 1919. [353] J. Y. Martin, D. D. Dean, D. L. Cochran, J. Simpson, B. D. Boyan, Z. Schwartz, Clin. Oral Implants Res. 1996, 7, 27. 1801215 (28 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advancedsciencenews.com www.aem-journal.com [354] M. Yamada, M. Shiota, Y. Yamashita, S. Kasugai, J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82B, 139. [355] L. Montanaro, C. R. Arciola, D. Campoccia, M. Cervellati, Biomaterials 2002, 23, 3651. [356] M. Takemoto, S. Fujibayashi, M. Neo, J. Suzuki, T. Kokubo, T. Nakamura, Biomaterials 2005, 26, 6014. [357] A. Scislowska-Czarnecka, E. Menaszek, B. Szaraniec, E. Kolaczkowska, Tissue Cell 2012, 44, 391. [358] C. Zhao, X. Zhu, K. Liang, J. Ding, Z. Xiang, H. Fan, X. Zhang, J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 387. [359] G. Li, H. Cao, W. Zhang, X. Ding, G. Yang, Y. Qiao, X. Liu, X. Jiang, ACS Appl. Mater. Interfaces 2016, 8, 3840. [360] Y. Li, W. Yang, X. Li, X. Zhang, C. Wang, X. Meng, Y. Pei, X. Fan, P. Lan, C. Wang, ACS Appl. Mater. Interfaces 2015, 7, 5715. [361] T. Zhao, R. Yang, C. Zhong, Y. Li, Y. Xiang, J. Mater. Sci. 2011, 46, 2529. Adv. Eng. Mater. 2019, 21, 1801215 [362] V. N. Hodorenko, T. L. Chekalkin, J.-S. Kim, S.-B. Kang, G. T. Dambaev, V. E. Gunther, Artif. Cells Nanomed. Biotechnol. 2016, 44, 704. [363] M. Dettin, A. Bagno, R. Gambaretto, G. Iucci, M. T. Conconi, N. Tuccitto, A. M. Menti, C. Grandi, C. Di Bello, A. Licciardello, J. Biomed. Mater. Res. Part A 2009, 90, 35. [364] Y. Seol, Y. Park, S. Lee, K. Kim, J. Lee, T. Kim, Y. Lee, Y. Ku, I. Rhyu, S. Han, J. Biomed. Mater. Res. Part A 2006, 77, 599. [365] H. Ao, Y. Xie, H. Tan, X. Wu, G. Liu, A. Qin, X. Zheng, T. Tang, J. Biomed. Mater. Res. Part A 2014, 102, 204. [366] H. Gollwitzer, K. Ibrahim, H. Meyer, W. Mittelmeier, R. Busch, A. Stemberger, J. Antimicrob. Chemother. 2003, 51, 585. [367] A. M. Gallardo-Moreno, M. A. Pacha-Olivenza, L. Salda~ na, C. PerezGiraldo, J. M. Bruque, N. Vilaboa, M. L. González-Martín, Acta Biomater. 2009, 5, 181. [368] P.-H. Chua, K.-G. Neoh, E.-T. Kang, W. Wang, Biomaterials 2008, 29, 1412. 1801215 (29 of 29) © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim