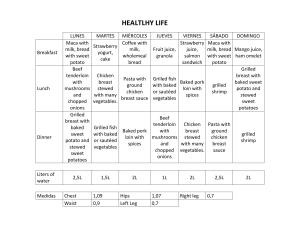
Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer
Anuncio

Reviews Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer Juliet Richman * and Mitch Dowsett Abstract | Women with early-stage oestrogen receptor (ER)-positive (ER+) breast cancer who receive standard endocrine therapy for 5 years remain at risk of distant recurrence for at least 15 years after treatment discontinuation. The extension of the duration of adjuvant endocrine therapy to 10 years has been shown to reduce the risk of recurrence only in a subset of women and, to date, predictive biomarkers of benefit from therapy do not exist. In this Review, we briefly explore the epidemiology of late recurrence (>5 years after diagnosis) in patients with ER+ breast cancer. The mechanisms underlying this phenomenon remain poorly understood; we discuss the evidence currently available on processes such as alterations of gene expression or specific genomic aberrations and examine several models used for risk prognostication and for estimating the presence of minimal residual disease, as well as the relevance of these prediction tools for clinicians and patients. Our aim is to enable clinicians to make well-informed decisions on whether to extend endocrine therapy for each individual patient. Ralph Lauren Centre for Breast Cancer Research, Royal Marsden Hospital, London, UK. *e-mail: juliet.richman@ icr.ac.uk https://doi.org/10.1038/ s41571-018-0145-5 296 | MAY 2019 | volume 16 A unique tenet of oestrogen receptor (ER)-positive (ER+) breast cancer is its ability to recur up to 20 years after diagnosis1,2. Unlike many other cancers, including ER-negative (ER−) breast cancer, the recurrence risk of ER+ breast cancer remains stable beyond 5 years after diagnosis. This means that 4,000–5,000 distant recurrences beyond 5 years can be expected among the ~46,000 new cases of ER+ breast cancer diagnosed in the UK in 2015 (refs3,4) (Fig. 1). The overall incidence of invasive breast cancer is projected to reach >71,000 new cases per year in the UK in 2035 (ref.5) and ~280,000 new cases per year in the USA by 2030 (ref.6). Late recurrence, therefore, is and will remain a considerable health problem in the foreseeable future. In this Review, we define late recurrence as recurrence beyond 5 years from diagnosis. Several unanswered questions surround late recurrence, such as whether differences other than the time elapsed after diagnosis exist between early and late recurrence and, if so, whether late recurrence can be better prevented by defining different patient subgroups according to risk of recurrence. In this Review, we explore the importance of late recurrence as an entity. We discuss the methods currently used to estimate risk of late recurrence and the validity and utility of prognostic biomarkers and aim to provide guidance for clinicians on how to incorporate these methods into clinical practice in order to make decisions that improve the outcomes for patients with ER+ breast cancer. Adjuvant endocrine therapy Endocrine therapy, aimed at suppressing oestrogen signalling, is recommended at diagnosis and for at least 5 years in virtually all women with ER+ primary breast cancer owing to the survival benefits derived from this therapeutic modality. In postmenopausal women, treatment with tamoxifen or aromatase inhibitors for 5 years is associated with an ~30% or an ~40% reduction, respectively, of mortality from breast cancer7. Several studies have addressed the question of whether patient outcomes would be improved with an extension of endocrine therapy beyond 5 years (Table 1). Tamoxifen beyond 5 years From the late 1990s, the recommendation has been to cease tamoxifen treatment at 5 years because, in the NSABP-B14 study8, patients with early-stage ER+ breast cancer who received 5 years of placebo later had a significant improvement in disease-free survival (DFS) over those who received further tamoxifen (82% versus 72%; P = 0.03); a trend (not significant) towards improved overall survival (OS) was also observed in the same group (94% versus 91%)8. Over the past 5 years, data from two studies with larger cohorts9,10 subsequently showed a benefit of tamoxifen beyond 5 years. In the ATLAS study9, an absolute reduction in disease recurrence (21.4% versus 25.1%; P = 0.002) and reduced breast cancer-related mortality (12.2% versus 15%; P = 0.01) at 10 years of follow-up were observed in patients www.nature.com/nrclinonc Reviews Key points • Oestrogen receptor (ER)-positive (ER+) breast cancer is at least as likely to recur beyond 5 years as it is before 5 years from diagnosis. • Extended endocrine therapy is likely to improve survival in a subgroup of women with ER+ breast cancer; all women should be evaluated for their likely risk of recurrence at the completion of 5 years of endocrine therapy. • Clinical and genomic expression models can help stratify patients for their risk of late recurrence, but most are not predictive of benefit from extended endocrine therapy. • Dormancy of ER+ breast cancer occurs through a multitude of mechanisms; microscopic disease can emerge from dormancy in some women in response to unidentified triggers. • Monitoring of minimal residual disease through circulating tumour cells and circulating tumour DNA is likely to prove beneficial for anticipating late recurrence. • An individualized, patient-centred approach to long-term risk management is essential owing to the protracted length of survivorship and its ensuing complexity. receiving an additional 5 years of tamoxifen compared with those who stopped treatment at 5 years. The difference between the extended tamoxifen and placebo arms became more apparent after 10 years of follow-up, highlighting the importance of long-term follow-up durations in studies involving use of adjuvant therapy. The incidences of pulmonary embolism and endometrial cancer were significantly higher among patients in the extended therapy group than in the discontinuation group (Table 1). These findings were consistent with those from the UK-based aTTom study10, in which patients with ER+ breast cancer who received tamoxifen for up to 10 years had a significant reduction in disease recurrence (16.7% versus 19.2%; P = 0.003) and a nonsignificant trend towards reduced overall mortality (24.5% versus 26.1%) compared with those who underwent tamoxifen discontinuation at 5 years. Consistent with the ATLAS study, incidence of endometrial cancer was significantly greater compared with those who discontinued tamoxifen at 5 years. Aromatase inhibitors beyond 5 years In the pivotal MA.17 study11, women who had received 5 years of adjuvant tamoxifen were randomly allocated to receive either the aromatase inhibitor letrozole or placebo for up to 5 years. Letrozole treatment was un­equivocally associated with an absolute reduction in the risk of recurrence of 4.6% (HR 0.58) compared with placebo. The incidence of toxicities was largely comparable in the two trials. Women receiving letrozole experienced significantly more hot flushes and arthralgia and/or myalgia than those receiving placebo. A significantly greater incidence of new-onset osteoporosis was observed in the letrozole arm than with placebo (8.1% versus 6.0%; P = 0.003), but this difference did not translate into an increased rate of bone fractures. The aromatase inhibitor exemestane was tested in a study with a similar design, NSABP-B33 (ref.12), in which therapy extension with an aromatase inhibitor was associated with improvements in 4-year DFS (91% versus 89%; P = 0.07) and in 4-year relapse-free survival (96% versus 94%; P = 0.004) compared with placebo. The incidence of toxicities was similar to that observed in MA.17 (ref.11). NAtuRe Reviews | ClInIcAl Oncology The benefits of extending a 5-year course of adjuvant aromatase inhibitors appear to be less substantial. In the MA.17R study13, 1,918 women without disease recurrence after 5 years of therapy with letrozole were randomly assigned to receive either letrozole or placebo for a further 5 years. An absolute reduction in disease recurrence of 3.3% was seen in the letrozole group, but this difference was weighted heavily in favour of a reduction in the incidence of contralateral second cancers. Patients in the letrozole group had a higher incidence of osteo­ porosis and skeletal-related adverse events; the incidence of other toxicities was unremarkable. In the DATA study14, a borderline significant improvement in 5-year DFS (83.1% versus 79.4%; P = 0.066) and no improvement in OS (90.8% versus 90.4%; P = 0.6) were observed with 6 years versus 3 years, respectively, of anastrozole following 2–3 years of tamoxifen. Nevertheless, in post hoc analyses, a subgroup of patients with both ER+ and progesterone receptor-positive disease with lymph node involvement in the 6-year treatment group had improved outcomes. In the IDEAL study15, 5 years of treatment with letrozole was not superior to 2.5 years of treatment after an initial 5 years of endocrine therapy; no prognostic subgroups or biomarkers were identified in this study. Furthermore, the analysis of DFS events in IDEAL showed that the main differences in outcomes between treatment arms were related to second breast cancers and not to distant disease. Taken together, these results suggest that postmenopausal women who have received 5 years of tamoxifen and switch to an aromatase inhibitor are those most likely to derive benefit from extended endocrine therapy. For those who have received 5 years of therapy with an aromatase inhibitor, the benefits appear modest and, therefore, appropriate patient selection methods are clearly needed. Endocrine therapy is cost effective and often well tolerated, and thus therapy extension beyond 5 years should only be avoided in patients with the lowest risk of recurrence. In some patients, however, toxicities can be substantial and must be considered in decisions regarding treatment extension. Future of adjuvant therapy The majority of distant recurrences seen after 5 years of endocrine therapy cannot be prevented by extending treatment beyond the initial 5 years; thus, other treatments are needed for this purpose. In a neoadjuvant study, the addition of the mTOR inhibitor everolimus to letrozole was associated with a trend towards higher overall response rates than letrozole plus placebo (68.1% versus 59.1%; P = 0.062)16. The results of an ongoing adjuvant trial of this combination (NCT01674140) might show improved outcomes. Results from studies of other agents in the adjuvant setting, such as the CDK4/6 inhibitors palbociclib (tested in the PALLAS trial: NCT02513394) and abemaciclib (tested in the MonarchE trial: NCT03155997), are eagerly anticipated. Ongoing neoadjuvant trials such as the iSPY 2 study (NCT01042379) involving multiple targeted agents, as well as immunotherapies, might pave the way for the use of tailored, risk-stratified approaches to adjuvant therapy in the future. In this regard, the ultimate goal of volume 16 | MAY 2019 | 297 Reviews Diagnosis and primary surgery 0–5 years of endocrine therapy* Clinically detectable breast cancer No response owing to resistance Treated primary breast cancer Distant recurrence (beyond 5 years) Women with early (0–5 years) or late (>5 years) distant recurrence e Micrometastases 50% of women with micrometastases Early distant recurrence Early acquired g resistance d b f 40% Partial response dormancy k Exit before 5 years h l Enter dormancy Exit dormancy 5 years Micrometastases Dormant (distant diseasefree at 5 years) i Maintain dormancy c Early ER+ breast cancer Late distant recurrence 50% of women with residual Women with no distant recurrence a 60% Complete response j Cured Cured Dormant Long-term dormant Cure and long-term dormancy are clinically indistinguishable Fig. 1 | Outcomes of women with oestrogen receptor-positive breast cancer. Out of ~46,000 women diagnosed with oestrogen receptor (ER)-positive (ER+) breast cancer annually in the UK, ~60% will be cured with surgery alone (part a), and ~40% will have residual micrometastatic disease after surgery (part b). The majority of these women will be treated with endocrine therapy and might subsequently have a complete response (part c), no response (part d), which can lead to metastatic outgrowth (part e) within a short period of time, or a partial response (part f). A partial response would be associated with the presence of residual micrometastatic disease, which would either acquire resistance leading to early recurrence (part g) or enter into a dormant state (part h). Dormant micrometastatic disease (part i) can be maintained, a process that can continue beyond 5 years (part j), or exit from dormancy can occur within 5 years (part k) or beyond 5 years (part l). The percentages given after 5 years are for the population of women with micrometastatic disease after surgery and not the overall population. *Sometimes preceded by adjuvant radiotherapy and/or chemotherapy. clinical research in this setting is to identify biomarkers to predict what the best tailored treatment is for each individual patient. Molecular and cellular mechanisms Numerous factors can influence the emergence of late recurrence (Fig. 2). The potentially long period that precedes distant recurrence in patients with ER+ breast cancer is often referred to as a period of latency or tumour dormancy. Preclinical research into tumour dormancy is currently a highly active area (reviewed previously17,18). In this section, we do not discuss molecular 298 | MAY 2019 | volume 16 mechanisms in detail but rather present the main principles underlying tumour dormancy with a focus on evidence derived from preclinical models of ER+ breast cancer and from translational studies with samples from patients with ER+ breast cancer. Mechanisms underlying dormancy The term dormancy sometimes refers to reversible cellular quiescence and, in other cases, to tumour mass dormancy19–21 (Fig. 3). In the former state, the concept is that individual cells with metastatic potential are maintained in a state of cell cycle arrest characterized by a www.nature.com/nrclinonc Reviews Table 1 | Randomized trials involving extended endocrine therapy Study (dates recruited, n and median follow-up duration (months)) Intervention (first-line therapy; investigational arm versus control arm) Absolute benefita HR (95% CI) for disease recurrence in the investigational arm Toxicities in investigational versus control and/or placebo armsb NSABP-B14 (1988–1994, 1,172 and 81) 5 years tamoxifen; 5 years tamoxifen versus placebo 4% 7-year DFS benefit in placebo arm (P = 0.03) and 3% 7-year OS benefit in placebo arm (P = 0.07) 1.4 (0.9–2.2) • Grade 3–4 AEs 5% versus 3%95 • Endometrial cancer incidence 1.4% versus 0.7%8 • Hot flushes 64% versus 48%95 • Venous thromboembolism 1.4% versus 0.2%95 ATLAS (1996–2005, 6,846 (ER+) and 91.2c) 5 years tamoxifen; 5 years tamoxifen versus no treatment extension 3.7% 10-year recurrence benefit for extended endocrine therapy (P = 0.002) and 2.8% 10-year breast cancer-specific survival benefit for extended endocrine therapy (P = 0.01) 0.84 (0.76–0.94) • Endometrial cancer incidence 116 versus 63 patients; P = 0.0002 • Pulmonary embolism 41 versus 21 patients; P = 0.01 aTToM (1991–2005, 6,953 and NA) 5 years tamoxifen; 5 years tamoxifen versus no treatment extension 2.6% recurrence benefit for extended endocrine therapy (P = 0.003) and 1.6% OS benefit for extended endocrine therapy (P = 0.1) 0.99 (0.86–1.15) in years 5–6, 0.84 (0.73–0.95) in years 7–9 and 0.75 (0.66–0.86) in year 10 or after Endometrial cancer incidence 102 versus 45 patients; P < 0.0001 10 NSABP-B33 (2001–2003, 1,598 and 30)d 5 years tamoxifen; 5 years exemestane versus placebo 2% 4-year DFS benefit for extended endocrine therapy (P = 0.07) and 2% 4-year RFS benefit for extended endocrine therapy (P = 0.004) 0.68 (NA) • Grade 3 AEs 9% versus 6%; P = 0.03 • Bone fractures 28 versus 20 patients; P = 0.33 12 MA.17 (1998–2002, 5,187 and 30)d 4.5–6.0 years tamoxifen; 5 years letrozole versus placebo 4.6% 4-year DFS benefit for extended endocrine therapy (P < 0.001) and 0.4% 4-year OS benefit for extended endocrine therapy (P = 0.3) 0.58 (0.45–0.76) • Hot flushes 58% versus 54%; P = 0.003 • Arthralgia 25% versus 21%; P < 0.001 • Alopecia 5% versus 3%; P = 0.01 • Osteoporosis 8.1% versus 6.0%; P = 0.03 • Bone fractures 5.3% versus 4.6%; P = 0.25 11 ABCSG 6a (1996–2001, 856 and 62) 5 years tamoxifen ± aromatase inhibitors in first 2 years; 3 years anastrozole versus no further treatment 4.7% 6-year RFS benefit for extended endocrine therapy (P = 0.031) and 1.4% 6-year OS benefit for extended endocrine therapy (P = 0.57) 0.62 (0.40–0.96) • Hot flushes 39.0% versus 22.4%; P < 0.001 • Fatigue 10.6% versus 4.3%; P < 0.001 • Joint pain 24.5% versus 18.3%; P = 0.009 • Hair loss 9.0% versus 2.1%; P < 0.001 96 NSABP-B42 (2006–2010, 3,923 and 83) 5 years any endocrine therapy; 5 years letrozole versus placebo 3.4% 7-year DFS benefit for extended endocrine therapy (P = 0.048) and 0.5% 7-year OS benefit for placebo (P = 0.22) 0.85 (0.73–0.99) Bone fractures 91 versus 72 patients; P = 0.27 97 MA.17 R (2004–2009, 1,918 and 75) 5 years aromatase inhibitors ± prior tamoxifen; 5 years letrozole versus placebo 4% 5-year DFS benefit for extended endocrine therapy (P = 0.01) and 1% 5-year OS benefit for extended endocrine therapy (P = 0.83) 0.66 (0.48–0.91) • Bone pain 18% versus 14%; P = 0.01 • New-onset osteoporosis 11% versus 6%; P < 0.001 • Bone fractures 14% versus 9%; P = 0.001 13 DATA (2006–2009, 1,860 and 50) 2–3 years tamoxifen; 6 years anastrozole versus 3 years anastrozole 3.7% 5-year DFS benefit for extended endocrine therapy (P = 0.06) and 0.4% 5-year OS benefit for extended endocrine therapy (P = 0.6) 0.79 (0.62–1.02) • Arthralgia and/or myalgia 58% versus 53% • Osteopenia and/or osteoporosis 21% versus 16% 14 IDEAL (2007–2011, 1,824 and 79) 5 years any endocrine therapy; 5.0 years anastrozole versus 2.5 years anastrozole 1.4% DFS benefit for 5.0 years (P = 0.49) and 0.8% OS benefit for 2.5 years (P = 0.79) 0.92 (0.74–1.16) • Overall grade 3–4 AEs 8.8% versus 10.0%; P = 0.43 • Arthralgia 14.7% versus 13.2% • Hot flushes 13.1% versus 10.5% • Osteoporosis 12.7% versus 7.5% • Bone fractures 5.0% versus 2.8% • Fatigue 9.7% versus 7.5% 15 Refs 8,95 9 AE, adverse event; DFS, disease-free survival; ER+, oestrogen receptor-positive; NA , not available; OS, overall survival; RFS, recurrence-free survival. aFirst result provided is the primary end point of the study ; time shown is time from randomization. bToxicities of all grades have been reported when data were available. c Mean. dRecruitment ceased at 30 months from start of recruitment, and crossover to experimental arm was allowed. dStudy ceased at first interim analysis at 62 months from start of recruitment, and crossover to experimental arm was allowed. NAtuRe Reviews | ClInIcAl Oncology volume 16 | MAY 2019 | 299 Reviews major reduction or complete absence of proliferation, whereas tumour mass dormancy is conceptualized as an equilibrium between a proliferating cellular population and another population with a higher cellular death rate. Both types of dormancy are likely to coexist, with each one doing so as part of the same biological continuum (Fig. 3). However, much of the evidence supporting these mechanisms is preclinical; further translational studies linking these mechanisms to patients with ER+ breast cancer are needed. The study of the mechanisms underlying both types of tumour dormancy is hampered by difficulties in developing cell lines and mouse xenograft models that accurately reflect the long latency period that can precede late-recurring ER+ breast cancer and by the length of time needed to reach the end point in these studies. Reference to studies involving tumour types other than breast cancer, therefore, are included in this Review. Another feature that has not been fully reproduced yet in experimental breast cancer models — and is particularly relevant to ER+ breast cancer — is the effect of oestrogen deprivation imposed by adjuvant endocrine treatment. The differential response to adjuvant endocrine therapy will be a key determinant in the behaviour of a micrometastasis, which in some patients will be fully ablated and in others will respond but then relapse with a variable duration of latency in between (Fig. 2). One particularly relevant xenograft study, however, has highlighted the unique behaviour of ER+ micrometastases22. In this study, the authors developed ovariectomized mouse models in which injected ER+ tumour cells seemed to be dormant for several weeks until stimulation with exogenous estrogen or progesterone triggered the outgrowth of macrometastases. This behaviour contrasted to that observed with ER− tumour cells, which developed metastases very quickly with no reliance on hormone stimulation. The ER+ micrometastases showed a low level of proliferation as measured by Ki67, thereby suggesting true cellular quiescence. These findings could reflect the emergence from dormancy seen in some patients upon cessation of endocrine therapy. The mechanisms that underlie both types of dormancy involve complex interactions between tumour cells and their microenvironment23–26. Other key components are cancer stem cells27, the tumour vasculature28 and the host immune system29–31. The immune system can both maintain and promote exit from tumour dormancy. Data from translational studies have revealed that lymphocytic infiltration into ER+ breast tumours from patients who received endocrine therapy was associated with a poor antiproliferative response to neoadjuvant aromatase inhibitors29, in contrast to observations from studies involving patients with ER− tumours treated with chemotherapy30. The spatial distribution, and not merely the abundance, of tumour-infiltrating lymphocytes has prognostic relevance in ER+ breast cancer and could be useful in defining patients with a higher risk of late recurrence31. The ability of a tumour to develop its own blood supply is fundamental to its growth and has been widely investigated. In a mouse model developed to study breast cancer dormancy, a similar balance between the 300 | MAY 2019 | volume 16 levels of the proliferation marker Ki67 and apoptotic activity was found in both angiogenic and nonangiogenic tumours, suggesting that suppression of angiogenesis during dormancy is not always associated with cellular quiescence28. However, in another preclinical study, a gene expression signature for dormancy, which was found to be more highly expressed in ER+ versus ER− cell lines, was enriched with genes that downregulate angiogenesis and has been associated with the maintenance of a quiescent phenotype in ER+ breast cancer-derived cell lines32. Exit from dormancy is an urgent research challenge, particularly because therapies based on preventing this process might be more feasible than those aimed at eradicating dormant cells. Again, preclinical evidence strongly implicates tumour microenvironment-mediated signalling as a facilitator of exit from dormancy25,26,33, in tandem with changes in immunosurveillance34 and the re-emergence of highly tumorigenic cancer stem cells35 (Fig. 3). Despite the preclinical evidence pertaining to the molecular mechanisms associated with dormancy, to our knowledge, limited preclinical and translational studies have been conducted to determine the specific changes in microenvironmental factors that can trigger the exit of breast cancer cells from dormancy. A combination of the ongoing acquisition of mutations and other environmental factors (such as physiological stress and ageing) is likely to tip the balance towards exit from dormancy over time. For example, haematopoietic stem cells might be released from quiescence into proliferation by conditions simulating those associated with inflammation or chronic blood loss36. Certain chronic conditions, which might be directly related to tumour progression, or events associated with high levels of physiological stress might lead to the awakening of tumour cells from dormancy; however, robust experimental data are not available. Therapeutic targets of dormancy Dormant cells are generally considered to be resistant to cytotoxic chemotherapy because, in most cases, efficacy is dependent on malignant cells actively going through the cell cycle37. Therefore, therapeutic agents targeting different elements in the multiple signalling pathways that enable cells or cell clusters to enter and maintain a dormant state have become a focus of drug development strategies aimed at perpetuating that state38–40. The development of most of these potential treatments is at the preclinical stage. While the level of angiogenesis seems certain to have a role in both maintaining and exiting dormancy, trials of adjuvant and neoadjuvant bevacizumab have, on the whole, failed to show conclusive evidence of benefit in patients with breast cancer41. An exploratory sub-analysis revealed that bevacizumab had a greater effect in ER+ patients, but tests for interaction between hormone receptor positivity and effect of bevacizumab on DFS and OS were not significant. This lack of data does not necessarily negate the importance of the development of the vasculature but, rather, might reflect the ability of bevacizumab to target this mechanism. Nonetheless, endocrine therapy, as is discussed further, is currently the mainstay of eliminating, www.nature.com/nrclinonc Reviews Disease presentation and surgery Endocrine therapy Chemotherapy Level of detection 5 years a Micrometastases 4 7 5i 5ii 6 8 9 Dormancy 2 1 2 Ablation 3 b Level of detection Micrometastases 7 5i 1 c 5ii 6 8 9 Dormancy Ablation Level of detection Micrometastases Dormancy Ablation Fig. 2 | Potential effects of a 5-year course of adjuvant endocrine therapy with or without chemotherapy on the natural history of subclinical micrometastatic disease. In this diagram, the slopes of the blue lines represent the growth rate of the metastases. a | Behaviour of a large-sized micrometastasis. Different outcomes can occur: cure owing to complete response to chemotherapy (1) or endocrine therapy (3), partial response to chemotherapy and endocrine therapy (2), innate resistance to endocrine therapy (4), stable dormant disease followed by early-stage (5i) or late-stage (5ii) acquired therapeutic resistance, beginning of disease recurrence with subsequent disease regression as a withdrawal response to adjuvant tamoxifen treatment (6), which would lead to disease to become dormant or be fully ablated, emergence of distant disease upon cessation of endocrine therapy (7) or at a later stage of follow-up monitoring (8), or establishment of stable, long-term dormancy (9). b | Behaviour of a small-sized micrometastatic lesion, including large lesions that have been reduced in size by adjuvant therapy. The outcomes in this situation have been described in panel a, but the emergence of recurrent disease is delayed in comparison with that scenario because of the smaller size of the metastatic lesions at the time of surgery. c | Multiple factors leading to the outgrowth of metastatic lesions and overt late-stage recurrence. In the example in the red dotted circle, six recurrent lesions emerge at a similar time (~7 years after surgery). The dotted blue and orange lines denote a patient in which a withdrawal response might lead to tumour ablation. NAtuRe Reviews | ClInIcAl Oncology volume 16 | MAY 2019 | 301 Reviews Exit from dormancy Macrometastases g Micrometastasis with two different, equal size cellular clones b Micrometastasis with selection of orange clone c Emergence from quiescence • Angiogenic switch • Microenvironment signalling • Changes in immune system Cell proliferation Balanced proliferation and cell death • Microenviroment signalling • Immune equilibrium • Stem cell and/or luminal differentiation equilibrium a e Cellular quiescence • ↓ Angiogenesis • Dormancy-permissive microenvironment signals f Emergence from balanced proliferation and cell death • Acquisition of mutations • Dominance of tumorigenic stem cells • Immune system changes • Other host changes Cell death Cell death d Maintained dormancy Fig. 3 | Coexistence of two modes of dormancy: cellular quiescence and balanced proliferation and cell death. Quiescence is represented herein by a micrometastatic lesion with two clonal populations that are not actively going through the cell cycle (with no cellular proliferation or death). This lesion can remain in this state, switch to balanced proliferation and cell death (part a) or exit dormancy (part b). In balanced proliferation and cell death, equivalent low levels of cellular proliferation (part c) and cell death (part d) exist in the cell populations of the lesion, and no selection between the two clonal populations occurs. The lesion can remain in this state or switch to cellular quiescence (part e) or exit dormancy (part f), which can be followed by the emergence of a dominant clone that is likely to be resistant to endocrine therapy (part g). or at least controlling, micrometastatic, proliferating ER+ tumour cells. Overall, much remains to be discovered within the scientific conundrum of dormancy in ER+ breast cancer. Newly developed organoid-based models and patient-derived xenografts might enable better reproduction of the biology of late recurrence to further advance the study of this area. Future studies should be aimed at elucidating the specific molecular triggers that bring about escape from dormancy. Predicting risk of late recurrence Clinicopathological predictors Individual clinical predictors of recurrence, often termed classical variables, have been extensively investigated. Classical variables include tumour size, tumour grade, Ki67 index, patient age and degree of lymph node involvement. The burden of micrometastatic disease and its proliferation rate likely affect the risk and timing of disease recurrence (Fig. 2); thus, clinical variables that inform on disease burden and proliferation rate are likely to be good predictors of the risk of late recurrence. The only independent clinical variable consistently shown to be associated with the risk of late recurrence is lymph node burden1,42,43. Tumour size has also been shown 302 | MAY 2019 | volume 16 to be independently associated with late recurrence in some studies43, including a meta-analysis involving >60,000 women who were alive and free from distant recurrence at 5 years after diagnosis2. One of the most notable findings of the latter study was that the cumulative risk of recurrence among women with the lowest stage tumours (T1N0) was as high as 13%. Clinicopathological data such as tumour size, tumour grade and lymph node burden have been incorporated into several prognostic models, such as the Nottingham Prognostic Index and NHS Predict, which are both designed to be used at the time of diagnosis to guide decisions on adjuvant endocrine therapy and/or chemotherapy. These methods are attractive because they represent an accessible, immediate and cost-free approach to risk prognostication that can be used in most routine clinical settings worldwide. The clinical treatment score at 5 years (CTS5), reported in 2018, has been specifically developed to predict risk of late recurrence in women who have not had distant disease recurrence 5 years after diagnosis44. This score was based on the original clinical treatment score at diagnosis (termed CTS), which itself was a model of clinical variables developed to be used alongside the immunohistochemistry 4 (IHC4)45. In the validation data set for CTS5, an increasing score www.nature.com/nrclinonc Reviews had a positive correlation with risk of late recurrence. Additionally, the CTS5 divided the study cohort into three risk groups, the lowest of which had a 3.6% risk of distant recurrence at years 5–10. Consistent with the findings of previous studies1,42,43, having a high burden of lymph node metastases was a strong predictor of late recurrence, hence its heavy weighting in the CTS5, confirming the prognostic relevance of this factor in the late disease setting. Individual molecular biomarkers Very limited evidence exists on the association between the molecular mechanisms proposed to underlie tumour dormancy and the likelihood of late recurrence, but data are available on individual biomarkers and gene signatures that should be considered both to understand the biology of late recurrence and, potentially, as prognostic tools. Several studies have been conducted retrospectively in cohorts of patients with ER+ breast cancer, some of whom received endocrine therapy for 5 years46–51. A study by Bianchini et al. has been particularly insightful in its examination of oestrogen signalling-related genes that might differentiate early recurrence from late recurrence46. In this study, specimens of early-stage ER+ breast cancers derived from patients treated with tamoxi­ fen for 5 years were classified according to the average expression levels of 12 genes encoding mitotic kinases and four ER-associated genes analysed in the gene signature Oncotype DX (ESR1, PGR, SCUBE2 and BCL2). Among patients with a high mitotic kinase-related score, a high ER-associated gene expression score was indicative of improved DFS 0–5 years after diagnosis but of worse DFS 5–10 years after diagnosis, a result that was consistent across patients regardless of whether they had lymph node involvement. A similar analysis of genes and gene modules from the Oncotype DX signature in patients from the TransATAC study47 revealed that high expression of the same ER-related genes was indicative of a favourable prognosis in years 0–5 but not in years 5–10 — ESR1 and SCUBE2 were the genes whose prognostic relevance changed the most in this regard47. Furthermore, high levels of PGR expression, a favourable prognostic feature in the early recurrence setting, lost prognostic value in the late recurrence setting, a finding that was also reported in the EBCTCG meta-analysis2, in which Ki67 retained a moderate level of prognostic relevance for late recurrence. SCUBE2 encodes a tumour suppressor protein involved in the signalling processes that reverse epithelial-to-mesenchymal transition (EMT), which leads to reduced levels of tumour cell invasiveness48. The potential of SCUBE2 as a biomarker has been explored in a study of 156 samples from patients with breast cancer in which positive SCUBE2 expression was independently associated with an improved DFS hazard ratio for distant recurrence (0.26 (95% CI 0.13–0.49); P = <0.0001); however, only 58% of the tumours analysed were ER+, and the median follow-up duration was 44 months49. In agreement with the results of TransATAC47, in a translational study of 750 patients with high-risk hormone receptor-positive breast cancer receiving adjuvant NAtuRe Reviews | ClInIcAl Oncology chemotherapy50, the levels of expression of SCUBE2 were almost twofold higher in the subgroup of patients with a reduced risk of recurrence than in those in the high-risk group at 0–5 years after diagnosis, a result that was independently validated in samples from the METABRIC study50. These results, however, are not informative of the risk of late recurrence because the median follow-up duration of this study was 76 months. Taken together, the results of these studies of individual molecular biomarkers46,47 suggest that adjuvant endocrine therapy maintains tumour dormancy in a population of residual cancer cells in a subgroup of women with primary breast tumours with high levels of ER or ER-related gene expression. We estimate this to be ~40–50% of those women who are free from disease 5 years after diagnosis. On cessation of endocrine therapy, the dormant cell population would then proliferate in response to resumed oestrogen signalling, resulting in late recurrence. This model is not entirely concordant with the results of a retrospective study assessing the expression of genes from the EndoPredict signature52 in samples from the ABCSG 6 and ABCSG 8 trials51, in which patients with hormone receptor-positive breast cancer received 5 years of endocrine therapy. An increased expression of proliferation genes was associated with a significantly increased risk of recurrence in years 0–5 (P < 0.001) but not in years 5–10 (ref.51). In contrast with the observations of Bianchini et al.46 and data from the TransATAC study47, the expression of genes related to ER signalling was not prognostic of recurrence in years 0–5 but, similar to the other two studies46,47, was significantly associated with an increased risk of recurrence in years 5–10 (P < 0.001). An explanation for these differences could be that the oestrogen-related genes in Oncotype DX and EndoPredict have different biological relevance. Molecular profiles: IHC4 The identification of ER, progesterone receptor and HER2 (also known as ERBB2) as prognostic biomarkers in patients with breast cancer has transformed the management of patients in both early and metastatic disease settings. These biomarkers and Ki67, all evaluated using immunohistochemistry, have been integrated in the IHC4 score, which has been validated as a prognostic model for risk of recurrence at 0–10 years45. Most of the information captured in IHC4 has prognostic value in the early recurrence setting, but in the late recurrence setting, it does not have prognostic value superior to that derived from standard clinical variables43,53. This difference can be explained by the strong weight that the ER component has in the IHC4 algorithm, which has been validated in the early recurrence setting; the shift in prognostic importance of ER-related genes after 5 years has already been discussed for Oncotype DX. Molecular profiles Several molecular tests enable quantification of the levels of the RNAs from multiple genes in a primary tumour sample and have shown prognostic value for the evaluation of risk of distant recurrence in years 0–10 after diagnosis. Promising results have also been obtained when several of these gene signatures volume 16 | MAY 2019 | 303 Reviews Table 2 | Commercially available molecular assays for risk prognostication in patients with breast cancer tested for late recurrence Refs Patient characteristics (number of patients alive and with no distant disease recurrence after 5 years of endocrine therapy) Absolute risk (% (95% CI)) of distant recurrence for patients in lowest-risk group Percentage of patients in lowest-risk group TransATAC (5–10) • 535 LN− and 154 LN+ (separate analysis) • Postmenopausal • No prior chemotherapy LN− 4.8 (2.9–7.9) and LN+ 17.9 (11.5–27.3) LN− 65.6 and LN+ 61.0 58 NSABP-B28 (5–10) • 832 • 48% <50 years of age (menopausal status not specified; data correct at the time of randomization and not at 5 years) • 100% LN+ • 100% received chemotherapy 11.7 (8.6–15.7) 40.5 59 NSABP-B14 (5–15) • 564 • 29% <50 years of age (menopausal status not specified; data correct at the time of randomization and not at 5 years) • 100% LN− • No prior chemotherapy • 4.8 (2.8–7.9) in years 5–10 • 6.7 (4.3–10.4) in years 5–15 54.8 59 TransATAC (5–10) • 535 LN− and 154 LN+ (separate analysis) • Postmenopausal • No prior chemotherapy LN− 1.4 (0.5–3.8) and LN+ 0 (n = 15; no events) LN− 54.6 and LN+ 9.7 58 ABCSG 8 (5–15) • 1,246 • Postmenopausal • 74% LN− • No prior chemotherapy Overall 2.4 (1.1–5.3), LN− 2.5 (1.1–5.4) and LN+ 0 (n = 12; no events) 36.9 69 TransATAC and ABCSG 8 (5–10) • 2,137 • Postmenopausal • 74% LN− • No prior chemotherapy 2.4 (1.6–3.5) 55.4 62 TransATAC (5–10) • 535 LN− and 154 LN+ (separate analysis) • Postmenopausal • No prior chemotherapy LN− 4.3 (2.6–7.1) and LN+ 3.3 (0.5–21.4) LN− 73.5 and LN+ 26.0 58 ABCSG 6 and ABCSG 8 (5–10) • 998 • 71% LN− • Postmenopausal • No prior chemotherapy 1.8 (0.2–3.5) 64.3 51 TransATAC (5–10) • 535 LN− and 154 LN+ (separate analysis) • Postmenopausal • No prior chemotherapy LN− 2.6 (1.3–5.0) and LN+ 9.5 (8.3–23.9) LN− 63.6 and LN+ 54.6 58 Stockholm TAM (5–10) • 285 • 100% LN− • Postmenopausal • No prior chemotherapy 2.8 (0.3–5.2) 65 66 Multi-institutional cohort (5–10) • 312 • 100% LN− • 70% postmenopausal • 32% received chemotherapyb 2.5 (0–5.0) 58 66 NA 57 53 3.0% (2.4–3.6) 42.7 Cohort in which test was used and follow-up duration after diagnosis (years)a Oncotype DX PAM50 EPClin Breast Cancer Index Immunohistochemistry 4 (IHC4) score TransATAC (5–10) • 596 • 100% postmenopausal • 100% LN− • No prior chemotherapy Clinical treatment score at 5 years (CTS5) ATAC and BIG 1-98 (5–10) • 11,446 • 64% LN− • 100% postmenopausal • 21.8% received chemotherapy 44,98 LN−, patients without lymph node involvement; LN+, patients with lymph node involvement; NA , not available. aIn all cohorts, distant recurrence-free survival was the end point. bData correct at the time of randomization and not at 5 years. 304 | MAY 2019 | volume 16 www.nature.com/nrclinonc Reviews have been used to estimate the risk of late recurrence (Table 2). MammaPrint, an important signature54, is not discussed herein because it has not been evaluated in the setting of late recurrence. Oncotype DX recurrence score. The Oncotype DX recurrence score is a purely molecular model that is evaluated using reverse transcription PCR (RT-PCR) assays and incorporates 21 genes (including 6 housekeeping genes) related to proliferation, survival, invasion and oestrogen signalling55. Oncotype DX has shown prognostic value for the risk of recurrence 0–10 years after diagnosis55 and has been argued to enable the prediction of benefit from adjuvant chemotherapy56,57. Regarding late recurrence, however, Oncotype DX showed poor performance in several retrospective studies of the TransATAC patients43,53,58 when used to discriminate between patients with a high and a low risk of recurrence 5 years after treatment and did not improve on the performance of the CTS, probably owing to reasons already discussed. Oncotype DX was reported to have significant prognostic performance for late recurrence (5–15 years after diagnosis) in patients who had ESR1 levels that were described as higher (HR for recurrence 2.23 (95% CI 1.11–4.47); P = 0.04), a cut-off that actually excluded only the 10% of patients with the lowest ER levels from the overall population59. Of note, although the risk of distant recurrence in years 0–5 was 2.1% and 22.1% in the low-risk and high-risk groups, respectively, the difference between groups was reduced after 5 years (6.7% and 13.6%), consistent with the reduced separation for late recurrence seen in TransATAC (where the difference between high and low was 5.4%)43. PAM50. The PAM50 score combines the expression levels of 50 genes and tumour size, which are used to define the intrinsic subtypes of breast cancer, and information from a set of proliferation-related genes for prognostic purposes60. PAM50 has prognostic value for the prediction of recurrence risk between year 0 and year 10 (ref.61), with similar performance for the prediction of risk at years 0–5 and 5–10 (refs43,58). The combined analysis of the ATAC and ABCSG 8 trial populations also demonstrated that PAM50 had prognostic value in the late recurrence setting, independent of the CTS62. Of note, the net reclassification index (a tool used to compare the performance of one prognostic tool with another) for the prognostic performance of PAM50 plus CTS versus CTS alone was significant (9.3% reclassified; P < 0.001), indicating that the combination of PAM50 and CTS improves risk classification compared with CTS alone. In this analysis, 24.6% of women with lymph node-positive disease were allocated to the low-risk group, with a 5–10-year risk of recurrence of only 2.4%. EndoPredict. EndoPredict is a 12-gene expression panel that is combined with clinical parameters to generate an EPClin score52. EPClin has good prognostic value for the prediction of disease recurrence 0–10 years after diagnosis52,63 and of late recurrence in a combined cohort from the ABCSG 6 and ABCSG 8 studies51. In samples from the TransATAC study, EPClin was found to have a higher NAtuRe Reviews | ClInIcAl Oncology prognostic value for late recurrence than EndoPredict alone (likelihood ratio for distant recurrence in years 5–10 of 59.3 versus 23.6; P < 0.001 (ref.63)). Breast Cancer Index. The Breast Cancer Index (BCI) is a molecular score that incorporates the ratio of HOXB13:IL17BR levels and a proliferation-related five-gene module referred to as Molecular Grade Index64. When tested in the Stockholm TAM data set65, a heterogeneous cohort (in terms of breast cancer risk) of 1,374 postmenopausal women treated with tamoxifen, BCI had independent prognostic value for recurrence beyond 5 years66. In TransATAC53, BCI had independent prognostic value for late recurrence in women with no lymph node involvement. In an analysis of the prognostic ability of BCI in a cohort of 547 patients with T1N0 ER+ disease, ~30% of the patients were classified as having a high risk of recurrence with a significantly reduced DFS at 5–15 years after diagnosis compared with those who were deemed to have a low risk according to BCI67. Uniquely among breast cancer molecular profiles, the predictive value of the HOXB13:IL17BR ratio has been tested for its ability to predict responses to extended letrozole therapy. In patients involved in the MA.17 study11, a high ratio in the placebo group was independently associated with a significantly increased risk of recurrence (OR 2.24 (95% CI 1.09–4.61); P = 0.03). In the same study, patients with a high HOXB13:IL17BR ratio derived a significantly greater recurrence-free survival benefit from extended letrozole than those with a low ratio (OR for distant recurrence in high HOXB13:IL17BR ratio group 0.35 (95% CI 0.16–0.75); P = 0.007)68. Thus, the BCI not only enabled the identification of patients with a higher risk of late disease recurrence, but it also enabled those who could benefit from extending the duration of adjuvant endocrine treatment to be identified. Of note, the study of BCI performance in the MA.17 cohort was a small, retrospective, case–control comparison that requires validation in an independent, larger data set. Comparison of molecular profiling tools. A comparison of several molecular prognostic assays within TransATAC showed the additional prognostic value of BCI, EPClin and, in particular, PAM50 in the late recurrence setting above that of CTS alone58. By contrast, Oncotype DX and IHC4 did not improve on the prognostic performance of CTS in this setting. When PAM50, BCI and EPClin were each individually used to stratify the patients with lymph node-negative disease into risk groups, the lowest-risk group included at least 50% of patients with no lymph node involvement, who have been defined as having a <5% risk of late recurrence in years 5–10 after diagnosis. Taken together, despite not being developed specifically for the evaluation of the risk of late recurrence, PAM50, EPClin and BCI all have good prognostic value in this setting. All three models enable clinically valid risk groups to be defined, the lowest of which includes women for whom extended endocrine therapy might not be warranted. Of note, the existence of these molecular models challenges the concept that single clinicopathological volume 16 | MAY 2019 | 305 Reviews variables, such as tumour size and lymph node involvement, can be used for recurrence prognostication — findings from several studies mentioned above showed that women with lymph node-positive disease can be deemed at low risk and vice versa. One common thread running through the patient cohorts used in the validation studies mentioned above is that the large majority of patients are postmenopausal women with low-risk baseline features. While these results are highly informative, the patients included probably do not completely reflect the heterogeneous patient populations seen in routine clinical settings. In subgroup analyses of data from many of the translational studies58,62,69, the prognostic value of the genomic tests was weaker for women with lymph node-positive disease, a feature that might reflect the low patient and event numbers analysed or lymph node burden having a dominant prognostic effect over that of other variables. Despite this limitation, BCI, EPClin and PAM50 remain good tools for risk stratification in patients with lymph node involvement58. More data are needed from premenopausal women and those at higher risk of recurrence who were treated with chemotherapy, who are probably the patient populations most likely to be offered extended endocrine therapy on the basis of molecular prognostic models. Late recurrence and dormancy signatures. Noncommercial signatures specific to late recurrence and dormancy have been developed and tested in addition to the commercially available gene expression models described above. A signature comprising the expression of 49 genes relating to tumour cell quiescence and failure of angiogenesis has been derived from experimental models and tested in a combined set of ER+ tumours32. Those with higher dormancy scores were associated with improved metastasis-free survival with a 2.1-fold hazard ratio compared to those with low dormancy scores. Another signature that was found to be associated with late recurrence contained epithelial and stromal genes involved in EMT and with expression levels that were directly related to epithelial–mesenchymal plasticity in primary tumour cells70. The stromal component alone had a strongly significant association with time to recurrence, thus reflecting the importance of the surrounding microenvironment to tumour dormancy. How these two signatures perform in comparison with the established commercial signatures remains to be established. However, in a large study of time-dependent gene expression signatures for the prediction of late recurrence, a specific 5–10-year signature did not improve risk estimation above that provided by a 0–10-year model. The authors conclude that further specific late recurrence signatures are unlikely to improve on the performance of the existing risk prediction models developed to study recurrence 0–10 years after diagnosis71. Mutations and copy number alterations DNA mutations in the form of point mutations, copy number alterations (CNAs; which include amplifications or deletions) and translocations are common in all tumour types and accumulate over time. The incidence of these alterations depends on the proliferative 306 | MAY 2019 | volume 16 activity of cancer cells, and therefore, in a dormant but not fully quiescent tumour, they are likely a determinant of late recurrence risk. When DNA alterations affect a driver gene, the situation can lead to the emergence of a dominant clone. Such an alteration could facilitate the conversion of a small, dormant micrometastatic deposit into an overt metastatic lesion and therefore lead to late recurrence. A very limited amount of data from direct observations support this hypothesis, probably because a small number of studies have undertaken comprehensive, comparative sequencing of material from primary breast tumours and their associated metastases. A study by Yates et al.72 stands out in this regard, even though the researchers did not distinguish between early and late recurrence. Consistent with the findings of other studies73, they reported that the most common mutations in early-stage ER+ breast cancer affected PIK3CA and TP53; mutations in many other genes were described but generally with an incidence <5%73. Overall, the genomic landscape of metastases was found to be closely related to that of the primary tumour because the authors found that clonal mutations tended to persist in the metastases in patients with relapsed disease — suggesting that therapeutic decisions made on the basis of mutational signatures in the primary tumour can be extrapolated in the context of metastatic disease. In addition to this observation, however, the authors reported that metachronous metastases had, on average, mutations in two-thirds more genes (including driver genes) than the primary tumour, with a higher rate of mutation acquisition. This result demonstrates that, despite similarities in clonal mutations, heterogeneity exists in subclonal mutations that have arisen after micrometastatic colonies have seeded at distant sites. Mutations in ESR1 that confer constitutive activation of the mutated ER protein independent of ligand binding have been detected in ~20% of tumour samples derived from patients with metastatic ER+ breast cancer treated with an average of two prior lines of endocrine therapy74 and in ~40% of circulating tumour DNA (ctDNA) samples from patients with metastatic ER+ breast cancer with disease progression on treatment with an aromatase inhibitor75. The frequency of ESR1 mutations in a cohort of women treated with tamoxifen with no prior aromatase inhibitor exposure was reported as 0/22 in a retrospective study of consecutive patients with ER+ advanced-stage breast cancer76. This study showed that 95% patients with ESR1 mutations had received an aromatase inhibitor as part of their treatment for metastatic disease. While limited data exist on emergence of ESR1 mutations during or after adjuvant endocrine treatment, the evidence suggests that some late recurrences might be driven by an ESR1 mutation in patients treated with aromatase inhibitors but not with tamoxifen. However, with treatment discontinuation at 5 years, the loss of the selective pressure derived from oestrogen deprivation could lead to clones harbouring ESR1 mutations no longer having an advantage. A retrospective study involving 42 women treated for at least 2 years with aromatase inhibitors in the adjuvant setting and who subsequently had disease recurrence showed that only two patients had ESR1 mutations that were detectable in plasma ctDNA at the www.nature.com/nrclinonc Reviews time of recurrence77. Interestingly, those two recurrences occurred at 20 months and 48 months after discontinuation of endocrine therapy that had been maintained for at least 5 years indicating that, in those two patients, the mutated resistant clone was able to survive for many months and eventually propagate. Most genetic alterations driving the progression of breast cancer appear to arise from CNAs; their contribution to metastatic dissemination has been characterized in a number of detailed studies, albeit with modest-sized cohorts78–80. CNA in clinical samples from two studies of ER+ breast cancer in the neoadjuvant setting were analysed; amplification of CHKA was associated with a poor response to neoadjuvant aromatase inhibitor therapy79. This gene was reported to drive ER-modulated cell cycle progression, and its amplification was considered to drive overexpression. Additionally, CNAs have been incorporated into a molecular signature for recurrence prognostication from time of diagnosis. Genes with both changes in expression and copy number variation have been incorporated into a molecular signature80. Internal validation has demonstrated the ability of the score associated with this signature to separate the study cohort into three distinct risk groups, the lowest of which had a distant recurrence rate of 11% over 7 years (at a median follow-up duration of 99 months)80. Similar to the role of point mutations, while the acquisition of CNAs can be expected to drive early or late recurrence in some patients, this process remains to be analysed in large-scale studies of the molecular features of recurrent disease in relation to time since diagnosis and the effect of treatment. Minimal residual disease. Given the incidence and eventual mortality of ER+ breast cancer, the proportion of women with micrometastatic or minimal residual disease after surgery is estimated at 40% (Fig. 1). Currently, following surgery, patients are recommended to receive adjuvant systemic endocrine therapy and/or chemotherapy purely on the basis of baseline risk factors owing to the lack of a clinically available method for measuring minimal residual disease (MRD). The same situation occurs in patients who have received endocrine therapy for 5 years, and thus, the decision to extend therapy is based on risk prognostication using both clinical variables (such as age) and pathological variables (such as tumour size and grade of the primary tumour). In all types of breast cancer, the presence of disseminated tumour cells (DTCs) within the bone marrow is associated with an approximately twofold increase in the risk of recurrence and death81. Methods based on the measurement of circulating tumour cells (CTCs) and ctDNA are being developed for prognostic purposes in patients with early-stage breast cancer after promising results were obtained in those with metastatic disease75,82,83. Levels of CTCs have been demonstrated to be important factors for both prediction of progression-free survival (PFS) and OS at the initiation of a new treatment in the metastatic setting and, more importantly, as part of on-treatment monitoring83. Results from studies in metastatic breast cancer have shown that ctDNA detection of mutant ESR1 was associated with improved PFS with fulvestrant compared with exemestane following NAtuRe Reviews | ClInIcAl Oncology progression on an aromatase inhibitor75 and that early suppression in levels of mutant PIK3CA during palbociclib treatment can predict improved PFS substantially earlier than changes in tumour size82. CTCs can be detected using flow cytometry with CELLSEARCH. In patients with early-stage breast cancer, the presence of CTCs was shown to be a biomarker of innate resistance to chemotherapy84 and was prognostic of less favourable DFS and OS outcomes up to 5 years after diagnosis85. In a report published in 2018 (ref.86) on the prevalence of CTCs in patients with breast cancer, CTCs were detected at 5 years in 7% of those with ER+ disease (all of whom had received endocrine therapy)86. Interestingly, the majority of patients (36/47) who had detectable CTCs at 5 years had negative results at 2 years, suggesting that the CELLSEARCH assay could be useful for tracking re-emergence of resistant disease. The prognostic value of CTCs for late recurrence was analysed in a study of 353 women treated for early-stage ER+ breast cancer who had no distant disease recurrence after 5 years of endocrine therapy87. The study, with results published in 2018, demonstrated that the presence of CTCs at 5 years was associated with a 13-fold increase in the risk of recurrence (independent of clinical co-variates) thereafter compared with patients without detectable CTCs; the number of CTCs was directly correlated with the risk of recurrence87. While the number of ER+ patients involved in the study was low and the follow-up duration was <5 years, this is, to our know­ ledge, the first study providing evidence of the prognostic value of CTCs for late recurrence in ER+ breast cancer. As such, this study supports the feasibility of CTC detection as a tool for monitoring women who are free from distant disease at 5 years. To further establish the prognostic value of CTCs in late recurrence, longer follow-up durations, as well as serial measurements during treatment with endocrine therapy and after cessation, are needed. Over the past few years, data have emerged suggesting that ctDNA might have a similar role to CTCs in enabling detection of MRD. The authors of a study in the neoadjuvant setting88 reported that post-surgical serial tracking of mutations in ctDNA had a significant prognostic value for early recurrence with improved lead times for detection compared with imaging-based monitoring88. Longer follow-up durations and different study designs are required to assess the prognostic value of monitoring ctDNA following adjuvant treatment and extrapolate its relevance for late recurrence. The majority of the current evidence in this regard comes from studies involving MRD detection following surgery and immediately after, but it is likely to be at least as useful for women who have reached 5 years without distant recurrence to better distinguish between patients who are likely to be cured and those with MRD after 5 years of therapy (Fig. 1). However, a critical link yet to be made is that between earlier detection of MRD and improved patient outcomes, thereby justifying long-term, serial disease tracking, which might extend over several decades. CTCs are detectable in the blood >20 years following curative treatment for breast cancer89, and, given their short half-life, DTCs must exist in an inactive state and be reactivated at a later stage volume 16 | MAY 2019 | 307 Reviews in some women by triggers that have yet to be identified. Owing to this uncertainty, the questions of which patients should be monitored and how to investigate positive results must be addressed. Clinical management of late recurrence In an epidemiological study of >430,000 women with varying stages of invasive and non-invasive breast cancer diagnosed between 1973 and 2000, including >116,000 women with localized (node-negative) or regional (node-positive) invasive ER+ disease, cause of death was analysed90. Of those women with localized ER+ disease who died in the follow-up period (n = 11,406), only 22% of deaths were breast cancer related. For those with regional ER+ disease who died in the follow-up period (n = 3,465), 56% of deaths were breast cancer related. Overall, therefore, the majority of women who died did so owing to causes unrelated to their breast cancer. For those women who survive their ER+ breast cancer, survivorship extends through a long period of time during which they face other non-breast cancer health-related events. Therefore, clinicians must make recommendations for the prolonged management and follow-up of their patients with a history of breast cancer. In most circumstances, the biology of late recurrence will be only of academic interest to the clinician, but the risk of late recurrence will be of central importance and will need to be evaluated alongside other clinical factors when discussing long-term management with a patient. Several key points in decision-making include the absolute risk of late recurrence, the likelihood of risk reduction with endocrine therapy (or another systemic therapy), the toxicities associated with systemic therapy, the patient’s long-term risks from continuation of endocrine therapy (endometrial cancer, bone density and venous thrombo­ embolism risk) and personal preferences. In terms of risk estimation, several clinical algorithms have been validated for the majority, but not all, patients. Several molecular profiling assays can also be used to stratify patients into risk groups in relation to late recurrence. Whether patients are stratified into meaningful groups (clinical validity) and whether the resulting treatment changes ultimately improve outcomes (clinical utility) need to be established. Clinical validity has been shown for PAM50, EPClin and BCI, as discussed above, but clinical utility and the predictive benefit of extended endocrine therapy on the basis of these assays are yet to be proved. Of note, the annual risk of relapse is generally appreciable, even in patients deemed at low risk, and therefore, the cumulative risk can become substantial over a time span of decades. We propose a decision matrix for the combined use of clinical and genomic tests for risk stratification of patients upon completion of 5 years of endocrine therapy without distant recurrence (Fig. 4). This model can assist patient management if used in the context of patient–clinician conversations, taking into account the patient’s preferences and any adverse effects of endocrine therapy that they might have experienced. Specific cut-off values for risk are not proposed herein. Clinicians need to estimate the likely absolute benefit from extending adjuvant endocrine therapy based on the estimates of risk without such treatment. 308 | MAY 2019 | volume 16 When extended adjuvant therapy is deemed appropriate, aromatase inhibitors should be continued in women who were postmenopausal at diagnosis. To our know­ ledge, no studies have specifically addressed a switch from aromatase inhibitor-based to tamoxifen-based therapy, but it is unlikely that this approach provides a survival benefit; it could be recommended for some patients owing to its different toxicity profile. Tamoxifen should be continued in women who are premenopausal at diagnosis and remain premenopausal 5 years after starting treatment; those who become postmenopausal during treatment can switch to an aromatase inhibitor or remain on tamoxifen — again, this decision should be driven by the patient’s preferences after considering potential toxicities. The importance, and the complexity, of communicating risk of recurrence to patients has markedly increased with the advent of genomic prognostic models. Explaining the concept of risk over long periods of time can be particularly challenging. The use of plain language, quoting absolute risk instead of relative risk, and the use of pictographs are currently considered the most effective methods of risk communication91. As data from preclinical and translational studies have shown, early and late recurrence are, to a certain degree, different phenomena associated with distinct risk factors; for some patients, this difference must also be adequately explained. A further complication in the long-term management of breast cancer survivors is that in some health-care systems (including in the UK), they do not receive follow-up monitoring in routine hospital-based settings but instead via patient-led, managed self-care or open-access programmes, with rapid contact with clinical teams should the need arise. This approach has been shown to be as effective as more-intensive, hospital-based follow-up monitoring in terms of DFS, OS and patient-recorded quality of life outcomes92. These models have therefore been enshrined in guidance in both the USA and the UK. In these programmes, however, discussions of late recurrence might be conducted by correspondence or telephone, rather than in person, which carries the risk that some women might have become disengaged from their health-care providers. The attitude of survivors of breast cancer to the self-management of their long-term risk of recurrence has not been well characterized and is likely to vary between individuals in terms of both personal attitudes towards the adverse events derived from long-term treatment and psychological aspects. Patients frequently cite endocrine therapy as a daily reminder of their cancer that they would much rather forget about and, therefore, committing to a further 5 years could have important psychological as well as physical implications but also uncertain levels of benefit. Fear of recurrence is a well-characterized phenomenon associated with increased anxiety, frequent medical consultations and reduced ability to plan for the future93. In one study, investigators reported that one-third of breast cancer survivors overestimate their risk of recurrence by twofold, a phenomenon which is correlated with frequent anxiety94. Grappling with the potential risks of disease recurrence beyond 5 years could considerably increase www.nature.com/nrclinonc Reviews CTS5 result No genomic test required (patient unlikely to benefit from extended endocrine therapy) Completed Low clinical risk 5 years endocrine therapy Borderline Intermediate clinical risk Offer genomic test for late recurrence (e.g. PAM50, BCI or EPClin) Stop endocrine therapy Genomic test result Low genomic risk Intermediate genomic risk Borderline Calculate CTS5 High clinical risk Patient unlikely to benefit and potential toxicity issues Risk of late recurrence might be great enough to justify extended endocrine therapy High genomic risk Risk of late recurrence high enough to warrant extended endocrine therapy No genomic test required; extended endocrine therapy should be offered Stop endocrine therapy Consider extended endocrine therapy Recommend extended endocrine therapy Recommend extended endocrine therapy Fig. 4 | Decision-making aid for clinical and genomic testing. The clinical treatment score at 5 years (CTS5) should be calculated for all women upon completion of 5 years of adjuvant endocrine therapy. For the majority of women with a low clinical risk score, endocrine therapy can be discontinued because extended therapy is very unlikely to benefit them. For the majority of women with a high risk of recurrence, extended endocrine therapy up to 10 years is recommended if the toxicity profile is not unfavourable. In both situations, genomic testing is unlikely to add further prognostic information and is not recommended. Women with an intermediate clinical risk and those at borderline low–intermediate or high– intermediate clinical risk should receive a genomic test to enable integrated clinical–genomic stratification of their risk of late recurrence. Following genomic testing, the following scenarios are possible: discontinuation of endocrine therapy for patients with a low risk of recurrence or recommendation of extended endocrine therapy for up to 10 years in patients with a high risk. Women who remain at an intermediate level of risk should discuss toxicities and personal preferences with their clinician. Of note, the role of genomic testing as a predictor of benefit from extended endocrine therapy remains to be established in prospective studies. BCI, Breast Cancer Index. the incidence of comorbidities associated with physical and/or psychological factors in some survivors of breast cancer. Clearly, the greater awareness of the continuous risk of late recurrence in patients with ER+ breast cancer that has unfolded over the past few years has provided multiple new, previously unappreciated challenges in clinical management. We are optimistic and expect that, in the foreseeable future, this appreciation will result in increased basic, translational and clinical research efforts to reduce the risk of late recurrence. Conclusions The late recurrence of ER+ breast cancer is a major clinical challenge. The elucidation of the mechanisms that maintain or trigger exit from dormancy will be key in the near future — the development of appropriate models is fundamental in this regard. Advances in the field of RNA quantification have led to the development of molecular prognostic models, which, coupled with clinicopathological models, hold promise in identifying patient subgroups with distinct risk of recurrence. Currently, perhaps the most pertinent questions are how NAtuRe Reviews | ClInIcAl Oncology to monitor and treat patients with a high risk of recurrence. The extension of endocrine therapy beyond 5 years has not yet shown definitive results but, in selected subgroups, is probably associated with improved survival. Therefore, the identification of women that would benefit from treatment (or not) is essential. The existence of improved risk prediction tools is already assisting treatment-related decisions. Decision making might be further improved with new tools such as the detection of plasma ctDNA. The increasing scientific understanding of dormancy and late recurrence is yet to affect therapeutic options but is likely to do so in the foreseeable future. The central element in these decisions is the cancer survivor, including how she understands and wishes to manage her risk. This concept can be approached with patients in several ways and, ultimately, largely depends on the individual and on the guidance she receives from clinicians — in the era of personalized medicine, an individualized approach must permeate all aspects of clinical management. Published online 18 December 2018 volume 16 | MAY 2019 | 309 Reviews 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. Esserman, L. J. et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res. Treat. 129, 607–616 (2011). Pan, H. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017). Cancer Research UK. Breast cancer statistics. CRUK http://www.cancerresearchuk.org/health-professional/ cancer-statistics/statistics-by-cancer-type/ breast-cancer (2018). Dodson, A. et al. ER, PR and HER2 biomarkers in UK and Irish clinical breast cancer testing: analysis of results from>168,000 patients. Cancer Res. 78, (Suppl.), PR-08-16 (2017). Cancer Research UK. Breast cancer incidence (invasive) statistics. CRUK https://www. cancerresearchuk.org/health-professional/ cancer-statistics/statistics-by-cancer-type/ breast-cancer/incidence-invasive (2018). Rosenberg, P. S., Barker, K. A. & Anderson, W. F. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J. Natl Cancer Inst. 107, djv159 (2015). Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386, 1341–1352 (2015). Fisher, B., Dignam, J., Bryant, J. & Wolmark, N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J. Natl Cancer Inst. 93, 684–690 (2001). Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013). Gray, R. G. et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J. Clin. Oncol. 31, S5 (2013). Goss, P. E. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl Cancer Inst. 97, 1262–1271 (2005). Mamounas, E. P. et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J. Clin. Oncol. 26, 1965–1971 (2008). Goss, P. E. et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N. Engl. J. Med. 375, 209–219 (2016). Tjan-Heijnen, V. C. G. et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 18, 1502–1511 (2017). Blok, E. J. et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006–2005). J. Natl Cancer Inst. 110, 40–48 (2018). Baselga, J. et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 27, 2630–2637 (2009). Gomis, R. R. & Gawrzak, S. Tumor cell dormancy. Mol. Oncol. 11, 62–78 (2017). Zhang, X. H., Giuliano, M., Trivedi, M. V., Schiff, R. & Osborne, C. K. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin. Cancer Res. 19, 6389–6397 (2013). Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 7, 834–846 (2007). Sosa, M. S., Avivar-Valderas, A., Bragado, P., Wen, H. C. & Aguirre-Ghiso, J. A. ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin. Cancer Res. 17, 5850–5857 (2011). Yeh, A. C. & Ramaswamy, S. Mechanisms of cancer cell dormancy—another hallmark of cancer? Cancer Res. 75, 5014–5022 (2015). Ogba, N. et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 16, 489 (2014). Fluegen, G. et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary 310 | MAY 2019 | volume 16 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. tumour hypoxic microenvironments. Nat. Cell Biol. 19, 120–132 (2017). Johnson, R. W. et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol. 18, 1078–1089 (2016). Gao, H. et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–779 (2012). Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013). Lawson, D. A. et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135 (2015). Naumov, G. N. et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J. Natl Cancer Inst. 98, 316–325 (2006). Dunbier, A. K. et al. Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin. Cancer Res. 19, 2775–2786 (2013). Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28, 105–113 (2010). Heindl, A. et al. Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+breast cancer. J. Natl Cancer Inst. 110, 166–175 (2018). Kim, R. S. et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLOS ONE 7, e35569 (2012). Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011). Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011). Gawrzak, S. et al. MSK1 regulates luminal cell differentiation and metastatic dormancy in ER( + ) breast cancer. Nat. Cell Biol. 20, 211–221 (2018). Walter, D. et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520, 549–552 (2015). Naumov, G. N. et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res. Treat. 82, 199–206 (2003). Hurst, R. E., Bastian, A., Bailey-Downs, L. & Ihnat, M. A. Targeting dormant micrometastases: rationale, evidence to date and clinical implications. Ther. Adv. Med. Oncol. 8, 126–137 (2016). Hurst, R. E. et al. Identification of novel drugs to target dormant micrometastases. BMC Cancer 15, 404 (2015). Goss, P. E. & Chambers, A. F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 10, 871–877 (2010). Bear, H. D. et al. Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol. 16, 1037–1048 (2015). Lee, E. S. et al. Factors associated with late recurrence after completion of 5-year adjuvant tamoxifen in estrogen receptor positive breast cancer. BMC Cancer 16, 430 (2016). Sestak, I. et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J. Natl Cancer Inst. 105, 1504–1511 (2013). Dowsett, M. et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J. Clin. Oncol. 36, 1941–1948 (2018). Cuzick, J. et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J. Clin. Oncol. 29, 4273–4278 (2011). Bianchini, G. et al. Proliferation and estrogen signaling can distinguish patients at risk for early versus late relapse among estrogen receptor positive breast cancers. Breast Cancer Res. 15, R86 (2013). Dowsett, M. et al. Estrogen receptor expression in 21-gene recurrence score predicts increased late 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. recurrence for estrogen-positive/HER2-negative breast cancer. Clin. Cancer Res. 21, 2763–2770 (2015). Lin, Y. C., Lee, Y. C., Li, L. H., Cheng, C. J. & Yang, R. B. Tumor suppressor SCUBE2 inhibits breast-cancer cell migration and invasion through the reversal of epithelial-mesenchymal transition. J. Cell Sci. 127, 85–100 (2014). Cheng, C. J. et al. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 69, 3634–3641 (2009). Wilson, T. R. et al. The molecular landscape of high-risk early breast cancer: comprehensive biomarker analysis of a phase III adjuvant population. NPJ Breast Cancer 2, 16022 (2016). Dubsky, P. et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br. J. Cancer 109, 2959–2964 (2013). Filipits, M. et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res. 17, 6012–6020 (2011). Sgroi, D. C. et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 14, 1067–1076 (2013). van de Vijver, M. J. et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002). Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004). Paik, S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24, 3726–3734 (2006). Sparano, J. A. et al. Prospective validation of a 21-gene expression assay in breast cancer. N. Engl. J. Med. 373, 2005–2014 (2015). Sestak, I. et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 4, 545–553 (2018). Wolmark, N. et al. Prognostic impact of the combination of recurrence score and quantitative estrogen receptor expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: results from NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J. Clin. Oncol. 34, 2350–2358 (2016). Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009). Dowsett, M. et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J. Clin. Oncol. 31, 2783–2790 (2013). Sestak, I. et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J. Clin. Oncol. 33, 916–922 (2015). Buus, R. et al. Comparison of EndoPredict and EPclin with Oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J. Natl Cancer Inst. 108, djw149 (2016). Jerevall, P. L. et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br. J. Cancer 104, 1762–1769 (2011). Rutqvist, L. E. & Johansson, H. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 46, 133–145 (2007). Zhang, Y. et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin. Cancer Res. 19, 4196–4205 (2013). Schroeder, B. et al. Risk stratification with Breast Cancer Index for late distant recurrence in patients with clinically low-risk (T1N0) estrogen receptor-positive breast cancer. NPJ Breast Cancer 3, 28 (2017). Sgroi, D. C. et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the www.nature.com/nrclinonc Reviews 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. HOXB13/IL17BR biomarker. J. Natl Cancer Inst. 105, 1036–1042 (2013). Filipits, M. et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin. Cancer Res. 20, 1298–1305 (2014). Cheng, Q. et al. A signature of epithelial-mesenchymal plasticity and stromal activation in primary tumor modulates late recurrence in breast cancer independent of disease subtype. Breast Cancer Res. 16, 407 (2014). Buus, R. et al. Novel 18-gene signature predicts early and late relapse in ER+/HER2- breast cancer patients [abstract B14]. Presented at the 2015 NCRI Cancer Conference in Liverpool. Yates, L. R. et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32, 169–184 (2017). Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012). Jeselsohn, R. et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 20, 1757–1767 (2014). Fribbens, C. et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 34, 2961–2968 (2016). Schiavon, G. et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl Med. 7, 313ra182 (2015). Allouchery, V. et al. Circulating ESR1 mutations at the end of aromatase inhibitor adjuvant treatment and after relapse in breast cancer patients. Breast Cancer Res. 20, 40 (2018). Miller, C. A. et al. Aromatase inhibition remodels the clonal architecture of estrogen-receptor-positive breast cancers. Nat. Commun. 7, 12498 (2016). Lopez-Knowles, E. et al. Integrative analyses identify modulators of response to neoadjuvant aromatase inhibitors in patients with early breast cancer. Breast Cancer Res. 17, 35 (2015). Zhang, Y. et al. Copy number alterations that predict metastatic capability of human breast cancer. Cancer Res. 69, 3795–3801 (2009). Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005). NAtuRe Reviews | ClInIcAl Oncology 82. O’Leary, B. et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat. Commun. 9, 896 (2018). 83. Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004). 84. Rack, B. et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl Cancer Inst. 106, dju066 (2014). 85. Janni, W. J. et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin. Cancer Res. 22, 2583–2593 (2016). 86. Bauer, E. C. A. et al. Prevalence of circulating tumor cells in early breast cancer patients 2 and 5 years after adjuvant treatment. Breast Cancer Res. Treat. 171, 571–580 (2018). 87. Sparano, J. et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/ jamaoncol.2018.2574 (2018). 88. Garcia-Murillas, I. et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl Med. 7, 302ra133 (2015). 89. Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004). 90. Schairer, C., Mink, P. J., Carroll, L. & Devesa, S. S. Probabilities of death from breast cancer and other causes among female breast cancer patients. J. Natl Cancer Inst. 96, 1311–1321 (2004). 91. Fagerlin, A., Zikmund-Fisher, B. J. & Ubel, P. A. Helping patients decide: ten steps to better risk communication. J. Natl Cancer Inst. 103, 1436–1443 (2011). 92. Moschetti, I., Cinquini, M., Lambertini, M., Levaggi, A. & Liberati, A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst. Rev. 5, CD001768 (2016). 93. Custers, J. A. et al. Towards an evidence-based model of fear of cancer recurrence for breast cancer survivors. J. Cancer Surviv. 11, 41–47 (2017). 94. Hawley, S. T. et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res. Treat. 161, 557–565 (2017). 95. Fisher, B. et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J. Natl Cancer Inst. 88, 1529–1542 (1996). 96. Jakesz, R. et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J. Natl Cancer Inst. 99, 1845–1853 (2007). 97. Mamounas, E. et al. Effect of extended adjuvant endocrine therapy with letrozole (L) in postmenopausal women with hormone receptor (+) breast cancer after prior adjuvant therapy with an aromatase inhibitor (AI): NRG Oncology/NSABP B-42. Breast J. 32, S25–S26 (2017). 98. Sestak, I. et al. in Highlights from the 40th Annual San Antonio Breast Cancer Symposium (ed. Lathrop, K.) 4–5 (UT Health San Antonio, AACR and Baylor College of Medicine, 2017). Acknowledgements J.R. is a Cridlan Ross Smith Charitable Trust clinical research fellow. The authors acknowledge support from the UK National Institute for Health Research Royal Marsden– Institute of Cancer Research Biomedical Research Centre. The authors are thankful to C. Isacke and A. Ring for providing internal review and feedback on this manuscript. Author contributions J.R. researched data for the article. Both authors made substantial contributions to discussions of the content, wrote the article and reviewed and edited the manuscript before submission. Competing interests The authors declare no competing interests. Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Reviewer information Nature Reviews Clinical Oncology thanks J. Cortes, G. Viale and the other anonymous reviewer(s) for their contribution to the peer review of this work. Related links ClinicalTrials.gov database: https://clinicaltrials.gov/ct2/home CTs5 Online Calculator: https://www.cts5-calculator.com Nottingham Prognostic index: http://www.pmidcalc.org/?sid=3689666&newtest=Y NHs Predict: http://www.predict.nhs.uk/technical.html volume 16 | MAY 2019 | 311