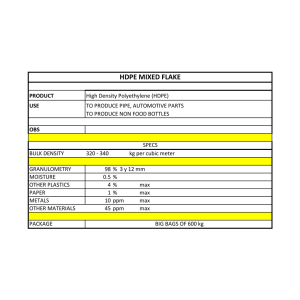
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/280738164 Testing of Scale Inhibitor Efficiency in a Pre-Scaled Environment Conference Paper · March 2015 CITATION READS 1 2,606 2 authors: Karl Fredrik Alnes Issam Aarag Haukeland University Hospital Age.Na.S. Agenzia nazionale per i servizi sanitari regionali 7 PUBLICATIONS 14 CITATIONS 1 PUBLICATION 1 CITATION SEE PROFILE All content following this page was uploaded by Karl Fredrik Alnes on 07 August 2015. The user has requested enhancement of the downloaded file. SEE PROFILE Testing of Scale Inhibitor Efficiency in a Pre-Scaled Environment Issam Aarag (CLARIANT OIL SERVICES SCANDINAVIA AS) Dr. Karl Fredrik S. Alnes (CLARIANT OIL SERVICES SCANDINAVIA AS) Abstract: Scale inhibitor selection and ranking for a proposed field application can be made more effective by employing laboratory test techniques that will better simulate and reflect the realfield scaling environment that the inhibitor will encounter on application. One of the most often overlooked but important parameters with respect to scale inhibitor selection is the effect of pre-formed inorganic mineral scales located on wetted production surfaces. Pre-scaled surfaces will impact scale inhibitor performance and ranking in two ways; firstly the scaled surface will provide an ideal high crystal surface area for attraction and interaction-retention of bulk scale inhibitor molecules, essentially acting as a scale inhibitor ‘thief zone’, and secondly, the crystal surfaces will simultaneously present an energetically more favorable environment for further scale growth and also act as a potential locus for new scale and seed crystal development to promote more aggressive bulk scaling. The following technical paper reports the application of the Kinetic Turbidity (KT) Test technique as part of a conventional scale inhibitor laboratory test suite for ranking and selection of a downhole scale inhibitor in a slightly unorthodox carbonate scale control scenario. The KT test allowed for rapid performance screening of the candidate scale inhibitors using pre-scaled and non-scaled (clean) KT test cells, and demonstrated the importance of testing using pre-scaled and ‘clean’ wetted test surfaces when determining the minimum inhibitor concentration (MIC) needed for effective scale control. Introduction: The range of scale inhibitor performance tests used to select an inhibitor for a defined carbonate and / or sulphate scaling scenario generally falls under one of a number of industry accepted test suite programs. Each component of the test suite contributes specific performance data, and when viewed together, provides the basis for ranking of candidate scale inhibitor products according to the specific minimum performance criteria agreed with the client. The traditional ‘basic’ scale inhibitor selection test suite usually comprises; Page 1 of 18 Brine and Materials Compatibility Tests Static Jar Tests Tube Blocking (Dynamic) Tests Thermal Stability Tests For scale inhibitor squeezing, the following additional tests can be included; Static Adsorption Tests Formation Damage Coreflood Isotherm Derivation Coreflood A range of more exotic and specific scale inhibitor performance tests can be considered for inclusion that are either particular to the mode of scale inhibitor application and / or potential for interaction with other materials of construction / production constituents. All scale inhibitor tests have their own particular merits but tend to be performed using sterile, solids free synthetic waters created in the laboratory using high purity grade reagents dissolved according to recipe in distilled or deionized water, and the static jar tests performed in clean disposable laboratory glassware. The clinical scaling environment created in laboratory jar and tube blocking tests differs considerably from the actual field situation, particularly with respect to brines and wetted surfaces; real field produced waters tend to contain a myriad of additional dissolved and suspended organic and inorganic species, and real field wetted production surfaces in contact with scaling brines can often present some degree of mineral scale deposition. Real field produced waters are far too complex to reproduce exactly within the laboratory, therefore we need to use an aseptic analogue which is hoped will provide a response in scale inhibitor performance tests similar to the real field brine. Pre-formed scale presence on wetted production surfaces can, to a certain degree, be reproduced and assessed in the laboratory and included as part of the routine scale inhibitor testing program. Pre-Scaling and Scale Inhibitor Testing: The impact of pre-scaled surfaces on scale inhibitor performance is a known and well understood phenomenon, as the presence of solid scale fouling on a wetted surface in contact with a scaling brine will provide an ideal high crystal surface area for attraction and interaction-retention of bulk scale inhibitor molecules, essentially acting as a scale inhibitor ‘thief zone’. The pre-scaled surface will also present an energetically more favorable environment for further scale growth and act as a potential locus for new scale and seed Page 2 of 18 crystal development to promote more aggressive bulk scaling. Pre-scaling can be incorporated into conventional tube blocking performance tests as an additional variable. There are concerns about establishing repeatability of pre-scaling in tube blocking tests and this can, on occasion, lead to uncertainty when comparing results from tube blocking test ranking exercises. Pre-scaling is generally not used in static jar tests for scale inhibitor performance determination. Scale inhibitors are designed to rapidly locate themselves at growing scale crystal surfaces and edges, and in doing so ‘quench’ any further growth at that crystal surface location. The process of crystal growth however will continue to develop at other points on the crystal surface as long as super-saturation criteria are met and / or the concentration of bulk free scale inhibitors falls below a minimum threshold value for that system. If the MIC is artificially lowered by loss of scale inhibitor through surface adsorption / location then the applied MIC dose will fail to meet the inhibitor demands of the system and the system will scale – even though a laboratory derived MIC is being applied. Likewise in a produced water production scenario where the waters have been laboratory evaluated to show supersaturation but borderline scaling potential, the presence of the pre-scaled surface may be sufficient to provide the energetic impetus to initiate scale precipitation processes from the slightly supersaturated system, and in doing so confounding the initial assessment of the very low MIC demand for the system. The KT test provides a capability for performance testing scale inhibitors in the presence and absence of pre-scaled wetted test surfaces, and also employs a ‘quasi-dynamic’ aspect to the testing via in-situ stirring. The KT instrument was originally created to provide an alternative technique for determining scaling onset and development for use in scale inhibitor performance ranking, without relying on conventional scaling indicators such as changes in differential pressure (tube blocking test) or changes in brine ion concentration (static jar test). Scaling Scenario: Well ‘A’ in offshore platform ‘T’ was configured for downhole scale inhibitor chemical injection, and the incumbent scale inhibitor ‘I’ was dosed into the well continuously from surface to provide control of calcium carbonate scale from point of injection and downstream, up-well. A modest calcium carbonate scaling potential had been identified below the scale inhibitor injection point in Well ‘A’ which was treated periodically via washing with mineral acid. No scaling had been identified from wellhead to injection point. Page 3 of 18 More recently, calcium carbonate scale build-up had been identified in the well flowline to separator, and after a period of 8 weeks had accumulated sufficiently to raise the flowline pressure by 60 psi. Mineral acid washing of the flowline resulted in the pressure returning to normal. Scale inhibitor ‘I’ was dosed throughout but had failed to provide acceptable protection from calcium carbonate scaling. Flowline scaling was identified again after resumption of production and on this occasion a section of flowline tubing was isolated and removed for inspection. Examination revealed that calcium carbonate mineral scaling was developing within the tubing and layering was evident in the 1 cm thick deposit. A scale inhibitor selection exercise was performed at the request of the operator to identify a suitable replacement for scale inhibitor ‘I’ for carbonate scale control downhole in Well ‘A’ and Well ‘A’ flowline. As part of the selection program the operator requested calcium carbonate scale inhibition performance testing in the presence of pre-formed calcium carbonate scaling of wetted surfaces. Page 4 of 18 Test Water: Table 1 provides a representative produced water chemistry for Well ‘A’. Table 1: Well ‘A’ Produced Water Ion Composition Element Concentration (mg/L) Sodium 15700 Potassium 600 Calcium 1200 Magnesium 70 Strontium 55 Barium 1 Chloride 27100 Bicarbonate 300 Sulphate 400 pH 7.0 Scale Inhibitors: Four off-the-shelf scale inhibitor products were selected and identified here as inhibitors 1, 2, 3 and 4. Inhibitors 1, and 4 were water soluble polymers and inhibitors 2 and 3 were generic phosphonate scale inhibitor formulations. All were pre-screened for suitability for application at 130°C via continuous downhole injection via injection line and valve. Laboratory Test Suite: The laboratory test suite used in the selection and ranking exercise included brine compatibility tests, static jar tests for calcium carbonate scale efficacy and also tube blocking dynamic performance tests. All tests were performed at 130°C using 100% well ‘A’ produced water composition, identified in Table 1. Page 5 of 18 Results: Brine Compatibility Tests The brine compatibility tests showed all candidate products were 100% compatible with well ‘A’ produced water at 130°C at all doses across a 24 hour test period, see Figure 1. Dose At mix 0.5 h 1h 2h 4h 24 h Dose 50 ppm 50 ppm 100 ppm 100 ppm 500 ppm 500 ppm 1000 ppm 1000 ppm 1% 1% 5% 5% 10 % 10 % 20 % 20 % 50 % 50 % 90% 90% Dose At mix 0.5 h 1h 2h 4h 24 h Dose 50 ppm 50 ppm 100 ppm 100 ppm 500 ppm 500 ppm 1000 ppm 1000 ppm 1% 1% 5% 5% 10 % 10 % 20 % 20 % 50 % 50 % 90% 90% At mix 0.5 h 1h 2h 4h 24 h At mix 0.5 h 1h 2h 4h 24 h Figure 1: Brine Compatibility Results in Well ‘A’ Produced Water at 130°C. Incompatibility is indicated via greyscale shading. No incompatibility = white circle. (Top LHS = inhibitor 1, Top RHS = inhibitor 2, Bottom LHS = inhibitor 3, Bottom RHS = inhibitor 4) Page 6 of 18 Static Jar Tests The static jar tests provided better discrimination of product character with respect to their calcium carbonate scale inhibition performance at 130°C. Scale inhibitor 3 proved most effective for calcium carbonate scale control with inhibitors 1 and 2 performing equally and were therefore tied for second position, and last was inhibitor 4 which showed the poorest performance of all 4 scale inhibitors for calcium carbonate scale inhibition in well ‘A’ produced water at 130°C across a 24 hour test period, see Figure 2. 1 2h 2 24 h 2h 3 24 h 2h 4 24 h 2h 24 h Bl a nk Control 10 ppm 20 ppm 30 ppm 40 ppm 50 ppm Figure 2: Static Jar Tests Results in Well ‘A’ Produced Water at 130°C for calcium carbonate scaling. The degree of scaling is indicated via greyscale shading. No scaling = white background. (1 = inhibitor 1, 2 = inhibitor 2, 3 = inhibitor 3, 4 = inhibitor 4) Page 7 of 18 Tube Blocking Dynamic Performance Tests The results of the tube blocking test clearly discriminate between the dosed and non-dosed tube blocking test runs, with the ‘Blank’ duplicates scaling at virtually the same time in repeat blank runs, see Figure 3 at approximately 28 minutes run time. Blank 1 Blank 2 Inhibitor 1 Inhibitor 2 Inhibitor 3 Inhibitor 4 8 Differential Pressure (psi) 7 6 20 ppm 5 18 ppm 16 ppm 12 ppm 14 ppm 10 ppm 8 ppm 4 ppm 6 ppm 2 ppm 0 ppm 4 3 2 1 0 0 75 150 225 300 375 450 525 600 675 750 825 Time (minutes) Figure 3: Tube Blocking Test Results in Well ‘A’ Produced Water at 130°C for CaCO3 scaling. The tube blocking test results showed that inhibitor 3 performed best, and suggested a dynamic MIC of approximately 2 ppm. Scale inhibitors 2 and 4 performed similarly, indicating dynamic MICs of approximately 8 ppm. Last was inhibitor 1 which performed poorly in the tube blocking test, showing a dynamic MIC of approximately 14 ppm for well ‘A’ calcium carbonate scale control. Page 8 of 18 Test Suite Summary: The test suite clearly identified inhibitor 3 as best candidate for recommendation as continuous downhole scale inhibitor replacement to incumbent chemical ‘I’ for deployment in well ‘A’ for calcium carbonate scale control. Ideally the tests should have been performed using the incumbent product as reference throughout, however this was not possible and therefore the test suite was completed in its absence. Kinetic Turbidity Testing: All four of the candidate scale inhibitors were performance tested via the KT testing instrument, an Agilent Cary Win UV configured with a 6x6 Multi Cell Holder Peltier Series II adapter, see Figure 4. Tests were performed with and without pre-scaling of the test cell to assess the potential impact on scale inhibitor demand created by pre-scaling the KT test cell. Figure 4: Kinetic Turbidity Test equipment. Left: The Agilent Cary Win UV instrument. Middle: Is the 6x6 Multi Cell Holder Peltier Series II adapter. Right: is the cuvette test cell complete with microstirring pip in the base. In 2012, Baugh et.al. (OTC-23150-MS) presented a new method for screening scale inhibitors using a multi-cell UV-Vis spectrometer with constant monitoring of turbidity at 500 nm. The instrument used the same configuration as described in Figure 4, which can heat the sample cuvettes up to 97°C and also provide constant stirring via magnetic stirring bar. The KT test presents a useful additional tool for the rapid screening of a range of scale inhibitors in selection programs. The KT test has since been adopted and adapted to create, a new method for testing scale inhibitor performance using pre-scaled test cuvettes; the goal being to mimic scale inhibitor performance in production environments where wetted surface prescaling is suspected. Page 9 of 18 KT Test Results: Scale Inhibitor 1 Scale inhibitor 1 provided a good example with respect to comparing the impact that prescaling might have on scale inhibitor performance, see Figures 5 and 6 for conventional KT and pre-scaled KT tests respectively. In the absence of pre-scaling, the Blank and Control test cells behave as expected. The Blank test cell absorbance profile, see Figure 5, shows rapid significant particle development identified by the sharp rise absorbance. The absorbance / turbidity declines across the following 2 hour test period as the scale particles become so large that the stirring bar is incapable of maintaining them in stirred solution, and they drop to the bottom of the cell. The scale inhibitor cells show scaling for both the 4 ppm and 6 ppm dosed cells, with scale control established at approximately 8 ppm applied. 0.25 Absorbance 0.2 DI water 0.15 Blank 4 ppm 6 ppm 0.1 8 ppm 10 ppm 12 ppm 0.05 0 0 20 40 60 Time (minutes) 80 100 120 Figure 5: KT Test Results for Inhibitor 1, NO PRE-SCALING. In the pre-scaled test, the Blank and Control test cells behave as expected, see Figure 6. Again the Blank absorbance profile declines across the 2 hour test period as the scale particles form and enlarge and then drop to the bottom of the cell as their mass becomes too large to keep them in stirred solution, see Figure 6. It is interesting to note that there appears to be relatively low surface deposition of scale on the cell walls of the cuvette. At 4 ppm dose of inhibitor 1 the solution turbidity increases with time signifying solid scale precipitation which does not appear to drop from solution but is either maintained in suspension or adheres to the Page 10 of 18 cell walls resulting in no net decrease in turbidity with time as was evident in the Blank. A 6 ppm dose presents a similar story however the profile shows a more gradual increase in turbidity, see Figure 5. At doses above 6 ppm the KT test response shows a very small increase in turbidity with time across the 2 hour test period. As the tube blocking dynamic MIC was determined as approximately 14 ppm, see Figure 3 above, it was decided to dose the pre-scaled cell with 10-18 ppm of inhibitor 1. The inhibitor 1 pre-scaled KT test results show a different performance with the spectra initiating at higher absorbance values compared to non-pre-scaled analogues and the DI water KT test cell, see Figure 6. The Blank response is again characteristic of zero inhibition and rises, peaks and then falls in absorbance with time. 0.16 0.14 0.12 Absorbance 0.1 DI water Blank 0.08 10 ppm 0.06 14 ppm 12 ppm 16 ppm 18 ppm 0.04 0.02 0 0 20 40 60 Time (minutes) 80 100 120 Figure 6: KT Test Results for Inhibitor 1 WITH PRE-SCALING. The dosed cell results appear quite challenging to interpret, however the absorbance spectra from the 16 and 18 ppm dosed samples appears to be very similar in profile, and also appear to maintain a stable linear profile response after initially experiencing a very slow tapering decline. The other dosing tests show continued drop throughout the dosing period suggesting scale formation and loss as the particles grow large enough, see Figure 6. An alternative interpretation regarding the slow tapering decline is the connection between solution pH, the pH sensitive pre-scaled coating and the pH of the scale inhibitor dosed into the cell. The addition of low pH scale inhibitor may exert a pH-effect on the existing carbonate scale Page 11 of 18 deposited on the test cell wall, and as such may cause a reduction in absorbance as the scale film on the cell wall is dissolved with time as the pH equilibrates. In the absence of any other obvious significant scale related changes then a steady decrease in absorbance with time for acidic scale inhibitors will need to be seriously considered as the most likely cause of spectra slow decline. The lower the pH of the scale inhibitor dosed into the cell coupled to actual dose applied will combine to create the most significant effect on the spectra. The KT derived MIC therefore roughly correlates with the tube blocking dynamic MIC for scale inhibitor 1, with the combined MIC approximating to between 14 and 16 ppm applied dose. Scale Inhibitor 2 KT testing of inhibitor 2 in the absence of pre-scale, showed the Blank and Control cells behaving as expected, see Figure 7. The control showed rapid particle development which declined with time across the 2 hour test time period. The scale inhibitor dosed cells indicated scaling at both 2 and 4 ppm applied doses, see Figure 7, with scale control established at approximately 6 ppm applied, see Figure 7. The 6 ppm MIC value is reasonably close to the tube blocking derived 8 ppm ‘approximate’ dynamic MIC for inhibitor 2, see Figure 3. 0.25 Absorbance 0.2 DI water 0.15 Blank 2 ppm 4 ppm 0.1 6 ppm 8 ppm 10 ppm 0.05 0 0 20 40 60 Time (minutes) 80 100 120 Figure 7: KT Test Results for Inhibitor 2, NO PRE-SCALING. The KT results for tests performed in pre-scaled cells dosed with scale inhibitor 2 are presented, see Figure 8. The 2 ppm and 4 ppm applied dose tests clearly fail. The 6 ppm and Page 12 of 18 8 ppm dosed sample cells appear to increase in absorbance suggesting scale development, and then reduces to give a level profile for the latter stages of the experiment. The very slight decline observed for both 6 and 8 ppm dosed tests may be attributed to the pH-effect mentioned above. 0.14 0.12 Absorbance 0.1 DI water 0.08 Blank 2 ppm 0.06 4 ppm 6 ppm 8 ppm 0.04 0.02 0 0 20 40 60 Time (minutes) 80 100 120 Figure 8: KT Test Results for Inhibitor 2 WITH PRE-SCALING. The KT test and tube blocking MIC results combined suggest that a dose of 6–8 ppm of inhibitor 2 will provide protection against calcium carbonate scaling in well ‘A’, even in the presence of pre-scaled tubing. Scale Inhibitor 3 For inhibitor 3, the absorbance spectra in Figure 9 indicated that a 1 ppm dose of inhibitor 3 fails, while all other higher scale inhibitor 3 doses pass and show symmetry with the DI water absorbance profile. Page 13 of 18 0.3 0.25 Absorbance 0.2 DI water Blank 0.15 1 ppm 3 ppm 4 ppm 0.1 6 ppm 0.05 0 0 20 40 60 Time (minutes) 80 100 120 Figure 9: KT Test Results for Inhibitor 3, NO PRE-SCALING. When pre-scaling is included we see a change in performance for inhibitor 3, see Figure 10. Doses of 2-8 ppm were used, as a preliminary KT pre-scaled test run performed at lower concentration identified possible scaling issues at 2 ppm applied dose. The 2-8 ppm dosed KT tests performed in pre-scaled cells are presented, see Figure 10. All profiles appear to show the tapered decay pattern that indicates a slow loss of absorbance with time, without showing the dramatic pulse of scale formation and increase in turbidity at the start of the test (as per the Blank). It is therefore considered most likely that the tapered spectral profiles shown here have resulted from the impact of dosing a low pH scale inhibitor into a scaled cell containing acid soluble scale film on the cell wall surface. The cells were inspected before and after test and there appeared to be some indications of increased solid accumulation on the cell floor of the cells dosed with 1-2 ppm scale inhibitor 3, while no increase in cell floor deposit was evident in cells dosed with higher concentrations of inhibitor 3. Page 14 of 18 0.16 0.14 0.12 0.1 DI water Blank 0.08 2 ppm 4 ppm 0.06 6 ppm 8 ppm 0.04 0.02 0 0 20 40 60 80 100 120 -0.02 Figure 10: KT Test Results for Inhibitor 3 WITH PRE-SCALING. Combining test results from the dynamic tube blocking test MIC, see Figure 3, and the KT test derived MIC, a combined MIC of 4 ppm of scale inhibitor 3 would be recommended for control of scale in well ‘A’. Scale Inhibitor 4 Scale inhibitor 4 KT testing in the absence of pre-scaling showed the Blank absorbance profile response to behave as expected, see Figure 11. The Blank cell absorbance shows rapid particle development which then decays across the following 2 hour test time period. The DI water absorbance profile and the inhibitor 4 tests all showed the self-same absorbance profile trend characterized by a slow steady rise in absorbance in all cases, see Figure 11. This occurrence is difficult to explain. The pre-scaled test results for inhibitor 4 are presented in Figure 12, and appear to be quite challenging to interpret. The Blank and DI water test cell responses behave as expected, and unlike the zero pre-scaled DI water profile, pre-scaled DI water control cell provides a flat baseline. Page 15 of 18 0.25 Absorbance 0.2 0.15 DI water Blank 4 ppm 6 ppm 0.1 8 ppm 10 ppm 0.05 0 0 20 40 60 Time (minutes) 80 100 120 Figure 11: KT Test Results for Inhibitor 4, NO PRE-SCALING. 0.14 0.12 Absorbance 0.1 DI water 0.08 Blank 2 ppm 0.06 4 ppm 6 ppm 8 ppm 0.04 0.02 0 0 20 40 60 Time (minutes) 80 100 120 Figure 12: KT Test Results for Inhibitor 4 WITH PRE-SCALING. The 6 and 8 ppm higher applied dose cells absorbance profiles appear to parallel one another closely while the 4 ppm and 2 ppm tests show quite different profiles with steady decline with time for 4 ppm and decline with an initial absorbance rise for the 2 ppm dosed cell. Combining tube blocking and KT test results suggests an MIC of approximately 6 ppm for scale inhibitor 4 for scale control in Well ‘A’. Page 16 of 18 Discussion: The Kinetic Turbidity tests were performed as a supplement to the conventional scale inhibitor performance testing suite and have provided much additional data regarding the candidate scale inhibitor chemical performances for control of calcium carbonate scale under well ‘A’ high temperature downhole application conditions, particularly for pre-scaled and non-prescaled tests. In general there was fairly close agreement between the candidate scale inhibitor MICs determined via conventional Tube Blocking test and then Kinetic Turbidity test evaluation, and also a looser MIC correlation between pre-scaled and non-pre-scaled KT test MICs. The KT technique is still in its infancy and will benefit from increased use during scale inhibitor selection studies to increase the knowledge base when using this technique and in particular improve interpretation of absorbance profiles to enhance the scale inhibitor selection process. The absence of the incumbent scale inhibitor from the testing suite has robbed the study of an additional dataset which would have allowed comparison in conventional performance testing against the candidate scale inhibitors, and unfortunately also removed any possibility of explaining why the incumbent scale inhibitor chemical stopped providing scale control in the case of well ‘A’. The KT tests were an add-on and were therefore performed after the initial selection test program was completed. In retrospect it would have been interesting and helpful to the discussion to have repeated the tube blocking tests, but on this occasion to have included some pre-scaling. Page 17 of 18 Conclusions: The Kinetic Turbidity test has been used successfully to enhance a scale inhibitor selection exercise which was focused on selecting a suitable scale inhibitor species for continuous application downhole for an unusual calcium carbonate scaling scenario. The KT technique tested scale inhibitors using both pre-scaled and non-pre-scaled tests. The KT tests generated MIC results similar to that achieved via conventional tube blocking test. The KT test results showed some correlation between tests performed in the absence and presence of cell pre-scaling. The KT test technique has shown considerable promise for being included as an add-on to the conventional scale inhibitor selection program. The technique is still in its infancy and will benefit markedly through use and subsequent build-up in the knowledge database. Acknowledgements: The authors of this paper want to thank the following people for all their help and assistance in creating this paper: Dr. Alex Thornton for proof-reading and all his comments and changes. Stina Storebråten for doing most of the leg work. Terje Bratseth for discussions. Vasan Singaravel for performing the initial tests on the method. The authors would also like to thank Clariant Oil Services and the Oilfield Chemistry Symposium committee for permission to publish this paper. Page 18 of 18 View publication stats