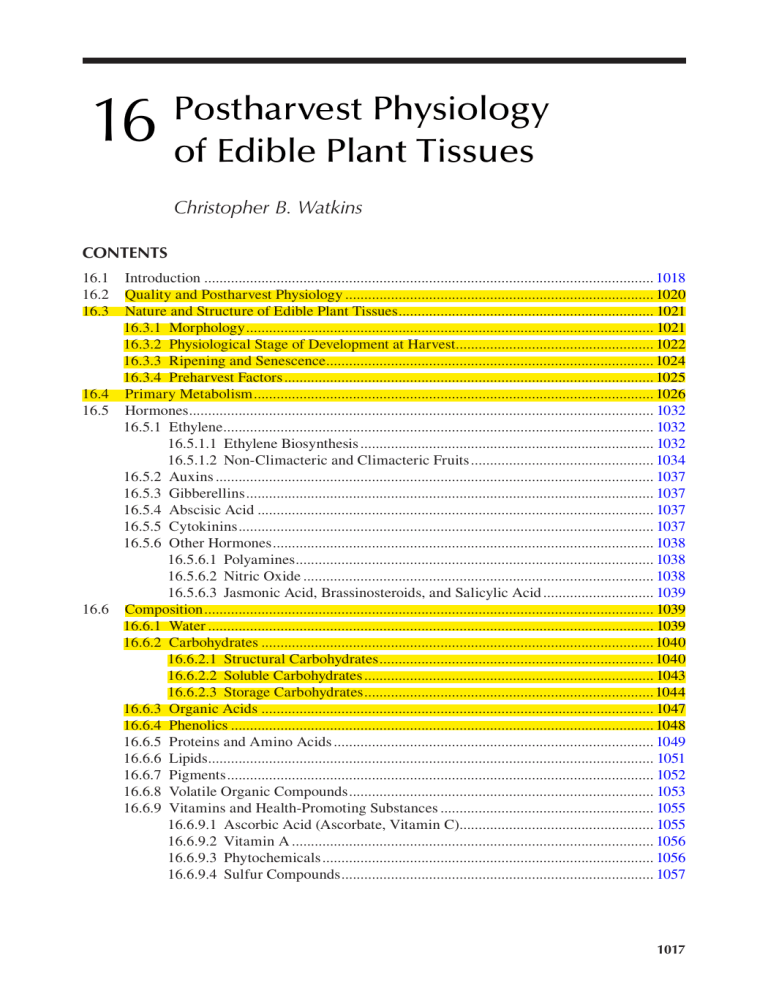
16 Postharvest Physiology of Edible Plant Tissues Christopher B. Watkins CONTENTS 16.1 16.2 16.3 Introduction....................................................................................................................... 1018 Quality and Postharvest Physiology.................................................................................. 1020 Nature and Structure of Edible Plant Tissues.................................................................... 1021 16.3.1 Morphology............................................................................................................ 1021 16.3.2 Physiological Stage of Development at Harvest..................................................... 1022 16.3.3 Ripening and Senescence....................................................................................... 1024 16.3.4 Preharvest Factors.................................................................................................. 1025 16.4 Primary Metabolism.......................................................................................................... 1026 16.5 Hormones........................................................................................................................... 1032 16.5.1 Ethylene.................................................................................................................. 1032 16.5.1.1 Ethylene Biosynthesis.............................................................................. 1032 16.5.1.2 Non-Climacteric and Climacteric Fruits................................................. 1034 16.5.2 Auxins.................................................................................................................... 1037 16.5.3 Gibberellins............................................................................................................ 1037 16.5.4 Abscisic Acid......................................................................................................... 1037 16.5.5 Cytokinins.............................................................................................................. 1037 16.5.6 Other Hormones..................................................................................................... 1038 16.5.6.1 Polyamines............................................................................................... 1038 16.5.6.2 Nitric Oxide............................................................................................. 1038 16.5.6.3 Jasmonic Acid, Brassinosteroids, and Salicylic Acid.............................. 1039 16.6 Composition....................................................................................................................... 1039 16.6.1 Water...................................................................................................................... 1039 16.6.2 Carbohydrates........................................................................................................ 1040 16.6.2.1 Structural Carbohydrates......................................................................... 1040 16.6.2.2 Soluble Carbohydrates............................................................................. 1043 16.6.2.3 Storage Carbohydrates............................................................................. 1044 16.6.3 Organic Acids........................................................................................................ 1047 16.6.4 Phenolics................................................................................................................ 1048 16.6.5 Proteins and Amino Acids..................................................................................... 1049 16.6.6 Lipids...................................................................................................................... 1051 16.6.7 Pigments................................................................................................................. 1052 16.6.8 Volatile Organic Compounds................................................................................. 1053 16.6.9 Vitamins and Health-Promoting Substances......................................................... 1055 16.6.9.1 Ascorbic Acid (Ascorbate, Vitamin C).................................................... 1055 16.6.9.2 Vitamin A................................................................................................ 1056 16.6.9.3 Phytochemicals........................................................................................ 1056 16.6.9.4 Sulfur Compounds................................................................................... 1057 1017 1018 Fennema’s Food Chemistry 16.7 Postharvest Technologies................................................................................................... 1057 16.7.1 Storage Temperature.............................................................................................. 1057 16.7.2 Relative Humidity.................................................................................................. 1062 16.7.3 Modified and Controlled Atmosphere Storage...................................................... 1062 16.7.4 Edible Coatings...................................................................................................... 1065 16.7.5 Ethylene.................................................................................................................. 1069 16.7.5.1 Ethylene Avoidance................................................................................. 1070 16.7.5.2 Ethylene Adsorbers, Oxidation, and Catalytic Decay............................. 1071 16.7.5.3 Inhibitors of Ethylene Action.................................................................. 1071 16.7.6 Heat Treatments..................................................................................................... 1073 16.7.7 Ionizing Radiation.................................................................................................. 1073 16.7.8 Other Technologies................................................................................................ 1074 16.8 Transgenic Plant Products................................................................................................. 1074 16.8.1 Genetically Modified Organisms........................................................................... 1074 16.8.2 Nutritionally Enhanced Food Crops...................................................................... 1075 16.8.3 Modification of Ripening and Senescence Processes............................................ 1076 16.9 Commodity Requirements................................................................................................. 1076 16.9.1 Cereals, Nuts, and Seeds........................................................................................ 1076 16.9.2 Whole Fruits and Vegetables................................................................................. 1077 16.9.3 Fresh-Cut (Minimally Processed) Fruits and Vegetables...................................... 1077 16.10 Conclusions........................................................................................................................ 1079 References..................................................................................................................................... 1080 16.1 INTRODUCTION Edible plant tissues include cereal grains, nuts, seeds, fruits, vegetables, and even flowers. Depending on the product, they may be consumed whole, fresh-cut, and/or processed. Except when processed, these products are metabolically active. O2 is used and CO2 is produced during respiration, the process by which carbohydrate and other substrates, such as organic acids, proteins, and fats, are metabolized to provide the energy necessary for cells to maintain structure and function, while also producing heat (“vital heat”) and water. The substrates cannot be replenished once the product has been removed from the plant. Therefore, faster respiration rates will result in loss of food nutritional value, loss of saleable weight, poorer flavor and texture, and, thus, reduced product quality. Tuber or root crops, grains, legumes, and other seeds are considered staple crops and are major contributors to the food supply (Table 16.1), and of these, rice, maize, and wheat contribute about two-thirds of human food consumption. The cereal grains have lower water content (averaging 6.8fold less), corresponding to higher energy, protein, and carbohydrate contents (3-, 6.5-, and 3.1-fold more, respectively) than tuber and root crops [1]. The staple crops derived from cereals (rice, maize, wheat, barley, sorghum), tubers (potato, sweet potato, yam), storage roots (cassava, taro), and seeds of beans and peas are major starch-storing organs, and much of our agricultural land is devoted to their growth [2]. Most of the estimated 2500 million tons of starch crops harvested annually are consumed directly as food or used as animal feed [3]. These staples make significant contributions of minerals and vitamins to human health, but the importance of fruits and vegetables in reducing risk of cardiovascular disease, stroke, diabetes, and cancers has become increasingly recognized. In addition to the major constituents of acids, sugars, and aroma volatiles that contribute to eating quality for any given product expected by the consumer, the contribution of antioxidants (vitamins A, C, and E), phenolics, and other phytochemicals is critical. Many fruits and vegetables also have aesthetic value (along with flowers and ornamental products) that contributes to human well-being. 1019 Postharvest Physiology of Edible Plant Tissues TABLE 16.1 Nutrient Content per 100 g Portion of Major Staple Foods Maize/ Rice Rice Soybean Sweet Corn (White) (Brown) Wheat Potato Cassava (Green) Potato Sorghum Yam Plantain Water (g) Energy (kJ) Protein (g) Fat (g) Carbohydrate (g) Fiber (g) 10 1528 9.4 4.7 74 7.3 12 1528 7.1 0.7 80 1.3 10 1549 7.9 2.9 77 3.5 13 1369 12.6 1.5 71 12.2 79 322 2.0 0.1 17 2.2 60 670 1.4 0.3 38 1.8 68 615 13.0 6.8 11 4.2 77 360 1.6 0.05 20 3 9 1419 11.3 3.3 75 6.3 70 494 1.5 0.2 28 4.1 65 511 1.3 0.4 32 2.3 Source: USDA Nutrient Data Laboratory, ndb.nal.usda.gov/ndb/search/list, 2014. Reliable estimates for losses of edible plant tissues after harvest are not easily obtained, but roughly one-third or about 1.3 billion tons of the food produced in the world goes to waste each year [4]. In general, grains, nuts, and seeds have high product stability associated with low moisture levels in the tissues. Postharvest management of these products is usually focused therefore on control of germination, mycotoxins, and insect infestations, for example, by appropriate drying after harvest. Storage periods for grains can be over 12 months under appropriate conditions. Nevertheless, losses of cereal grains in Africa average about 15% per year, due to factors such as grain shattering during harvesting and handling, spillage during transport, and quality loss at all steps in the postharvest chain, including storage, especially under warm and humid climatic conditions. The principal agents of quality loss are molds, insects, rodents, and birds. For fruits and vegetables, losses average about 32% [5]. These losses are similar in both developing and developed countries, but reasons for loss are different: in developing counties, lack of infrastructure leads to greater losses between production and retail (22%) compared with losses at retail, food service, and consumer sites (10%), while in developed countries greater losses occur at retail and beyond (20%) because of factors such as product deterioration, excess production, poor home storage, and “plate waste” (dissatisfaction, food preferences, “full stomachs”). Fruits and vegetables tend to be much less stable than cereal crops because their higher water content results in continuation of active metabolism after harvest, resulting in both desirable and undesirable changes during storage. Desirable changes include development of pigments: for example, lycopene synthesis in tomato, anthocyanin synthesis in apple and strawberry, and development of carotenoids (yellow and orange colors) in apricots and peaches. Other changes include softening to edible ripeness, loss of chlorophyll (de-greening), and development of aroma and flavor characteristics. The same processes can be positive in some situations and negative in others: loss of chlorophyll is desirable in tomatoes but undesirable in cucumbers and broccoli. Conversion of starch to sugars is desirable for apples but undesirable for potatoes (excessive browning during frying), whereas conversion of sugars to starch is desirable for potatoes but undesirable for peas and sweet corn (loss of sweetness). Depending on the physiological stage of the harvested product, growth processes may continue, such as undesirable sprouting of potato and geotropic curvature of asparagus. Fresh-cut or minimally processed fruits and vegetables have been an increasing component of the market in developed countries due to convenience, healthiness, attractive appearance, and ­flavor. Preparation of these products requires processes such as cutting, shredding, grating, and ­disinfecting prior to packaging, which results in injury to the plant tissues, evoking wound responses that typically shorten storage periods compared with the whole product. 1020 Fennema’s Food Chemistry Accordingly, utilization of postharvest technologies to slow down metabolic processes associated with senescence and ripening of perishable plant tissues, whether whole or fresh-cut, is essential to maintain quality after harvest. An understanding of the chemistry of postharvest physiology of these tissues is fundamental to the successful application of these technologies to preserve edible plant tissues. 16.2 QUALITY AND POSTHARVEST PHYSIOLOGY Postharvest technologies help maintain quality of edible plant tissues by linking production with consumption, adding value, extending marketing periods, and enabling new markets to be accessed [6]. However, different perspectives exist on what “quality” is, how it can be measured, and how it relates to consumer acceptability [7]. Definitions have included “fitness for use” and “meeting expectations of the consumer,” and “the degree of excellence of a product or its suitability for a particular use.” On a global scale, quality is increasingly integrated with economic, social, and environmental issues, to include factors such as worker-protection standards, chemical use, irradiation, cultural preferences, and genetic modification [6]. Many consumers believe that term “fresh” refers to products that are freshly harvested and sent directly to the market place, rather than stored. However, storage is a fundamental aspect of ensuring food supply year-round, and can vary in length from a few days in a cooler to many months under controlled atmosphere conditions. Any product that is “intact” and/or as marketed after harvest prior to the end of its natural shelf-life is “fresh.” The quality and health-promoting substances of a product after storage can be identical to that at harvest if the cultivar selection and storage conditions are optimal. However, as discussed in Section 16.3.4, factors such as cultivar selection and harvest timing can influence the degree of dissatisfaction with the quality of fruits and vegetables in the marketplace. The primary objectives of postharvest technologies are to maintain quality by: 1. Reducing metabolic rates that result in undesirable changes in color, composition, texture, flavor, and nutritional status, and undesirable growth such as germination, sprouting, or rooting 2. Reducing water loss that results in loss of saleable weight, wilting, shriveling, softening, and loss of crispness 3. Minimizing bruising, friction damage, and other mechanical injuries 4. Reducing spoilage caused by microbial decay, especially of damaged or wounded tissues 5. Preventing development of freezing injuries, or physiological disorders such as chilling injury or senescent disorders 6. Ensuring that handling, storage, and transportation methods minimize the risk of chemical or microbial contaminations that affect food safety These objectives can be met by understanding that maintaining quality from the time of harvest to when it is eaten by the consumer is an integrated process of handling, storage, transport, and retail display, which recognizes the biology of each type of edible plant tissue. Products must be harvested at optimum maturity or quality, handled carefully to avoid mechanical injury, cooled quickly to remove field heat, treated with postharvest chemicals if necessary and/or stored in modified atmospheres appropriate for the product, and maintained at acceptable temperatures during storage, transport, and marketing. Contaminating chemicals must be avoided or removed, and attention should be paid to avoid injurious effects of naturally occurring chemicals such as ethylene. All freshly harvested produce has naturally occurring bacteria, yeast, and molds, and these “contaminants” are associated with dust, insects, soil, rainfall, and sometimes human activity. Food poisoning outbreaks have been associated with contamination of produce by animals and by human contact. Therefore, attention to food safety protocols, such as Good Agricultural Practices (GAP), Hazard Analysis, and Critical Control Point (HACCP) programs, is becoming an increasingly important requirement for marketing of edible plant products. 1021 Postharvest Physiology of Edible Plant Tissues 16.3 NATURE AND STRUCTURE OF EDIBLE PLANT TISSUES 16.3.1 Morphology The range of plant tissues that are harvested for use by humans is vast, but may be classified by the plant part [8]. This classification includes intact plants, detached plant parts, aboveground structures (leaves, petioles, stems, spikes, flowers, dried and fleshy fruits, and other structures such as mushrooms [fungi]), and belowground structures (roots, rhizomes and tubers, bulbs, corms, and non-storage organs such as root cuttings and crowns). These tissues are discussed in detail elsewhere [8] and are summarized in Table 16.2. The structure of harvested products can further be subdivided into general tissue types of which the products are composed—dermal, ground, vascular, support, and meristematic (Table 16.3). The characteristics of these plant parts and tissues types are diverse but include differences in metabolic rates, presence or absence of carbohydrate reserves, and susceptibility to water loss and injury. Postharvest management protocols may also vary greatly from a focus on ripening and senescence-related processes to those associated with factors such as growth, for example, sprouting, rooting and germination, lignifications, or wound TABLE 16.2 Plant Parts Harvested for Use by Humans Based on Morphology Type of Plant Part Intact plants Detached plant parts—above ground Detached plant parts—below ground Examples Leaves Petioles Stems, shoots, and spikes Flowers Fruits—fleshy Fruits—dried Other structures Roots Rhizomes and tubers Bulbs Corms Bean or alfalfa sprouts; bare root seedlings and rooted cuttings Spinach, collards, Chinese cabbage, lettuce, cabbage Celery, rhubarb, pak-choi Asparagus, bamboo shoots, gladiolus, flowering ginger spikes Cauliflower, broccoli, lily blossoms Apple, banana, fig, orange, peach, pineapple, strawberry, tomato Wheat, rice, soybean, Brazil nut, poppy, soybean, walnut Mushroom, truffles Beet, carrot Ginger, lotus, potato, sweet potato Onion Chinese water chestnut, taro Source: Kays, S.J., Postharvest Physiology of Perishable Plant Products, Exon Press, Athens, Greece, 1997. TABLE 16.3 General Tissue Types and Cell Types of Edible Plant Tissues Tissue Type Cell Type Dermal Ground Vascular Support Meristematic Epidermis (stomata, trichomes, nectaries, hydathodes), periderm Parenchyma cells Xylem, phloem Collenchyma, sclerenchyma Meristematic Source: Kays, S.J., Postharvest Physiology of Perishable Plant Products, Exon Press, Athens, Greece, 1997. 1022 Fennema’s Food Chemistry 4 3 3 3 4 5 (a) (b) 4 5 (c) 1 1 1 2 5 2 3 3 4 4 (d) 2 2 2 2 3 4 1 1 1 (e) 5 (f ) FIGURE 16.1 Diagrammatic illustrations of anatomical structures of different types of fruit. (a) Pepo (cucumber, squash, and pumpkin) in cross section: (1) rind (receptacular), (2) flesh (ovary wall), (3) placenta, (4) seed, and (5) vascular bundle. (b) Drupe (cherry, peach, and plum) in longitudinal section: (1) pedicel, (2) skin (ovary wall), (3) flesh (ovary wall), pit (stony ovary wall), and (5) seed. (c) Aggregate (raspberry, strawberry, and blackberry) in longitudinal section: (1) fleshy ovary wall, (2) seed (stony ovary wall plus seed), (3) fleshy receptacle, (4) sepal, and (5) pedicel. (d) Legume (pea, soybean, and lima bean) in longitudinal section: (1) pedicel, (2) sepal, (3) vascular bundles, (4) seed, and (5) pod (ovary wall). (e) Pome (apple and pear) in longitudinal section: (1) pedicel, (2) skin and flesh (receptacle), (3) leathery carpel (ovary wall), (4) seed, and (5) calyx (sepals and stamens). (f) Hespiridium (citrus) in cross section: (1) collenchymatous exocarp (the flavedo), (2) parenchymatous mesocarp (the albedo), (3) seed, and (4) endocarp of juice sacs formed by breakdown of groups of parenchyma-like cells. periderm formation. Classification by plant part, and an understanding of the contribution of tissues types, provides important distinctions for the food chemist to conceptualize the physical and physiological characteristics that influence postharvest behavior and impact harvest, handling, and storage management of products for whole, fresh-cut, or processing uses. The morphology of edible plant products can also differ greatly by type [9]. For fruits, the fleshy part that is eaten is derived from many plant parts (Figure 16.1). The fruit can be derived from the pistil or accessory parts. Whatever its origin, fruit are largely composed of parenchymatous tissue. Cereals are examples of dried fruits or ovules of dried fruits. Rice, wheat, and barley grains are indehiscent fruits that are classed as caryopses: one-seeded fruits in which the thin pericarp and the seed are adherent (Figure 16.2). 16.3.2 Physiological Stage of Development at Harvest The physiological stage that horticultural products are harvested for commercial purposes ranges from sprouts and seedlings, which are harvested when the plant is in the very early stages of growth, to seeds and dry beans that are harvested at the senescent stage of development (Figure 16.3). The physiology of most product types is affected primarily by the degree of development, which affects factors such as the rate of respiration, epidermal development, and susceptibility to injury and pathogens, and also by postharvest factors such as storage temperatures. Decisions associated with how the commodity is harvested, handled, and stored can greatly affect the storage life of even the most long-lived fruit or vegetable. For fully developed fruits, two competing factors often exist. On one hand, characteristics of the products such as sweetness and 1023 Postharvest Physiology of Edible Plant Tissues flavor that are desired by the consumer increase as they mature and ripen. At the same time, the storability of the product continues to decrease (Figure 16.4). The outcome of these competing factors is that fruit destined for storage should be harvested earlier than that suitable for immediate consumption. Examples include apples, which if picked at full ripeness will have a low starch content and a high concentration of soluble solids and be highly aromatic, but will have a short storage life. In contrast, apples destined for long-term storage must be harvested less mature when the fruit has high starch content and a less developed aromatic character. Strawberries that are fully red and fully flavored will have a much shorter storage life than when harvested at the white tip stage of maturity. However, the fruit harvested at the white tip stage will have less intense flavor and aroma profiles than the fully ripened fruit. Therefore, the perceived quality of fruits in the supermarket during offseason is often lower than the locally grown ones because of the need to balance postharvest transit time to market with the rate of product deterioration. Hairs of brush Awn Lemma Endosperm (cells filled with starch in a protein matrix) Palea Aleurone cells Nuclear tissue Testa (seed coat) Tube cells Cross cells Hypodermis Epidermis Scutellum Sheath of shoot Rudementary shoot Primary shoot Root sheath Pericarp Tegmen Aleurone layer Starchy endosperm Scutellum Epiblast Plumule Radicle Sterile lammae Embryo Root cap Rachilla (a) (b) Bran Endosperm Endosperm Horny endosperm Floury endosperm Germ (cells filled with starch granules in protein matrix) Bran epidermis Mesocarp Germ Scutellum Cross cells Tube cells Testa (seed coat) (c) FIGURE 16.2 (c) corn. Aleurone (part of endosperm but separated with bran) Plumule (rudimentary shoot and leaves) Radicle (primary root) Tip cap Diagrammatic illustrations of cereal grains called caryopsis fruit: (a) rice, (b) wheat, (Continued) 1024 Fennema’s Food Chemistry Scutellar epithelium Vascular bundle Scutellum Nerve Endosperm Depleted cells Coleoptile Starchy endosperm Aleurone layer Embryonic leaves Provascular strands Testa Rootlets Pericarp Root cap Lemma Micropylar region Palea (d) Rachilla Pigment strand Musk Sheaf cells Ventral furrow Trichomes (hairs) Hull Hilum Cotyledon Endosperm Embryo Aleurone cells Bran Seed coat Scutellum Embryo Plumule Root Embryonic axis (e) Testa (f) Endosperm FIGURE 16.2 (Continued) Diagrammatic illustrations of cereal grains called caryopsis fruit: (d) barley, and (e) oat. An example of a true seed crop that does not contain a thin fruit shell is soybean (f). 16.3.3 Ripening and Senescence Harvested edible plant tissues typically undergo senescence processes that lead to cell death. Senescence may be regarded as the final stage of plant development during which the plant exhausts the organic resources accumulated during growth and development to sustain life processes or, in other words, the final stage of ontogeny* that leads to death and decrease of functional capacity. However, ripening is an additional developmental stage in fruits. Ripening involves distinct metabolically active processes, both anabolic and catabolic, that results in cell-wall softening and texture changes, color changes, and aroma production, which make the fruit more desirable for animals as food, or more susceptible to decay by the action of microorganisms, to ensure seed dispersal. By definition, a fruit is “mature” only if it can complete its normal ripening after it has been harvested. Fruits exhibit a wide range of developmental changes during the course of their development from * Ontogeny: course of development of an organism. 1025 Postharvest Physiology of Edible Plant Tissues Developmental stages Initiation Development Death Growth Maturation Physiological maturity Ripening Senescence Harvestable maturity Sprouts Stems and leaves Asparagus, celery, lettuce, cabbage Inflorescence Artichoke, broccoli, cauliflower Partially developed fruits Cucumber, green bean, okra, sweet corn Fully developed fruits Apple, pear, citrus, tomato Roots and tubers Seeds Carrot, onion, potato, cassava, sweet Potato FIGURE 16.3 Horticultural maturity in relation to developmental stage of the plant. (Modified from Watada, A.E. et al., HortScience, 19, 20, 1984.) the ovary to the mature fruit, even though their ripening patterns may be very different, for example, climacteric or non-climacteric (Section 16.5.1.2). 16.3.4 Preharvest Factors Although this chapter is concerned with postharvest physiology, many of the responses of edible plant tissues are greatly affected by cultivar selection and preharvest management. Growers usually select cultivars on the basis of marketability (visual qualities specific to the market of choice) and yield, because these factors directly affect economic sustainability. However, cultivars can vary greatly in storage and shelflife, including resistance to postharvest diseases and physiological disorders. Differences in storage potential within specific products result from different physiologies and biochemistries of each cultivar. Also, breeders have sometimes favored selections with better resistance to the handling abuses during harvest, 1026 Fennema’s Food Chemistry 100 Relative change 75 50 Storability (firmness, acidity, starch, background color) Quality (color, flavor, sugar/acid ratio, starch index) 25 0 Maturation and ripening period FIGURE 16.4 The relationship between the storability of a ripening horticultural product and the quality characteristics desired by the consumer. As a fruit ripens, the quality attributes such as color and flavor increase, but the potential storage period decline. handling, and transport, which result in bruising and skin damage. The strategy has sometimes yielded the development of cultivars that have tougher skins and sometimes reduced eating quality. In some cases, genes that control processes such as low ethylene production, low respiration, and slower softening have been bred into the commodity. For example, the rin mutant has been incorporated into most commercial tomato cultivars, resulting in firmer fruits with slower softening rates but with less aroma and flavor. The storability of fruits and vegetables is affected by the mineral composition at harvest [10]. High calcium levels are usually associated with good storability, and, conversely, low calcium with shorter storage life and susceptibility to storage disorders and pathogen infection. In tomato, for example, a disorder known as “blossom end rot” is associated with low calcium concentrations in the fruit. Nitrogen is often applied to increase yield, but it can negatively affect postharvest quality because respiration rates are higher, and calcium levels are lower because product size is often larger and therefore mineral levels may become limiting. Another example is onion, where high nitrogen improves yield but increases storage rots. High nitrogen causes the onions to develop thick necks that are prone to wounding when topped, and therefore greater decay development. High levels of potassium, magnesium, and boron, and low levels of phosphorus, can also lower storability. Other factors such as pest and pathogen management in the field or orchard affect the disease potential of the produce after harvest. 16.4 PRIMARY METABOLISM Photosynthesis is the source of carbohydrates for edible plant tissues either directly as in green leafy products or translocated as in fruits and tubers, but typically harvested products are removed from environments in which photosynthesis can occur. Many products that continue to have potential to photosynthesize, such as ornamentals and leafy cuttings, are usually not edible. Edible detached plant parts with photosynthetic potential, such as leaves, shoots, petioles, and chlorophyll-containing fruit such as apples and peppers, are stored in low-light environments. Low storage temperatures further eliminate any contribution of photosynthetic sources of carbon. Therefore, the carbohydrate in the product at the time of harvest is the sole source of energy for maintenance of cellular function. The primary metabolic process for utilization of carbohydrate is respiration, which is essential for the maintenance of adequate supplies of the high-energy compounds adenosine triphosphate (ATP), nicotinamide adenine dinucleotide (NADH), and pyrophosphate (PPi), which are necessary to maintain cellular organization of living cells. In plants, substrates such as starches, sugars, and organic acids are catabolized by glycolysis and associated pathways to simpler molecules such as CO2 and H2O, together with the release of heat (energy). 1027 Postharvest Physiology of Edible Plant Tissues TABLE 16.4 Examples of Linkages between Glycolytic Pathway and TCA Cycle with Other Pathways within the Cell Glycolytic Pathway Intermediate Derived Metabolite(s) Glucose-6-phosphate Fructose-6-phosphate Dihydroxyacetone phosphate 3-Phosphoglycerate Phosphoenolpyruvate Pyruvate Nucleotides Amino acids, glycolipids, glycoproteins Lipids Serine Amino acids, pyrimidines Alanine TCA cycle Citrate α-Ketoglutarate Succinyl CoA Oxaloacetate Amino acids, cholesterol, fatty acids, isoprenoids Glutamate, other amino acids, purines Heme, chlorophyll Aspartate, other amino acids, purines, pyrimidines Complex carbohydrates e.g., starch Pentose phosphate pathway Shikimic acid pathway Sugars m-Inositol Complex polysaccharides, e.g., cell wall Glycolysis Pyruvic acid Acetyl CoA Non-mevalonate pathway Mevalonate pathway Malonic acid pathway Aromatic amino acids Phenypropanoid pathway Phenolic compounds Lignins Tannins Terpenoid pathways Malonyl CoA Lipids Phospholipids e.g., wax, fats Terpenes Polyketides Quinones FIGURE 16.5 Overview of primary and secondary pathways in edible plant tissues. Intermediates formed during glycolysis provide the carbon skeletons that are utilized by the cell to synthesize amino acids, nucleotides, pigments, lipids, and flavor compounds (Table 16.4). In addition, carbohydrate is utilized through an intricate linkage of pathways (Figure 16.5) to produce many important compounds that impact quality and storability of harvested plant tissues. In glycolysis, if glucose is used as substrate, the overall equation for respiration can be written as follows: C6H12O6 + 6 O2 + 38 ADP + 38 Pi → 6 CO2 + 6 H2O + 38 ATP + 686 kcal 1028 Fennema’s Food Chemistry Glucose can come from simple sugars including sucrose, or complex carbohydrates such as starch. When the substrate is carbohydrate and respiration is aerobic, the respiratory quotient (RQ), which is the ratio of CO2 being produced to O2 consumed, is near 1. If the substrate is lipid, the RQ will be less than 1 (e.g., for palmitic acid it is 0.36), and for organic acid the RQ will be greater than 1 (e.g., for malic acid it is 1.33). O2 used in respiration diffuses from the surrounding atmosphere, while CO2 diffuses out of the tissue. Of the 686 kcal produced per mole of glucose, about 281 kcal (41%) is used to produce 38 ATP molecules, 13 kcal (2%) accounts for the increased entropy as glucose is converted to oxidized end products, and 392 kcal (57%) is lost as “vital heat.” Glucose 1 Glucose-6-phosphate (G6P) 2 Fructose-6-phosphate (F6P) 3 Fructose-1,6-bisphosphate (F1,6P) 4 3-phosphoglyceraldehyde (G3P) 5 Isocitrate 16 α-Ketoglutarate 15 17 Succinyl CoA Citrate Dihydroxyacetone-P (DHAP) 6 18 1,3-bisphosphoglycerate 7 3-phosphoglycerate Succinate 14 8 2-phosphoglycerate 19 Oxaloacetate 9 21 Phosphoenolpyruvate (PEP) Malate 20 Fumarate 10 Pyruvate 11 Acetaldehyde 13 Acetyl CoA Electron transport system 12 Ethanol FIGURE 16.6 The glycolysis pathway, TCA cycle, and electron pathway, with associated enzymes and reactants. Each molecule of glucose produces two molecules of ATP and two molecules of NADH. Each molecule of pyruvate produces one molecule of FADH2 and four molecules of NADH. Through the electron transport system, one NADH molecule produces three ATP molecules, while one FADH2 molecule produces two ATP molecules. (1) Hexokinase (glucose + ATP → G6P + ADP), (2), phosphohexose isomerase (G6P → F6P), (3) phosphofructokinase (F6P + ATP → F1,6P + ADP), (4) aldolase (F1,6P → DHAP + G3P), (5) isomerase (DHAP → G3P), (6) 3-phosphoglyceraldehyde dehydrogenase (G3P + NAD+ → 1,3-­bisphosphoglyerate + NADH, (7) phosphoglycerokinase (1,3-bisphosphoglycerate + ADP + Pi → 3-phosphoglycerate + ATP), (8) phosphoglyceromutase (3-phosphoglycerate → 2-phosphoglycerate), (9) enolase (2-phosphoglycerate → PEP + H2O), (10) pyruvate kinase (PEP + ADP → pyruvate + ATP), (11) pyruvate decarboxylase (pyruvate → acetaldehyde + CO2), (12) alcohol dehydrogenase (acetaldehyde + NADH → ethanol + NAD+), (13) pyruvate dehydrogenase complex (pyruvate + CoA-SH + NAD+ → ­acetyl CoA + CO2 + NADH + H+), (14) citrate synthase (acetyl CoA + oxaloacetate + H2O→ citrate + CoA), (15) aconitase (citrate + H2O → isocitrate + H2O), (16) isocitrate dehydrogenase (­isocitrate + NAD → α-ketoglutarate + NADH + CO2), (17) α-ketoglutarate dehydrogenase (α-ketoglutarate + NAD → succinyl CoA + NADH + CO2), (18) succinate thiokinase (succinyl CoA + Pi + nucleoside diphosphate (either GDP or ADP) → succinate + CoA + nucleoside triphosphate (either GTP or ATP)), (19) succinate dehydrogenase (succinate + H2O → fumarate), (20) fumarase (fumarate + FAD → malate + FADH2), (21) malate dehydrogenase (malate + NAD → oxaloacetate + NADH + H+). Postharvest Physiology of Edible Plant Tissues 1029 Respiration involves a series of three complex and interconnected metabolic pathways—­ glycolysis, tricarboxylic acid (TCA) cycle, and electron transport system [11] (Figure 16.6). Glycolysis, which occurs in the cytoplasm, results in the production of two molecules of pyruvate from each molecule of glucose. Each of the 10 distinct, sequential reactions in glycolysis is catalyzed by a specific enzyme that performs one of the following actions: add an energy-­containing phosphate group to the substrate molecule, rearrange the molecule, or break down the molecule to a simpler one. The two key enzymes in glycolysis are phosphofructokinase (PFK) and pyruvate kinase (PK). Cells can control their rate of energy production by altering the rate of glycolysis, ­primarily through controlling PFK and PK activity. One of the products of respiration, namely ATP, is used as a negative feedback inhibitor to control the activity of PFK. Glycolysis produces two molecules of ATP and two molecules of NADH from the breakdown of each molecule of glucose. The TCA cycle, which occurs in the mitochondrial matrix, involves the breakdown of pyruvate into CO2 in nine sequential enzymatic reactions. Pyruvate is decarboxylated to form acetate, which condenses with a coenzyme to form acetyl CoA (Figure 16.6). This compound then enters the cycle by condensation with oxaloacetate to form citric acid. Citric acid has three carboxyl groups from which the cycle derives its name. Through a series of seven successive rearrangements, oxidations, and decarboxylations, citric acid is converted back into oxaloacetate, which is then ready to accept another acetyl CoA molecule. In addition to producing the many intermediates that are used in the synthetic reactions of the cell, the TCA cycle also produces one molecule of flavin adenine dinucleotide (FADH2) and four molecules of NADH for each molecule of pyruvate metabolized. The electron transport system, which occurs on membranes in the mitochondria, involves the production of ATP from the high-energy intermediates FADH2 and NADH. In a series of reactions, one NADH molecule produces three ATP molecules, while one FADH2 molecule produces two ATP molecules. The production of ATP depends not only on the energy derived from NADH and FADH2 but also on the chemical environment (pH and ion concentrations) within the cell and mitochondria. In the absence of O2, NADH and FADH2 accumulate in the reduced form. As the oxidized forms (NAD+ and FAD) are consumed, the TCA cycle comes to a halt and glycolysis becomes the sole source of ATP production. Regeneration of NAD+ is absolutely essential for the survival of the anaerobic cell and occurs through the reductive decarboxylation of pyruvate to ethanol in fermentative metabolism. Fermentation, or anaerobic respiration, involves the conversion of hexose sugars into alcohol and CO2 in the absence of O2. Pyruvate produced through glycolysis (reactions that do not require O2) can be converted to lactic acid, malic acid, acetyl CoA, or acetaldehyde. The dominant pathway(s) engaged depends on cellular pH, prior stresses, and the metabolic needs of the cell. Acidification of the cytoplasm enhances the activity of pyruvic decarboxylase, which then shunts pyruvate to form CO2 and acetaldehyde. The acetaldehyde is converted by the enzyme alcohol dehydrogenase to ethanol with the regeneration of NAD+. Two molecules of ATP and 21 kcal of heat energy are produced in anaerobic respiration (alcoholic fermentation) from each molecule of glucose. To maintain the supply of ATP at the aerobic rate, 19 times as many glucose molecules would be needed, and glycolysis would increase 19-fold. However, only two molecules of CO2 are produced during glycolysis, instead of six during aerobic respiration, and therefore the rate of CO2 production increases by only 6.3-fold. The O2 concentration at which a shift from predominantly aerobic to predominantly anaerobic respiration occurs varies among tissues and is known as the anaerobic compensation point (ACP) (Figure 16.7) [12]. ACP represents the O2 concentration below which anaerobic conditions occur. Storage of edible plant tissues just above ACP will typically result in maximum storage periods, but exposure of tissues to extended periods at O2 concentrations below ACP will cause cell death. The oxidative pentose phosphate pathway can also break down sugars to CO2. The first step of the pathway is the irreversible oxidation of glucose-6-P from glycolysis to 6-phosphogluconic acid [8]. The pathway provides a source of ribose-5-phosphate for nucleic acid production, a source of reduced NADP for synthetic reactions, and a means of interconversion of sugars to provide three, four, five, six, or seven carbon skeletons for biosynthetic reactions. Both the pentose phosphate and TCA cycle pathways appear to be operative in harvested plant tissues, but the precise contribution 1030 Fennema’s Food Chemistry Relative respiration rate 100 ACP 80 60 Aerobic respiration 40 Overall respiration Anaerobic respiration 20 0 0 5 10 15 20 Oxygen (%) FIGURE 16.7 Aerobic, anaerobic, and overall respiration rate of a generalized plant product in response to oxygen in the storage environment. The anaerobic compensation point (ACP) represents the lowest oxygen concentration achievable without injurious fermentation and represents the concentration at which the maximum storage potential can be obtained. of each is not well known. However, while in tomato fruit the pentose phosphate pathway accounts for about 16% of the total carbohydrate utilized, in storage root tissues between 25% and 50% of carbohydrate may be oxidized via this pathway [8]. The respiration rate is tightly coupled to the rate of metabolism, and therefore its measurement provides an important method of monitoring the metabolic and physiological state of edible plant tissues. Respiration rates can be very low to very high depending on the product (Table 16.5), and TABLE 16.5 Selected Edible Plant Tissues Classified according to Their Relative Respiration Rates Class Range Respiration (mg CO2/kg · h) at 5°C Very low Low <5 5–10 Moderate 11–20 High 21–30 Very high >30 Intact Dates, dried fruits and vegetables, nuts Apple, beet, celery, cranberry, garlic, grape, honeydew melon, onion, papaya, potato (mature), sweet potato, watermelon Apricot, banana, blueberry, cabbage, cantaloupe, carrot (topped), celeriac, cherry, cucumber, fig, gooseberry, lettuce (head), nectarine, olive, peach, pear, pepper, plum, potato (immature), radish (topped), summer squash, tomato Blackberry, carrot (with tops), cauliflower, leeks, lettuce (leaf), lima bean, radish (with tops), raspberry, strawberry Artichoke, asparagus, bean sprouts, broccoli, Brussels sprouts, endive, green onions, kale, mushroom, okra, parsley, peas, snap bean, spinach, sweetcorn, watercress Fresh-Cut Diced pepper, grated red beet, potato slices Cantaloupe cubes, carrot sticks and slices, onion rings, Peeled garlic, shredded cabbage and head lettuce, squash slices Cauliflower florets, leek rings, cut-salad mixes of leafy lettuces, chicory, endive, arugula, and/or radiccio Broccoli florets, slice mushrooms, shelled peas Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002; Kader, A.A. and Saltveit, M.E., Respiration and gas exchange, in Postharvest Physiology and Pathology of Vegetables, Bartz, J.A. and Brecht, J.K., Eds, Marcel Dekker, New York, pp. 7–30, 2003. 1031 Postharvest Physiology of Edible Plant Tissues TABLE 16.6 Selected Edible Plant Tissues Classified according to Relative Perishability and Potential Storage Life in Air at Near-Optimal Temperature and RH Relative Perishability Potential Storage Life (weeks) Very low Low >16 8–16 Moderate 4–8 High 2–4 Very high <2 Edible Plant Tissue Tree nuts, dried fruits and vegetables, grains Apple and pear (some cultivars), potato (mature), dry onion, garlic, pumpkin, winter squash, sweet potato, taro; bulbs and other propagules of ornamental plants Apple and pear (some cultivars), grape (SO2-treated), pummelo, table beet, carrot, radish, potato (immature) Grape (without SO2 treatment), melons, nectarine, papaya, peach, pepino, plum; artichoke, green beans, Brussels sprouts, cabbage, celery, eggplant, head lettuce, okra, pepper, summer squash, tomato (partially ripe) Apricot, blackberry, blueberry, cherry, fig, raspberry, strawberry; asparagus, bean sprouts, broccoli, cauliflower, cantaloupe, green onion, leaf lettuce, mushroom, pea, spinach, sweet corn, tomato (ripe); most cut flowers and foliage; fresh-cut (minimally processed) fruits and vegetables Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002. respiration rates are sometimes related to the growth stage at harvest (Figure 16.3). While the range of average respiration rates for each product type can be affected by seasonal growing conditions, stage of development at which the product is harvested, and cultivar and postharvest management, the storage life of products is broadly consistent. As a general rule, the rate of deterioration of the many different edible plant tissues after harvest is associated with their respective respiration rates (Table 16.6), as higher respiration rates result in faster use of carbohydrate and other energy reserves in the tissues, thereby resulting in hastened loss of product quality. Products with very high respiration rates such as asparagus, mushroom, parsley, peas, spinach, and sweet corn deteriorate much more rapidly than those such as apple, beet, celery, garlic, grape, honeydew melon, and onion, with low respiration rates. Dried products have extremely low respiration rates and therefore long storage potential under proper storage conditions. Rice, for example, has a very low respiration rate of <1 mg CO2/kg ∙ h at optimum conditions, but rates increase markedly under high moisture and temperature conditions [13]. Importantly, the relationship between respiration rates and maintenance of quality sets inherent limitations to increase storage periods within any particular product type. A product that has a storage life of a week, for example, cannot be extended appreciably compared with one with a storage life of several months. Nevertheless, controlling the rate of respiration is a critical aspect of all postharvest technologies, as any treatments that reduce respiration rates will increase the storage potential. Decreasing the storage temperature is the major technology used to control the respiration of many edible plant tissues. Low O2 and elevated CO2 concentrations will also decrease respiration rates, but O2 levels must remain adequate to support aerobic respiration, and CO2 concentrations should be low enough to prevent injury development. If a fresh fruit or vegetable is kept in a sealed plastic bag, for example, cell death can occur as a result of inadequate O2 and excessive CO2 concentrations accumulating in the atmosphere around the product. Also, “vital heat” resulting from respiration will result in higher temperatures around the commodity and will reduce storage life if not removed by refrigeration or ventilation. Water is also a product of respiration, and uncontrolled loss can result in loss of quality, for example, wilting. The way in which these factors are incorporated into postharvest technologies to maintain quality of edible plant tissues is described in Section 16.7. 1032 16.5 Fennema’s Food Chemistry HORMONES Plant hormones are chemical compounds that potentiate a signaling network and regulate the metabolic systems involved in plant growth and development, and plant responses to biotic and abiotic stresses. The five “classic” plant hormones are ethylene, auxins, gibberellins, abscisic acid, and cytokinins (Figure 16.8), and are commonly regarded as plant growth regulators (PGRs). Many studies on postharvest quality of edible plant tissues have focused on ethylene because of its direct effects on ripening and senescence, and in part because of its ease of measurement. Relative to ethylene, less is known about the involvement of other hormones in ripening and senescence. Another confounding factor in interpreting hormones and their effects is that, while concentrations of hormones are important, the sensitivity of tissues to the hormones is also critical [14]. In this section, the roles of key hormones and their function are briefly outlined. However, it should be recognized that these divisions, while useful for understanding of postharvest processes, are artificial. It has become well recognized that metabolic processes are regulated in a complex way by the crosstalk of several hormones, including the more recently recognized families of plant hormones such as polyamines, nitric oxide, jasmonic acid, brassinosteroids, and salicylic acid [15–17]. Receptor mechanisms have been conclusively identified or discounted, hormone transport processes have been largely elucidated, and the cellular processes downstream of hormone signaling have been painstakingly dissected. The classification of hormones into developmental or environmental response categories has been replaced by mapping of hormonal signaling into transcriptional and post-transcriptional response networks [18]. 16.5.1 Ethylene Ethylene is a naturally occurring plant growth regulator that affects many aspects of growth and development of plants. It is a gas that can exert its effects at very low concentrations, from parts per billion (ppb; nL/L) to parts per million (ppm; µL/L). 16.5.1.1 Ethylene Biosynthesis Ethylene biosynthesis, perception, signal transduction, and regulation at the genetic and biochemical levels have been well documented [19]. In higher plants, ethylene is produced from methionine, which is first converted to S-adenosyl-l-methionine (S-AdoMet, or SAM) by the addition of adenine and utilization of ATP catalyzed by SAM synthetase (Figure 16.9). SAM is an important OH H C H C HO N H H Ethylene CH3 C CH3 HN CH3 CH3 Abscisic acid OH CO OH CH2 Gibberellic acid CH2 H C CH3 C N N COOH O CH3 Auxin (indoleacetic acid) OH O O O H CH2OH N N H Cytokinin (zeatin) FIGURE 16.8 Structures of major plant hormones: ethylene, auxin (indoleacetic acid), gibberellic acid, abscisic acid, and cytokinin (zeatin). 1033 Postharvest Physiology of Edible Plant Tissues 8 α-Keto-γ-methylthiobutyric acid (KMB) 5’ Methylthioribose1-phosphate (MTRP) Glutamate 9 Methionine 1 7 5’ Methylthioribose (MTA) S-Adenosyll-methionine (SAM) 6 5 N-Malonyl-ACC (MACC) 10 2 5’ Methylthioadenosine (MTA) 4 γ-Glutamyl-ACC (GACC) Ornithine (ORN) 3 Ethylene 18 Putrescine (PUT) 11 12 15 Agmatine (AGM) 14 S-Adenosyll-methioninamine (dcSAM) 1-Aminocyclopropanecarboxylate (ACC) Arginine (ARG) 13 16 N-carbamoylputrescine (CPUT) 17 19 Spermidine (SPD) 20 Spermine (SPM) FIGURE 16.9 The ethylene and polyamine biosynthetic pathways, with associated enzymes and reactants. (1) SAM synthase (MET + ATP → SAM + diphosphate (PPi) + phosphate (Pi)), (2) ACC synthase (SAM → ACC + MTA), (3) ACC oxidase (ACC + ½ O2 → Ethylene + CO2 + HCN + H 2O), (4) γ-glutamyltranspeptidase (ACC + glutathione → GACC + Cys-Gly), (5) ACC N-malonyl-transferase (ACC + malonyl coenzyme A → MACC + coenzyme A-SH), (6) MTA nucleosidase (MTA + H 2O → MTR + adenine), (7) MTR kinase (MTR + ATP → MTRP + ADP), (8) spontaneous reaction, (9) transaminase (KMB + amino acid → Methionine + 2-oxo acid), (10) SAM decarboxylase (SAM → dSAM + CO2), (11) SPD synthase (dSAM → SPD + MTA), (12) SPM synthase (dSAM → SPM + MTA), (13) arginase (ARG + H 2O → ORN + urea), (14) ORN decarboxylase (ORN → PUT + CO2), (15) ARG decarboxylase (ARG → AGM + CO2), (16) AGM iminohydrolase (agmatine deiminase) (AGM + H 2O → CPUT + NH3), (17) N-carbamoylputrescine amidohydrolase (amidase) (CPUT+ 2H 2O → PUT + CO2 + NH3), (18) agmatinase (AGM + H 2O → PUT + urea), (19) SPD synthase (PUT + dSAM → SPD + SAM), (20) SPM synthase (SPD + dSAM → SPM + SAM). metabolite, as it is a propylamine group donor in polyamine biosynthesis (Section 16.6.2.1) and a methyl group donor in transmethylation of lipids, nucleic acids, and polysaccharides. In addition, SAM in involved in the first dedicated step of ethylene biosynthesis, the conversion of SAM to aminocyclopropane-1-carboxylic acid (ACC). The enzyme involved in this step, ACC synthase, is normally rate-limiting. The by-product of this step, 5′-methylthioadenosine (MTA), is recycled to methionine via the Yang cycle, thereby allowing the production of ethylene to occur with a small pool of free methionine. ACC is then converted to ethylene by ACC oxidase (formerly known as the ethylene-forming enzyme). ACC can also be metabolized by the action of N-malonyltransferase and γ-glutamyltranspeptidase, to produce malonyl ACC (MACC) and γ-glutamyl-ACC (GACC), respectively. MACC cannot be metabolized back to ACC under physiological conditions. The significance of the conversion of ACC to MACC and GACC remains unclear, but conjugation of ACC may contribute to the regulation of ethylene formation. ACC itself may have an important role as a signaling molecule [20]. Ethylene has to be perceived by the plant cell to exert its action. As a result of the binding, signal transduction occurs via a series of gene expression regulators, resulting in the expression of genes and synthesis of proteins, many of which are important in senescence and ripening. Phenotypic* * Phenotype: visible characteristics. 1034 Fennema’s Food Chemistry Ethylene Ethylene receptors (ETR1, ETR2, ETR3/Nr, ETR4, ETR5, ETR6, ETR7) Protein kinase (CTR1) Nramp-like protein (EIN2) Transcription factors (EILs) Primary responsive genes (ERFs) Activation of ripening-associated genes FIGURE 16.10 Ethylene perception and signaling transduction pathway for the model fruit system: Tomato. Perception of ethylene is via binding to seven ethylene receptor (ethylene response) genes (ETR, ETR2, ETR3/ Never ripe (Nr), ETR4-7). The ethylene signaling cascade ends with transcriptional activation ethylene response factors (ERFs). changes in response to ethylene are determined by (1) the perception of the hormone, (2) the transduction of the signal through gene expression regulators, and (3) the expression of genes and synthesis of proteins sensitive to the received ethylene signal [21]. Perception of ethylene is via binding to receptors (Figure 16.10) on the endoplasmic reticulum. In tomato fruit, seven ethylene receptors (ethylene response) genes (ETR, ETR2, ETR3/ Never ripe (Nr), ETR4–7) have been identified, while there are five in Arabidopsis [19,22]. Differential expression of these genes may regulate ethylene receptivity in different tissues and mediate different biological processes including ripening. Ethylene signaling downstream of the receptors is mediated through a negative regulator constitutive triple response1 (CTR1) MAP kinase kinase kinase gene. The positive regulator ethylene insensitive 2 (EIN2) protein, negatively regulated by CTR1, mediates later steps of ethylene signaling via the EIN3 transcription factors. There is one EIN3 gene in Arabidopsis and four EIL (EIN3-like) genes in tomato (EIL1– 4). The ethylene signaling cascade ends with transcriptional activation ethylene response factors (ERFs). The ERFs then activate secondary response genes that are responsible for ripening. 16.5.1.2 Non-Climacteric and Climacteric Fruits Fruits can be divided into two categories, those that do not produce ethylene as part of ripening and senescence, and those where ethylene production is critical for normal ripening to occur. The two types of fruits have different respiration patterns during maturation and ripening (Figure 16.11). 1035 Postharvest Physiology of Edible Plant Tissues Rate of CO2 production Climacteric peak Non-climacteric Climacteric Climacteric rise Postclimacteric Preclimacteric Preclimacteric minimum Time FIGURE 16.11 Patterns of respiration in a non-climacteric fruit compared with a climacteric fruit. In a nonclimacteric fruit, the respiration rate declines over time, while in a climacteric fruit the rate declined from a pre-climacteric rate to a preclimacteric minimum before increasing to a peak rate. The respiration rates of non-climacteric fruits gradually decrease over time and they do not produce appreciable amounts of ethylene. In contrast, climacteric fruits produce a surge of respiration that is associated with autocatalytic ethylene production. The distinction between non-climacteric and climacteric fruits was originally based on differences in patterns of respiration, but is now often identified by differences in ethylene production. Two ethylene systems are thought to operate. System I ethylene exists in both non-climacteric and climacteric plant tissues as basal ethylene production. It is also responsible for the basal ethylene production in vegetables as well as for ethylene production that occurs as a result of wounding and other (a)biotic stresses. System II ethylene is responsible for the autocatalytic ethylene production that occurs in climacteric fruits. During ripening of climacteric fruit, System II ethylene production is associated with the upregulation of ACC synthase and ACC oxidase genes, as well as increased activities of these enzymes. Transcriptional regulation of ACC synthase is the major control point of ethylene biosynthesis; while ACC oxidase activity is not thought to be rate-limiting during normal ripening, repression of ACC oxidase gene activation in transgenic mutants reduces ethylene production and prevents or impedes ripening [19]. While the broad separation between climacteric and non-climacteric fruits is accepted, there is increasing evidence that such categorization is not absolute. Increases of ethylene production, albeit small, have been detected in the non-climacteric strawberry fruit [15]. Also, both ethylene-dependent and -independent ripening events have been described for climacteric fruits; for example, in melon, ethylene-independent expression of genes encode for flesh color, sugars, acidity, some cell-wall degrading enzymes, and some softening, and ethylene-dependent expression of genes encode for chlorophyll degradation, abscission, aromas, climacteric respiration, some cell-wall degrading enzymes, and the majority of softening [23]. Table 16.7 provides examples of climacteric and non-climacteric fruits. Many popular fruits are listed in the climacteric category, including apples, peaches, plums, and tomatoes. Ensuring low ethylene levels around these fruits can help delay the start of ripening, but normal ripening cannot occur in the absence of ethylene. For example, mature green tomatoes will not develop red color or soften without endogenous or exogenous ethylene. While non-climacteric fruits do not need increased ethylene production in order to ripen, they can be affected, usually negatively, by exposure to exogenous ethylene from ethylene-producing fruits and vegetables, damaged commodities, and contamination. Climacteric fruit can be relatively short- and long-lived, for example, peach and apple, respectively, as can non-climacteric fruits, for example, strawberries and lemons, 1036 Fennema’s Food Chemistry TABLE 16.7 Selected Fruits (Including Vegetable Fruits) Classified according to Climacteric and Non-Climacteric Respiratory Behavior during Ripening Climacteric Non-Climacteric Apple Apricot Avocado Banana Bitter melon Blueberry Breadfruit Cantaloupe Cherimoya Feijoa Kiwifruit Mango Muskmelon Nectarine Peach Pear Plum Tomato Watermelon Blackberry Cacao Cherry (sweet, sour) Cranberry Cacao Cranberry Cucumber Eggplant Grape Lemon Loquat Mandarin Olive Pepper Pineapple Raspberry Strawberry Summer squash Tamarillo respectively. Therefore, while fruits and vegetables vary greatly in the rates of ethylene production (Table 16.8), no clear association with these rates and storage life exists. However, within a fruit type there may be relationships between ethylene production and rate of ripening, with high production associated with rapid softening, for example, McIntosh apples. Stimulated ethylene production can also occur as a result of disease and decay, exposure to chilling temperatures, and wounding (including during fresh-cut processing). In addition, ethylene is produced by internal combustion engines, smoke, and other sources of pollution, and special care has to be taken TABLE 16.8 Fruits and Vegetables Classified according to Ethylene Production Rates Class Production Rate at 20°C (μL C2H4/kg · h) Very low Less than 0.1 Low 0.1–1.0 Moderate High 1.0–10.0 10.0–100.0 Very high More than 100.0 Edible Plant Tissue Artichoke, asparagus, cauliflower, cherry, citrus fruits, grape, jujube, strawberry, pomegranate, leafy vegetables, root vegetables, potato Blackberry, blueberry, casaba melon, cranberry, cucumber, eggplant, okra, olive, pepper (sweet and chili), persimmon, pineapple, pumpkin, raspberry, tamarillo, watermelon Banana, fig, guava, honeydew melon, lychee, mango, plantain, tomato Apple, apricot, avocado, cantaloupe, feijoa, kiwifruit (ripe), nectarine, papaya, peach, pear, plum Cherimoya, mammee apple, passion fruit, sapote Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002. Postharvest Physiology of Edible Plant Tissues 1037 to avoid contamination of sensitive products such as kiwifruit in the handling and storage environment. Other gaseous analogs such as propylene, carbon dioxide, and acetylene can exert similar effects to those of ethylene but only at much higher concentrations. Exposure of fruits and vegetables to ethylene can stimulate respiration and thereby increase use of carbohydrate reserves. Incorporating knowledge about responses of plant tissue to ethylene into postharvest technologies is described in Section 16.7. 16.5.2 Auxins Auxins (Figure 16.8) are the main hormones responsible for cell elongation in phototropism* and gravitropism,† and they control the differentiation of meristem into vascular tissue and promote leaf development and arrangement [24,25]. Auxins affect flowering, fruit set and ripening, and inhibition of abscission.‡ Indoleacetic acid (IAA) is the common form, being derived from tryptophan. Auxin is active at very low concentrations is plant cells; precise control of these concentrations are modulated by the rates of its synthesis, catabolism pathways (IAA oxidase), degradation (e.g., H2O2, light, direct oxidation), conjugation, and transport. Application of synthetic auxins is used to prevent premature drop of fruit such as apples, but can negatively affect fruit quality by stimulating ethylene production. However, in tomatoes, elevated auxin concentrations can delay ripening. Ripening in the non-climacteric strawberry occurs when the auxin concentration decreases to below a threshold level [26]. In strawberries, auxins are produced by the achenes,§ and anthocyanin accumulation and softening is accelerated by achene removal. 16.5.3 Gibberellins Gibberellins (GAs) (Figure 16.8) are a group of about 125 closely related plant hormones that stimulate shoot elongation, seed germination, and fruit and flower maturation. GAs are synthesized in the root and stem apical meristems, young leaves, and seed embryos. GAs also break dormancy in the seeds of plants that require exposure to cold or light to germinate. Other effects of GAs include gender expression, seedless fruit development, and the delay of senescence in leaves and fruits [27]. Maturing grapes are routinely treated with GA to promote larger fruit size as well as looser bunches (longer stems). 16.5.4 Abscisic Acid Abscisic acid (ABA) (Figure 16.8) is associated with responses of whole plants to different kinds of abiotic stresses such as drought, high temperature, chilling, and salinity; however, involvement of ABA in regulating abiotic stress in fruits has rarely been reported [28]. The evidence that ABA is involved in control of ripening is strong [15,28]: (1) a sharp increase in ABA accumulation is observed during the onset of fruit ripening and/or the ripening process in both climacteric and nonclimacteric fruits; (2) ABA application enhances the production of several metabolites that promote ripening; and (3) inhibition of ABA signaling in RNAi-silenced strawberry fruit impedes fruit ripening. Abscisic acid is a strong antagonist of GA action. 16.5.5 Cytokinins Cytokinins (Figure 16.8) are most abundant in growing tissues, such as roots, embryos, and fruits, where cell division (cytokinesis) is occurring. Cytokinins delay senescence and maintain the green color and freshness of many leafy vegetables, and can also delay fruit ripening [27]. * Phototropism: orientation in response to light. † Gravitropism: orientation in response to gravity. ‡ Abscission: natural detachment of plant parts. § Achenes: small, dry one-seeded indehiscent fruit. 1038 Fennema’s Food Chemistry 16.5.6 Other Hormones 16.5.6.1 Polyamines Polyamines (PAs) are small aliphatic amines positively charged at physiological pH in the cell. The diamine putrescine (PUT), triamine spermidine (SPD), and tetraamine spermine (SPM) are present in all living organisms (Figure 16.12). PAs are regarded as PGRs and have been implicated in a wide range of metabolic processes during plant growth and development, including senescence and ripening as well as abiotic and biotic plant stress responses [29]. The importance of PAs is related to their central role in multiple signaling pathways that drive various cellular functions. The intracellular levels of PAs in plants are mostly regulated by anabolic and catabolic processes, as well as by their conjugation with hydroxycinnamic acids. PUT is produced by two alternative pathways (Figure 16.9): from ornithine by the action of ornithine decarboxylase (ODC), or from arginine by the action of arginine decarboxylase (ADC) to agmatine and then by the actions of agmatine iminohydrolase and N-carbamoylputrescine amidohydrolase. The two pathways may be explained by differential compartmentalization of ADC and ODC in the chloroplast and cytoplasm, respectively. PUT is converted to SPD by the symmetrical addition of an aminopropyl residue from decarboxylated S-adenosylmethionine (dcSAM), by the action of SPD synthase. dcSAM is used in the same reaction, but catalyzed by SPM synthase to produce SPM. The free PA pools are modulated by catabolic pathways, degradation, conjugation, and transport. The utilization of SAM represents an important linkage to ethylene biosynthetic pathway, as this substrate also produces ACC (Figure 16.9) and exerts opposite effects on ripening and senescence. PA concentrations are often the highest in the early phases of fruit growth, and decreases in concentration may be signals for fruit ripening, but these relationships are not always consistent [29]. 16.5.6.2 Nitric Oxide Nitric oxide (NO) is a gaseous free radical with a relatively long half-life (3–5 s) compared with other free radicals in biological systems. In mammalian systems, NO is metabolized via the conversion of arginine to citrulline by nitric oxide synthase. It is an important signaling molecule with diverse physiological functions in plant growth and development, including ripening and senescence [16,30]. NO can provoke both beneficial and harmful effects in plant cells, depending on factors such as the local concentration of NO, the rate of synthesis, translocation, effectiveness of removal of this reactive nitrogen species, suppression of reactive oxygen species, as well as its ability to directly interact with other molecules and signals [16,31]. Exogenous NO can increase the storage and shelf-life of climacteric and non-climacteric fruits and vegetables, as well as fresh-cut products by delaying ripening and senescence, and inhibiting chilling injury development [30]. NH2 H2N Putrescine H N H2N NH2 Spermidine H2N H N N H NH2 Spermine FIGURE 16.12 Structures of the diamine putrescine, triamine spermidine and tetraamine spermine. 1039 Postharvest Physiology of Edible Plant Tissues O O O Jasmonic acid OH O COOH O OH O O Methyl jasmonate Salicylic acid OH Methyl salicylate OH H OH H HO H H HO H O O Brassinosteroids FIGURE 16.13 Structures of jasmonic acid, methyl jasmonate, salicylic acid, methyl jasmonate and brassinosteroids. 16.5.6.3 Jasmonic Acid, Brassinosteroids, and Salicylic Acid Jasmonates such as jasmonic acid (JA) and methylJA (Figure 16.13) are important cellular regulators of biological processes including senescence and ripening [15]. Postharvest application of jasmonates to fruits can increase sugar and anthocyanin concentrations, lignin biosynthesis, and ethylene production, and may regulate cell-wall degradation. Salicylic acid (SA), a monohydroxybenzoic acid, and methyl salicylate (MeSA) (Figure 16.13) are found in plants with roles in plant growth and development such as photosynthesis, transpiration, ion uptake and transport, and induction of disease and stress resistance. SA treatments can delay ripening of fruits such as apples, bananas, kiwifruit, mango, peach, persimmon, and tomato, probably by inhibiting ethylene production. However, there is insufficient evidence about endogenous SA changes to prove a role of the hormone during fruit ripening. Brassinosteroids (BRs) are growth-promoting steroids (Figure 16.13) that are now recognized as plant hormones; mutant analysis has demonstrated that the ability to synthesize, perceive, and respond to BRs is essential to normal plant growth and development [32]. While information about their role in ripening and senescence is limited, BRs have been implicated in ripening of grapes, strawberries, and tomatoes. 16.6 16.6.1 COMPOSITION Water Water is the major component of most fruits and vegetables. Fruits and vegetables can be regarded as “water inside pleasing packages” or “water with a mechanical structure”! Therefore, water loss or transpiration is a major factor affecting the quality of fruits and vegetables. In addition to lower saleable weight, loss of water can affect quality in many ways, including wilting, shriveling, flaccidness, soft texture, and loss of nutritional value. The rate of water loss and the impact of this loss varies from product to product. For example, maximum permissible losses can range from 3% for lettuce 1040 Fennema’s Food Chemistry to 10% for onions. Products vary in potential for water loss by morphological differences such as cuticle* thickness and composition and presence or absence of stomata† and lenticels,‡ which are structures that allow gases and moisture to move in or out of the plant. For some products, these differences are affected by developmental stage. 16.6.2 Carbohydrates Carbohydrates are a major constituent of edible plant tissues, making up 50%–80% of the dry ­matter. They can be classified into three general groups: structural, soluble, and storage carbohydrates. Carbohydrates are the predominant energy sources for cells and are the major translocated products of photosynthesis. Once an edible plant tissue is removed from the parent plant, or photosynthesis is interrupted in any way, carbohydrates represent the reserves necessary to maintain cellular function. The carbohydrate composition, especially the balance between complex carbohydrates and simple sugars (and acids), can have a major influence on product acceptability by the consumer. The roles of carbohydrates and their metabolism have been detailed by others [8,29,33–35] and summarized here. 16.6.2.1 Structural Carbohydrates Plant cell walls make up most of the dietary fiber in edible plant tissues. Structural carbohydrates form the foundation of the cell wall that surrounds the cell membrane and provides cells with structural support and protection. The primary cell wall generally is a thin, flexible, and extensible layer formed while the cell is growing. The secondary cell wall forms as a thick layer inside the primary cell wall after the cell is fully grown. The middle lamella forms at the interface between adjacent plant cells and promotes cell-to-cell adhesion. The cell wall is comprised of cellulose and a range of hemicellulosic and pectic polysaccharides. Collectively, these compounds represent approximately 90% of the dry matter in the cell wall. Other components include cell-wall proteins, both structural and enzymatic, mineral ions, and phenolic compounds, such as lignin, in secondary walls. Most cell types in edible plant tissues have non-­lignified primary walls, and usually only small amounts of the secondary wall are ­lignified. Examples of exceptions are the outer layers of wheat grains and parts of wheat bran, in mature asparagus where sclerenchyma fibers are responsible for the tough, stringy texture, and stone cells in pear and feijoas that give the fruits a gritty texture. Cell-wall proteins include extensins, a family of hydroxyproline-rich glycoproteins, which are necessary for cell wall expansion to occur. Extensins are highly abundant and form cross-linked networks in the cell wall. Cellulose, a linear polymer of β-1,4-linked d-glucose residues, is deposited in the wall in the form of crystalline/semicrystalline microfibrils, which result from lateral associations of individual cellulose chains by extensive hydrogen bonding. The resulting crystalline structure is resistant to chemical and enzymatic degradation. The microfibrils are laid down in helical arrays around the cell, and the pattern of their deposition provides control over the direction of cell expansion. Hemicelluloses are mainly composed of several neutral sugars, especially xylose but also include mannose, galactose, rhamnose, and arabinose. Also, in contrast to cellulose, hemicelluloses are branched. The most common matrix glycan in most fruits and vegetables is xyloglucan, a polymer made up of β-1,4-d-glucose with regularly spaced xylose side chains (mostly α-1,6-linked), to which other sugars are attached. Hemicelluloses associate with and noncovalently cross-link cellulose microfibrils. Pectic polysaccharides, or pectins, are linear or branched polymers that are rich in galacturonic acid and can contain up to 17 different monosaccharide types, including substituted species. These polymers are dispersed throughout the primary cell wall, and can form a gel matrix that is co-­extensive with the cellulose–hemicellulose network. Pectic polysaccharides include homogalacturonic acid (HGA), * Cuticle: outer layer of cutin and wax secreted by epidermis. † Stomata: openings in epidermis allowing for gas/vapor exchange. ‡ Lenticels: aggregates of cells forming pores at surface allowing for gas/vapor exchange. 1041 Postharvest Physiology of Edible Plant Tissues rhamnogalacturonan I (RG-I), rhamnogalacturonan II (RG-II), and xylogalacturonan, the ­latter three of which are branched heteroglycans. HGA is a contiguous 1,4-linked α-d-galacturonic acid polymer that is synthesized with a high degree of methyl-esterification at the C-6 position and carries acetyl groups on O-2 and O-3. HGA is found in the primary cell wall, and is also a major component of the middle lamella, where it is less esterified. The degree of esterification affects the ionic charge and calcium binding capacity, as well as availability of sites for enzyme action. The low esterification of HGA in the middle lamella promotes gel formation, and this is thought to be important for cell–cell adhesion. RG-I pectins contain a backbone of the repeating disaccharide of α-d-(1,4)-galacturonic acid and α-(1,2)-rhamnose. Side chains of various neutral sugars branch from many of the rhamnose residues. The neutral sugars are mainly d-galactose, l-arabinose, and d-xylose, with the types and proportions of neutral sugars varying with the origin of pectin. RG-I is also found in the primary cell wall and, generally, to a lesser extent in the middle lamella. The highly branched sidechains have conferred the term “hairy region.” The linkages that integrate the pectin superstructure in the wall include calcium bridges between uronic acid carboxyl groups creating the so-called pectin egg-box, and borate diester bonds spanning two RG-II monomers. There is some evidence that the RGI side chains can be covalently linked to hemicelluloses, forming a super-macromolecular polymeric network. RG-II is a complex, highly branched polysaccharide that is a minor component of primary cell wall and is absent from the middle lamella. Cell walls also contain non-polysaccharide constituents including structural proteins and numerous enzymes, and some cells have specialized walls, such as those with lignified secondary walls, or the walls of epidermal cells, which include large amounts of structural lipids, in the form of a cuticle. The composition of cell walls varies among plant taxa, tissues, cell types, and during development, and can influence the quality of the edible plant tissue. For example, cell walls of various grain tissues and their components have important impacts on the end uses of the grain—in milling, water uptake in the conditioning step and the breakage pattern is influenced by the cell-wall organization and composition in the pericarp-seed coat tissues [36]. The profiles of major components also are generally known. In contrast, the architecture of the cell wall is less understood, although a number of models of their structure have been proposed. These models typically represent the wall structural components, their orientations, interactions, and often their relative abundances, which collectively provide a static view of the overall architecture [37]. While several models have common components, they differ in their bonding interactions and polysaccharide distributions. The most frequently cited models describe the cell wall as composed of two polysaccharide networks [38]. One comprises cellulose microfibrils crosslinked with hemicelluloses (most often xyloglucans or xylans): a simple analogy of this network is that of the steel-and-wire grids in a reinforced concrete slab, while the other network, the pectin polysaccharides, would be the concrete (Figure 16.14). A weakness of all models is that Hemicellulose Pectic polysaccharides Cellulose microfibrils FIGURE 16.14 Idealized cell wall model showing the cellulose microfibrils, hemicellulose and pectic polysaccharides. 1042 Fennema’s Food Chemistry they do not reflect the dynamic nature of the cell wall where biosynthetic and degradative processes are occurring simultaneously, especially during plant growth. While changes in the cell-wall composition occur during normal plant growth and senescence processes, the most dramatic changes are probably those associated with softening and texture changes that occur during the ripening of many fleshy fruits [39]. These changes are often associated with those of color, aroma, and nutritional attributes, which make ripened fruits attractive as food for humans, to animals for seed dispersal, and more subject to decay. Development of a soft edible texture in fruit is often desired by consumers, with examples including avocados, kiwifruit, and pears, although softening is more limited in fruit such as apples. How cells fail in response to applied stresses such as mastication can greatly affect sensory perception, a good example being a mealy versus crisp apple. If the middle lamella is weaker than the cell wall, the cells will separate from each other, while if the middle lamella is stronger than the cell wall, the cell walls will fail. In the former case, the tissue will taste mealy compared with juicy in the latter case. Fruit texture is influenced by the structural integrity of the primary cell wall and the middle lamella, accumulation of storage polysaccharides such as starch, and the turgor pressure within cells resulting from osmosis. Changes in turgor pressure can have major effects on texture of some fruits (e.g., citrus), and hydrolysis of starch in mango and banana is associated with loosening of the cell wall structure. However, ripening-related changes in wall architecture generally involve degradation of the primary wall and middle lamella polysaccharides and alterations in the bonding between polymers, resulting in cell separation and wall swelling. Most polysaccharides undergo depolymerization, although the relative extent of pectin and hemicellulose degradation varies considerably between species, and it is not clear to what degrees cellulose is degraded. Similarly, there is some debate regarding which, if any, components of the wall are the first to undergo modification. Some reports suggest that the earliest events to be initiated are the loss of pectic galactan side chains and the depolymerization of matrix glycans, which may occur in the early ripening stages, followed by a loss of pectic arabinan side chains and pectin solubilization from the wall. The depolymerization of pectins may begin during early to midripening, but is usually most pronounced late in ripening [39]. However, some of these events may be absent or occur to limited extents in some species. During the ripening of various fruits, pectin depolymerization is absent to low in apple, banana, melon, pepper, strawberry, papaya, and watermelon, but moderate to high in avocado, peach, and tomato. Pectin solubilization is absent to low in apple and watermelon, but moderate to high in avocado, banana, blackberry, kiwifruit, plum, and tomato. Loss of pectic galactan and arabinan side chains also vary by the fruit type [39]. In addition, cell-wall swelling may be related to a loosening of the hemicellulose–cellulose ­network and to pectin solubilization, and these processes combined with the loss of pectic side chains increase wall porosity. Indeed, it has also been proposed that one of the early events in ripening is a decrease in the hydrogen bonding between hemicelluloses and cellulose microfibrils. An increase in porosity of the walls as a result of pectin depolymerization may allow increased access of degradative enzymes to their substrates. The importance of cuticle properties as an additional factor affecting fruit firmness has become increasingly recognized [40,41]. Cuticles can influence water loss, and hence cellular turgor, as well as play a central role in sensing and interaction of the edible plant product with the surrounding environment. A wide range of enzymes and nonenzymatic proteins mediate cell-wall changes during fruit ripening. One of the best studied in this regard is polygalacturonase (PG), which hydrolyzes demethylated HGA. Manipulation of activity by gene silencing or overexpression of the PG gene in tomato fruit has shown that it is not solely responsible for softening [19]. It is now clear that a suite of cell-wall-modifying enzymes is likely involved, including pectin methylesterases, pectate lyase, arabinanases and galactanases, and a spectrum of glycosidases, as well as the wall-modifying (“loosening”) protein expansin, which appears to act nonenzymatically. The exact contribution of 1043 Postharvest Physiology of Edible Plant Tissues each enzyme, or how they act synergistically, is not well defined. Moreover, their relative importance probably varies in accordance with the specific compositions of the cell walls and diverse array of textural states in fruits of different species, which in turn is associated with the difference in texture qualities of different fruits. 16.6.2.2 Soluble Carbohydrates Soluble carbohydrates in edible plant tissues are primarily the monosaccharides glucose and fructose and the disaccharide sucrose, which are found in different amounts and proportions in fruits and vegetables (Table 16.9). Other soluble carbohydrates found in varying amounts in certain species include xylose, mannose, arabinose, galactose, maltose, stachyose, and raffinose. Sugar alcohols such as sorbitol, can occur in significant amounts in fruits of the Rosaceae family such as apple, peach, and cherry. Sucrose is the primary soluble carbohydrate transported into the cells from leaves and other photosynthetic tissues. Other translocated compounds include sorbitol in the Rosaceae, mannitol in celery, and raffinose and stachyose in squash and muskmelon. Soluble carbohydrates are also derived from storage carbohydrates and processes such as cell-wall breakdown. The soluble carbohydrates are used in metabolic processes such as glycolysis or for secondary metabolic pathways including accumulation of storage compounds such as starch. In addition, low molecular weight carbohydrates contribute to the characteristic flavor and quality attributes of many edible plant products. The enzymes associated with metabolism of soluble carbohydrates are invertases (acid, alkaline, and neutral), sucrose synthase, and sucrose phosphate synthase. Invertases convert sucrose into glucose and fructose, sucrose synthase converts UDP-glucose and fructose into sucrose and UDP, while sucrose phosphate synthase converts UDP-glucose and fructose-6-P to sucrose-6-P and UDP. The role of these enzymes can shift depending on species, stage of development, and during postharvest storage (discussed in following section) [34]. TABLE 16.9 Major Soluble Sugars (mg/g FW) in Selected Fruits and Vegetables Product Sucrose Glucose Fructose Asparagus Cabbage Carrot Cucumber Eggplant Muskmelon Onion – sweet Potato Spinach Strawberry Sweet cherry Sweet potato Table grape Tomato 0.3–3.0 0.2–4.0 34–45 *tr–1.0 *tr–4.2 24–90 8.0–29 0.4–2.4 *tr–1.0 5.0–16 4.4–20 19–47 0.7–29 *tr–1.0 5.5–10 14–17 1.0–11 6.7–12 14–20 7.0–25 13–25 0.2–3.0 0.1–1.2 20–22 61–161 0.5–2.3 55–77 8.9–22 8.2–14 9–22 3.9–15 8.0–12 14–20 8.0–22 9.4–24 0.05–1.4 0.1–5.1 23–26 54–102 0.9–4.0 68–85 11–16 Sources: Modified from Maness, N. and Perkins-Veazie, P., Soluble and storage carbohydrates, in Postharvest Physiology and Pathology of Vegetables, Bartz, J.A. and Brecht, J.K., Eds, Marcel Dekker, New York, pp. 361–382, 2003; Lee, C.Y. et al., NY Food Life Sci. Bull, New York State Agricultural Experiment Station, Geneva, 1, 1–12, 1970; Paul, A.A. et al., J. Human Nutr., 32, 335, 1978; Li, B.W. et al., J. Food Comp. Anal., 15, 715, 2002. * tr = trace. 1044 Fennema’s Food Chemistry 16.6.2.3 Storage Carbohydrates Starch is an insoluble homoglucan composed of two polymers of glucose, amylopectin and amylose, the major storage carbohydrates in edible plant tissues. Amylopectin and amylose (Figure 16.15) together form semicrystalline, insoluble granules with an internal lamellar structure [42] (Figure 16.16). Amylopectin is the major component of starch, typically making up 75% or more of the starch granule and is responsible for its granular nature. Amylopectin is a large, branched molecule, with glucosyl residues linked by α-1,4-bonds to form chains of between 6 and >100 glucosyl residues in length. The α-1,4-linked chains are connected by α-1,6-bonds (branch points). The lesser abundant amylose is a linear structure comprised only of α-1,4-d-glucose units. The semicrystalline structure makes up the bulk of the matrix of the starch granule and is highly conserved in higher plant starches [2]. As the principal storage carbohydrate, starch plays important roles during the life cycle of the plant. In leaves, a fraction of the carbon assimilated through photosynthesis is retained in the chloroplasts as starch rather than being converted to sucrose for export to the sites of growth or organs that serve as sinks to accumulate sugars and/or storage carbohydrate. This transitory starch is degraded at night to provide substrates for leaf respiration and for continued sucrose synthesis for export to the rest of the plant. In non-photosynthetic organs such as stems, roots, tubers, and CH2OH CH2OH CH2OH OH CH2OH O OH O OH OH O O OH CH2OH OH x O OH O OH O OH OH OH O OH y OH CH2OH O OH O OH Amylose O OH CH2 OH CH2OH OH O O OH x O OH OH Amylopectin FIGURE 16.15 Structures of amylose and amylopectin. Amorphous Pseudo-crystalline band Amorphous band Starch chains 9 μm 1–100 μm FIGURE 16.16 Organization of starch within a starch granule. (From Mishra, S. et al., Food structure and carbohydrate digestibility, in Biochemistry, Genetics and Molecular Biology “Carbohydrates - Comprehensive Studies on Glycobiology and Glycotechnology,” C-F. Chang, Ed, InTech, Rijeka, Croatia, 2012.) 1045 Postharvest Physiology of Edible Plant Tissues seeds, sucrose may be converted to starch for longer term storage, often to high levels, in specialized plastids termed amyloplasts. Many climacteric fruits such as apples and bananas also accumulate starch during maturation, and hydrolysis of this starch to sugars is an important part of the ripening process. Postharvest technologies can also lead to undesirable changes in sugar–starch balance. Potato storage at less than 10°C can cause transformation of some starch to soluble sugar, which results in undesirable darkening of chips and French fries via the Maillard reaction [43]. In sweet corn, warm storage temperatures can lead to net starch biosynthesis and diminution of soluble sugar levels, resulting in loss of desired sweetness. In higher plants, starch is synthesized in plastids in both photosynthetic and nonphotosynthetic cells. Biosynthesis of both transitory and reserve starch is regulated by events involving interactions between metabolites and enzymes present in both the cytosol and plastids [2,44]. While aspects of starch biosynthesis are still being debated, classic models for photosynthetic and heterotrophic* cells are available [44,45]. In photosynthetic plant tissues, starch is the end product of a pathway directly linked to the Calvin cycle by means of plastid phosphoglucose isomerase (PGI), which exclusively takes place in the chloroplast. Photosynthetically synthesized triose-phosphate is exported to the cytosol and converted to sucrose, which is transported to heterotrophic parts of the plant. The mechanism of starch biosynthesis in both chloroplasts and amyloplasts has generally been considered to be a unidirectional and Glycolysis TCA cycle F6P 3 Fructose 5 UDP UTP ATP ADP 6 G6P G6P 4 G1P 2 9 3PGA 8 Pi Sucrose G1P ATP 10 PPi PPi 11 2Pi ADPG UDP-glucose (UDPG) 1 Sucrose 7 ADP 12, 13, 14 Starch Phloem Cell wall Cytosol Amyloplast FIGURE 16.17 Schematic representation of the conversion of sucrose to starch in heterotrophic tissues of dicotyledonous plants. (1) Sucrose synthase (sucrose + UDP → UDP-glucose + fructose), (2) UDP-glucose pyrophosphorylase (UDP-glucose + PPi → G1P + UTP), (3) Fructokinase (fructose + UTP → F6P + UDP), (4) Cytosolic phosphoglucomutase (F1P → G6P), (5) Phosphoglucoisomerase (G6P → F6P), (6) Hexose phosphate translocator, (7) Adenylate translocator, (8) Triose phosphate translocator, (9) Plastidic phosphoglucomutase (G6P → G1P), (10) ADP-glucose pyrophosphorylase (ATP + G1P → PPi + ADP-glucose), (11) Alkaline pyrophosphate (PPi → 2Pi), (12) Granule-bound starch synthase (ADP-glucose + (1,4-α-d-glucosyl)n → ADP + (1,4-α-d-glucosyl)n+1), (13) Soluble starch synthase (ADP-glucose + (1,4-α-d-glucosyl)n → ADP + (1,4-α-D-glucosyl)n+1), (14) Branching enzyme. * Heterotrophic: requiring complex sources of N and C for metabolic synthesis from other tissues because it cannot synthesize its own. 1046 Fennema’s Food Chemistry vectorial process wherein ADPG pyrophosphorylase (AGPase) exclusively catalyzes the synthesis of ADPG and PPi, and acts as the major rate-limiting step of the gluconeogenic process. In heterotrophic tissues, sucrose and UDP are transformed by sucrose synthase (SS) to produce UDPglucose (UDPG) and fructose. UDPG is then converted to glucose-1-P (G1P) by UDPG pyrophosphorylase (UGPase), and G1P is subsequently metabolized to glucose-6-phosphate (G6P) by means of the cytosolic phosphoglucomutase (PGM). G6P then enters the amyloplast, where it is converted to starch by the sequential activities of plastid PGM, AGPase, and starch synthase [44,45] (Figure 16.17). Starch hydrolysis involves the conversion of the insoluble semicrystalline matrix formed by amylopectin to glucose and G1P, which can feed into intermediary metabolism. Degradative reactions take place in photosynthetic tissues where biosynthesis occurs and therefore requires metabolic coordination, while in heterotrophic tissues biosynthesis and hydrolysis processes are often temporally separated. Starch hydrolysis requires the coordinated action of several enzymes at the starch granule surface, the major ones being α-amylase, β-amylase, phosphorylase, and α-glucosidase (maltase) (Figure 16.18). α-Amylases hydrolyze the α-(1–4) linkages of amylose to release oligosaccharide fragments of ~10 glucose subunits (maltodextrins), which are more slowly hydrolyzed exhaustively to maltose [8]. α-Amylases also hydrolyze α-(1–4) linkages of amylopectin, but is not active in regions of the α-(1–6) branch points, leaving limit dextrins (2–10 glucosyl units). β-Amylase hydrolyzes maltose units (two glucose units) from the nonreducing end of starch chains to yield maltose and limit dextrins. Starch phosphorylase attacks the α-(1–4) linkages to form G1P, incorporating P i. α-Glucosidase catalyzes the hydrolysis of maltose to glucose. Starch 1 Maltose 4 3 2 Glucose Glucose-1-P (G1P) 5 6 Glucose-6-P (G6P) 7 Fructose-6-P (F6P) Glycolysis or sucrose synthesis FIGURE 16.18 Pathways of starch breakdown. (1) β-amylase (starch + nH2O → n maltose), (2) α-glucosidase syn. maltase (maltose + H2O → 2 glucose), (3) α-amylase (starch → glucose), (4) starch phosphorylase (starch + n Pi → n G1P), (5) hexose kinase (glucose + ATP → G6P + ADP), (6) phosphoglucomutase (G1P → G6P), (7) phosphohexose isomerase (G6P → F6P). 1047 Postharvest Physiology of Edible Plant Tissues OH O O OH O OH O HO Malic acid OH O O HO OH O OH Tataric acid HO O O OH O OH Citric acid HO OH OH O OH HO OH Isocitric acid O Oxalic acid FIGURE 16.19 Structures of major organic acids found in edible plant tissues. 16.6.3 Organic Acids The most common organic acids in edible plant tissues are mono-, di-, or tri-carboxylic acids (Figure 16.19). Organic acids may be in the free acid or conjugate base (anionic) forms, as salts or chelates with cations, or covalently combined with other metabolites to form esters or glycosides. Metabolic functions of organic acids are many, including as intermediates in the TCA cycle (Section 16.3.1), as metabolites in photosynthesis (phosphoglyceric acid), and as respiratory substrate where they are found in high concentrations to serve as a source of energy for postharvest cellular function. Organic acids also accumulate in the vacuoles of edible plant tissues, especially in fruits where they contribute to flavor profiles and turgor pressure. In general, the accumulation of acids in vegetables is less pronounced. Organic acid accumulation is typically associated with less mature fruits, and decreasing acidity is a common feature of ripening. Exceptions exist, an example being the accumulation of acidity during on-tree ripening of sweet cherries [46]. The type of acid(s) and their relative proportion varies widely among and within fruit species, for example, a range from 0.4% to 1.7% and 0.7% to 1.6% in cultivars of tomato and plum, respectively [29]. Predominant acids in some edible plant tissues include malic acid in apples, apricot, artichoke, broccoli, cauliflower, onion, plum, nectarines, peach, loquat, pomegranate, and sweet cherry; citric acid in citrus fruits, tomato, persimmon, blueberry, strawberry, and mango; isocitric acid in blackberry; tartaric acid in avocado and grape; and oxalic acid in spinach and rhubarb. Tartaric acid is important in table and wine grapes, and the organoleptic properties and aging potential of wines are intimately linked to its concentration in the fruit, as well as that added during vinification. Other important organic acids include chlorogenic acid and ascorbic acid, discussed in relation to phenolics (Section 16.6.4) and antioxidative metabolism (Section 16.6.9.1), respectively. Organic acids are largely responsible for the sensory perception of tartness and sourness in both fruits and vegetables. However, the balance between sugars and acids is often a more important factor in taste perception rather than absolute concentrations of either set of compounds alone. If sugar concentrations are high, the perception of acidity can be diminished. Citric acid can mask the perception of sucrose and fructose, while malic acid enhances sucrose perception [47]. Organic acids also exist as esters with alcohols to yield characteristic tastes and aromas of fruits and vegetables. Esters are especially responsible for the aroma of many fruits, including apples, pears, bananas, pineapples, and strawberries. An extensive range of esters are found, and the predominant forms in some fruits are ethyl, propyl butyl, amyl, hexyl, and isobutyl acetates, butyl and amyl butyrates, and butyl propionates in apple, and methyl and ethyl butanoates and hexanoates in strawberry. Some of these alcohol and organic acid components are derived from amino acid catabolism. Malic acid synthesis occurs by the sequential action of phosphoenolpyruvate carboxylase and NAD-malate dehydrogenase, while degradation to pyruvate plus CO2 is catalyzed by the NADPmalic enzyme. Interestingly, the patterns of malic acid accumulation and degradation do not correspond with the typical classification into climacteric or non-climacteric fruits or the overall changes 1048 Fennema’s Food Chemistry in respiration rates [15]. Certain types of climacteric fruits use malate during the respiratory burst, while others continue accumulating malate throughout ripening. Malic acid metabolism is important for transitory starch metabolism during fruit development [48]. Citric acid is synthesized by the condensation of acetyl CoA with oxaloacetate, catalyzed by citrate synthase in the mitochondria via the TCA cycle and stored in vacuole. Degradation of citric acid occurs mainly in the cytosol, catalyzed by a cascade of enzymes, including aconitase, isocitrate dehydrogenase, glutamate decarboxylase, and glutamine synthase. Citrate is sequentially metabolized to isocitrate, 2-oxoglutarate and glutamate, and glutamate utilized for glutamine production and catabolized through the γ-aminobutyrate (GABA) shunt (Section 16.6.5) [49]. In addition to isomerization by cytosolic aconitase, citrate can be degraded by ATP-citric lyase to oxaloacetate and acetyl-CoA in cell cytosol to support synthesis of amino acids, fatty acids, isoprenoids, and other metabolites [50]. Tartaric acid synthesis from ascorbic acid is a catabolic reaction that proceeds via the conversion of ascorbic acid to 2-keto l-idonic acid, with successive reduction to l-idonic acid and oxidation to 5-keto d-gluconic acid. In the penultimate step, 5-keto d-gluconic acid is cleaved between carbons 4 and 5 to yield the four-carbon l-threo-tetruronate, which is oxidized spontaneously to form tartaric acid [51]. 16.6.4 Phenolics Phenolic compounds are plant secondary metabolites that affect the appearance (color), taste, and flavor of edible plant tissues. Phenolics are also strong antioxidants due to the electron delocalization over the aromatic ring and their high redox potential, which allows them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers [29]. Consequently, phenolics are recognized for their health-promoting properties such as anti-platelet, antioxidant, and anti-inflammatory activities [52]. There is a large variety in phenolic structure and occurrence, including very simple phenolics such as hydroxybenzoic acids, as well as large polymers such as condensed tannins and ­hydrolysable ­tannins with high molecular weights. Phenolic compounds include oleuropein and related compounds, hydroxybenzoic acid derivatives, cinnamates, flavonoids (flavones and isoflavones, flavanones, Erythrose-4-phosphate Phosphoenolpyruvate Tryptophan Shikimate Isochorismate Chorismate Phenylalanine Salicylic acid Tyrosine Cinnamic acid Stilbenes Lignans Lignin Simple phenols Tannins Suberin, cutin Chalcone FIGURE 16.20 Favonoids (flavones, flavonols isoflavones, anthocyanidins) Simplified pathway of phenolic acid synthesis and derived compounds. Postharvest Physiology of Edible Plant Tissues 1049 flavonols, and flavanols), lignans and stilbenes, anthocyanins, chalcones and dihydrochalcones, proanthocyanidins and tannin-like compounds, and ellagitannins. Most phenolic compounds are synthesized from phosphoenolpyruvate (from glycolysis) and ­erythrose-4-phosphate (from the pentose phosphate pathway) through shikimate in the shikimic acid pathway. 3-Deoxyarabino-heptulosonate 7-phosphate is biosynthesized by the corresponding synthase, which is the key enzyme that controls the carbon flow into the phenolic metabolite pathway (Figure 16.20). The aromatic amino acid phenylalanine is deaminated by the enzyme phenylalanine ammonia-lyase (PAL), the key enzyme in phenolic biosynthesis. PAL catalyzes the non-oxidative deamination of l-phenylalanine to form trans-cinnamic acid and a free ammonium ion. This reaction is the first step in the biosynthesis of a large range of phenylpropanoid-derived secondary products in plants, such as flavonoids and isoflavonoids, coumarins, lignins, wound-protective hydroxycinnamic acid esters, and other phenolic compounds. Regulation of PAL activity therefore is important in modulating phenylpropanoid biosynthesis in plants. The involvement of phenolics in postharvest physiology of edible plant tissues is extensive because of the aforementioned roles in appearance, taste, and flavor, and is discussed in Sections 16.6.7 through 16.6.9. However, an important additional involvement of phenolic compounds is in browning in fruits and vegetables. Enzymatic oxidation of phenolic compounds occurs by the action of polyphenol oxidase (PPO), which is mainly located in plastids in higher plants. PPO catalyzes two different reactions in the presence of molecular oxygen: the hydroxylation of monophenols to o-diphenols (monophenolase, cresolase, or hydroxylase activity) and the oxidation of o-diphenols to o-quinones (diphenolase, catecholase, or oxidase activity). The o-quinones nonenzymatically polymerize and give rise to heterogeneous black, brown, or red pigments commonly called melanins. Melanins are usually detrimental to product quality. Examples include browning of cut surfaces by wounding either by inappropriate handling (e.g., bruising) or deliberate activities (fresh-cut products), physiological disorders such as senescent breakdown of apples, or injuries by inappropriate postharvest temperature or atmosphere management. 16.6.5 Proteins and Amino Acids Proteins are critical to plant function as they regulate metabolism via their enzymatic functions in the cytoplasm, membranes, and cell walls. These enzymes are synthesized, activated, and/or degraded during the many normal cellular functions, as well as mediating responses to environmental changes, and changes in growth and development such as ripening and senescence. In addition to these catalytic proteins, others can have structural, regulatory, or storage functions. Proteins can also be lipoproteins (lipid prosthetic group), nucleoproteins (nucleic acid), metalloproteins (metal), or glycoproteins (carbohydrate). Free amino acids (protein and non-protein) and their water-soluble derivatives can play a major role in the storage of nitrogen for subsequent growth and development by plants. For example, they can account for 5% or more of the dry weight of a legume seed, for example, broad bean (arginine), or as in the seeds of lentils (γ-hydroxyarginine). Plants store proteins in the embryo and vegetative cells to provide carbon, nitrogen, and sulfur resources that are critical to the life cycle of plants. Mechanisms for protein storage and mobilization serve many different developmental and physiological functions. Storage proteins in cereals and grains are major contributors to human diets [53], but high-­ protein nuts such as peanuts, walnut, almonds, cashews, and Brazil nuts can also be important. While fruits typically have low protein concentrations, vegetables such as lentils, edamame, chickpeas, beans, green peas, corn, asparagus, and potatoes can have higher levels, though still relatively small percentages of the product composition. 1050 Fennema’s Food Chemistry Several types of non-protein nitrogen compounds are found in edible plant tissues, where, in addition to being a storage protein, they can act as a growth inhibitor and in defense against insects and herbivores. These compounds include • Canavanine (2-amino-4-guanidinoxybutanoic acid) in beans [54]. • Glycine betaine and proline, which are two major organic osmolytes that accumulate in a variety of plant species in response to environmental stresses such as drought, salinity, and extreme temperatures [55]. Both compounds are thought to have positive effects on maintaining enzyme and membrane integrity along with adaptive roles in mediating osmotic adjustment in plants. • Dopamine is found in a variety of food plants, with the highest concentrations in bananas, where the fruit pulp of red and yellow bananas contains concentrations of 40–50 ppm by weight [56]. Potatoes, avocados, broccoli, and Brussels sprouts may also contain dopamine at levels of 1 ppm or more, while oranges, tomatoes, spinach, beans, and some other plants contain less than 1 ppm. • Alkaloids are nitrogenous organic compounds, the most well known in edible plant tissues being solanine [57]. This glycoalkaloid is toxic and contained in leaves, fruits, and tubers of nightshade plants (Solanaceae) such as tomato, potatoes, and goji berries. Another non-protein amino acid, γ-aminobutyric acid (GABA), is found in a wide range of organisms. GABA has been characterized as a neurotransmitter or neuromodulator in the central nervous system of animals, but plays different roles in plant metabolism including carbon–nitrogen metabolism, energy balance, signaling, and development. GABA can accumulate under both biotic and NAD+ NADH 1 α-Ketoglutarate Glutamate (GLU) 2 CO2 TCA cycle γ-aminobutyric acid (GABA) α-Ketoglutarate Glutamate Succinic semialdehyde (SAA) 5 Succinate NADH 4 NAD+ 3 Pyruvate Alanine NADPH 6 NADP+ γ-Hydroxybutyric acid (GHB) FIGURE 16.21 The γ-aminobutyric acid (GABA) shunt. (1) Glutamate dehydrogenase (α-ketoglutarate + NH4+ + NADH → GLU + NAD+), (2) glutamate decarboxylase (GAD) (GLU → GABA + CO2), (3) GABA transaminase-TP (pyruvate dependent; GABA + pyruvate → SAA + alanine), (4) GABA transaminase-TK (α-ketoglutarate dependent; GABA + α-ketoglutarate → SAA + glutamate), (5) succinic semialdehyde dehydrogenase (SAA + NAD+ → succinate + NADH), (6) succinic semialdehyde reductase (SAA + NADPH → GHB + NADP+). Postharvest Physiology of Edible Plant Tissues 1051 abiotic stresses including anoxia, chilling, drought, salinity, and mechanical damage [58]. Recently, it has been found that GABA accumulates in fruits in response to postharvest treatments such as elevated CO2 and during development of physiological disorders. Also, exogenous GABA treatments may inhibit physiological disorders such as chilling injury. GABA is produced primarily via the GABA shunt (Figure 16.21). The GABA shunt is so intimately related to the TCA cycle that it might be considered a single entity with a major role in primary C/N metabolism [59]. Glutamate derived from the TCA cycle is decarboxylated to produce GABA and CO2 via pH- and calmodulin-dependent glutamate decarboxylase (GAD) activity in the cytosol. GABA is then transported to the mitochondria. GABA is then catabolized first by the activity of GABA transaminase, which can use either pyruvate (-TP) or α-ketoglutarate (-TK) to produce succinic semialdehyde (SAA) and alanine or glutamate, respectively. SAA is then catabolized by the NAD+-dependent enzyme succinic semialdehyde dehydrogenase to produce succinate and NADH, which are used for the mitochondrial respiratory pathway. Succinic semialdehyde dehydrogenase is highly sensitive to mitochondrial energy status, and activity would be inhibited under stress conditions in which the NAD+: NADH ratios are low. SAA would accumulate, resulting in feedback inhibition of GABA transaminase activity. SAA is also catabolized to γ-hydroxybutyric acid (GHB) through SAA reductase activity. Production of SAA may function in stress tolerance through detoxification of SAA. Another pathway for the production of GABA is from polyamines by the oxidation of putrescine [58]. 16.6.6 Lipids Lipids are a diverse group of hydrophobic compounds comprised of long hydrocarbon chains and often ester linkages in the molecule. Lipids have critical functions in whole plants and detached tissues where they have structural and metabolic roles as components of cellular membranes, oil bodies, and cuticular waxes. There are four general types of lipids in plants: triacylglycerols, phospholipids, waxes, and isoprene-derived lipids (See Chapter 4). Triacylglycerols are comprised of three fatty acids esterified to a glycerol molecule, are the most energy-rich form of food reserve, and exist in tissues as oil droplets or lipid bodies. Membrane lipids are comprised primarily of diacylglycerol esters with the third OH group of glycerol esterified to a polar group such as a carbohydrate/sugar (yielding a glycolipid) or (organo) phosphate compound (­yielding a phospholipid). The galactosyl-glycolipids are major and sometimes dominant lipids in the membranes of plastids (chloroplast, chromoplasts, amyloplasts); they are particularly abundant in grains such as wheat, and are believed to contribute to gluten development in doughs. Sterols and other isoprene-derived lipids such as tocopherols and carotenoids are found as part of membranes to provide necessary functionality for different tissue types. Cuticular lipids are a complex mix of hydrocarbons and esters of long-chain aliphatic acids (including diacids and oxygenated acids) and alcohols embedded in a lipid polymer called cutin. Waxes and cutin make up the cuticle, which regulates water and gas transmission between the plant and the environment. Also, while lipid concentrations are low in most edible plant tissues (Table 16.1), lipids represent storage compounds that can be used as a source of energy in fruits such as avocados and olives, cereal grains, and in seeds such as peanut and walnut [8]. Fats are more highly reduced than starch and provide almost twice the energy on a per-weight basis. When fatty acids are removed from glycerol, they can undergo β-oxidation to yield energy. The lipid composition of membranes determines their physical properties and functionality, and has a major role in quality maintenance of edible plant tissues. Phospholipids, mainly phosphatidylcholine and phosphatidylethanolamine, comprise most of the lipid matrix in many plant cell membranes. The fluidity of the lipid bilayer is determined largely by the fatty acid composition and positional distribution of the phospholipids. Membranes have barrier properties to water and ions, maintain compartmentalization of various cellular functions, and contain enzymes specific to these cellular functions. Changes in the quality of harvested plant tissues are greatly affected by 1052 Fennema’s Food Chemistry membrane function, as catabolism of membranes is a critical part of senescence. Water loss and the consequent loss of turgor pressure, which results in wilting and undesirable texture changes, are in part attributable to increased permeability of the plasmalemma (cell membrane) and tonoplast (vacuolar membrane). Responses of tissues to low storage temperatures, and especially susceptibility to chilling injury, are strongly associated with lipid composition of membranes. Generally, plant tissues with more fluid membranes (greater degree of unsaturation) are able to withstand lower, nonfreezing temperatures. Lipid peroxidation of membranes appears to be an integral part of ripening and senescence, and under stress conditions such as chilling-injury-inducing temperatures. Peroxidation can occur both as a part of the cascade of phospholipid catabolism and as a result of free-radical-mediated reactions initiated by reactive oxygen species. Lipid peroxidation and membrane deterioration occurs if the antioxidative defense systems are compromised, resulting in adverse effects on enzymatic activity and physical barrier properties of the membranes. These processes are similar for normal ripening, senescence, and in response to imposed stress conditions, and include one or more of the following: (1) a general decline in the glycerolipid fatty acid unsaturation due to lipid peroxidation and/or decreased unsaturation, (2) a change in the content and proportions of the phospholipid and galactolipid classes, (3) an accumulation of destabilizing lipid catabolites and peroxidation products, and (4) an increase in the level and/or changes in composition and conjugation of sterol lipids [60]. As components of waxes, cutin, and suberin, lipids also are critical components of plants and affect the storage behavior and shelf-life of many edible plant tissues. Waxes and cutin are found in the cuticle, provide protection from dehydration and pathogens, and influence gas diffusion from air to the inside of the product. Suberin is a lipid-derived polymeric material found on underground plant parts and on healed surface wounds. Like cutin, suberin is often embedded with waxes [8], and also provides protection from dehydration and pathogens. 16.6.7 Pigments The pigments found in edible plant tissues include a wide array of compounds that have essential roles in photosynthesis and/or provide visual attraction for animals and insects. Four primary classes of pigments based on their chemistry are chlorophylls, carotenoids, flavonoids (particularly anthocyanins), and betalains. Chlorophylls are the primary pigments in plants, the function of which is to capture light energy and convert it into chemical energy (carbon assimilation). It is the presence and relative abundance of chlorophyll that gives plants their green color. The green color of many leafy vegetables and some fruit, for example, green apples, is a major indicator of freshness and quality for the consumer. Therefore, postharvest technologies are designed to prevent loss of greenness. On the other hand, loss of green color and development of red, yellow, and orange coloration is a quality indicator for other products such as tomato, banana, pears, and citrus. Carotenoids, which are yellow, orange, or red tetraterpenoids, function as accessory pigments in plants, helping to fuel photosynthesis by gathering wavelengths of light not readily absorbed by chlorophyll. Carotenoids also protect the chlorophylls from photooxidation and have other important functions in plants, such as precursors to abscisic acid (ABA). Plants, in general, contain six ­ ubiquitous carotenoids: neoxanthin, violaxanthin, antheraxanthin, zeaxanthin, lutein, and ß-­carotene, together with the main two chlorophylls (Chl) Chl a and Chl b. The most familiar carotenoids in plants are ß-carotene (an orange pigment), lutein (a yellow pigment found in fruits and vegetables and the most abundant carotenoid in plants), and lycopene (the red pigment responsible for the color of tomatoes). The pigment composition and the resulting color are characteristic of the species and cultivar, but carotenoid composition and relative abundance in green leaf tissues of plants is somewhat conserved, probably because of the need for optimal function of photosynthesis. Flavonoids include the water-soluble red or blue anthocyanins and pale yellow compounds such as rutin, quercitin, and kaempferol. Anthocyanins (see Chapter 10), the largest and most diverse group of 1053 Postharvest Physiology of Edible Plant Tissues TABLE 16.10 Major Anthocyanins and Carotenoids in Various Edible Plant Tissues Anthocyanin Pelargonidin 3-glucoside Cyanidin 3-rutinoside Cyanidin 3-glucoside Cyanidin 3-glucoside Peonidin 3-glucoside Cyanidin 3-glucoside Malvidin 3-glucoside Malvidin 3-glucoside Carotenoid Capsanthin Lycopene β-Carotene β-Cryptoxanthin Violaxanthin Fruit or Vegetable Strawberry, sarsaparilla Sweet cherry Plum, blackberry, pomegranate Grapes Blueberry Red pepper Tomato, watermelon, papaya, guava, Deep Red and Star Ruby pummelo Peach, nectarine, plum, loquat, apricot, Mexican lime, citron, carrot, sweet potato Mandarin, lemon, Rangpur lime Chandler pummelo, orange Source: Modified from Valero, D. and Serrano, M., Postharvest Biology and Technology for Preserving Fruit Quality, CRC Press, Boca Raton, FL, 2010. plant pigments, are derived from the phenyl propanoid pathway. The color of anthocyanins is affected by pH in the host tissue. Anthocyanins occur in all tissues of higher plants, providing color in leaves, plant stem, roots, flowers, and fruits, though not always in sufficient quantities to be noticeable. Betalains are red or yellow pigments. Like anthocyanins they are water-soluble, but unlike anthocyanins the base structure is synthesized primarily from tyrosine and other amino acids. This class of pigments is found only in the Caryophyllales, and never co-occurs in plants with anthocyanins. Betalains are responsible for the deep red color of beets, and are used commercially as food-coloring agents. Examples of major carotenoids and anthocyanin species in edible plant tissues are shown in Table 16.10. Breeding and selection by humans have had a major influence on the composition of edible plant tissues. Examples include carrot, which had white roots, lacking carotenoids and having only traces of lutein and other carotenoids before domestication. Intensive breeding has generated the currently known carotenoid-rich varieties, including the widely popular orange carrots that accumulate high levels of the pro-vitamin A carotenoids β-carotene and, to a lesser extent, α-carotene [61]. The color of purple carrots is caused by the accumulation of anthocyanins, although these cultivars also accumulate carotenoids. A large diversity of citrus types and external color from the green of limes to the yellow of lemons, orange in mandarins and sweet oranges, and pink in red grapefruits exist. These colors typically reflect different compositions of chlorophylls and carotenoids, although anthocyanins provide a red to purple tint, in the flesh of a specific group, blood oranges [62]. Traditional and biotechnological approaches are being taken to increase concentrations of pigments such as anthocyanin, betalain, and lycopene in a variety of fruits and vegetables [63,64]. 16.6.8 Volatile Organic Compounds Plant volatile organic compounds (VOCs) are secondary metabolites that play important roles in biotic interactions and in abiotic stress responses. These volatile compounds contribute to the aroma of many vegetables, but are mostly recognized for their role in fruits. In climacteric fruits, VOCs typically increase during the onset of ripening and peak either at or shortly before full ripening, and 1054 Fennema’s Food Chemistry biosynthesis of many volatiles is regulated by ethylene. Accumulation of VOCs in fruits is presumably associated with seed dispersal in a similar manner to color development, but is an important facet of quality for human consumers where they interact with the olfactory epithelium in the nose to produce species-characteristic aroma sensations. Fruits and vegetables can contain hundreds of volatiles, but these are not necessarily perceived by the consumer. Differences in number and concentration of VOCs can be cultivar-specific within a genotype (e.g., apple) [65]. There are complex interactions between nonvolatile and volatile compounds, other taste sensations (sweet, sour, salty, bitter), and the balance of reactions that produce and degrade them when macerated and/or cooked. A relatively few VOCs are flavor-impact compounds, and the aroma thresholds (the concentration below which no aroma is perceived) vary enormously. For example, the aroma threshold for butyl ethanoate is 5000 ppb, but is only 0.13 ppb for its isomer ethyl butanoate. Important VOCs in vegetables include phthalides in celery, thiopropanal S-oxide in fresh onions, 2-propenyl isothiocyanate in cabbage, and 2,5-nonadienal in cucumber [66]. Apples produce a complex mixture of over 200 volatile compounds, including alcohols, aldehydes, ketones, sesquiterpenes, and esters. Esters are associated with “fruity” attributes of fruit flavor and typically increase to high levels late in the ripening process. Butyl acetate, hexyl acetate, and 2-methylbutyl acetate dominate the flavor of ripe fruit, with the latter two being identified by analytical sensory panels as having the greatest impact on the attractiveness of the fruit. The biosynthesis of VOCs is variable across different fruits and vegetables, but tomato can be used to illustrate several key pathways that have been identified and are shared among most edible fruits. The concentration of most, although not all, of the flavor volatiles in a tomato increases at the onset of ripening and peak either at or shortly before full ripening, suggesting that synthesis of flavor volatiles is highly regulated [67]. VOCs can be derived from carotenoids, fatty acids, terpenoids, and amino acids. 1. Carotenoid-derived volatiles: Among the most important VOCs in tomato fruits are apocarotenoids, which are derived from carotenoids such as β-ionone, 6-methyl-5-hepten-2-one, geranylacetone, and β-damascenone. Those carotenoid-derived VOCs are known to be produced by nonenzymatic oxidative cleavage of linear and cyclic carotenoids or by the cleavage action of carotenoid dioxygenase. Given their linkage to carotenoids, their abundance is well correlated with fruit ripening and they are highly abundant in red tomato fruits. 2. Fatty acid–derived volatiles: The odorous lipid breakdown products, such as cis-2penten-1-ol, trans-2-pentenal, cis-3-hexanal, trans-2-hexanal, and trans-2-heptenal, are among the most abundant aroma volatiles in tomato fruits. Hexanals are formed from the 13-hydroperoxides of linoleic-related fatty acids and generated by the coordinated and sequential reactions of a lipoxygenase, lyase, and isomerase. cis-3-Hexanal is associated with the fresh green aroma of tomato and it has an exceptionally low sensory threshold of 0.25 ppb. 3. Terpenoid volatiles: The major class of terpenoid VOCs is represented by lipophilic mono-, sesqui-, and diterpenoids, which are derived either from geranyl diphosphate or trans-­ farnesyl diphosphate. These compounds are generally volatile. Tomatoes are rich in terpenoids. During early fruit development, the cytosolic pathway of terpenoid biosynthesis is operational, producing the glycoalkaloids and sterols, and during ripening, the activity of the plastidic terpenoid pathway increases. However, ripe tomato fruits contain only minute quantities of monoterpenes and sesquiterpenes. Citrus essential oils are particularly rich in terpenoids. 4. Amino acid–derived volatiles: Tomato fruit volatiles are also derived from amino acids. Several branched chain amino acids and aromatic amino acids are associated with senescence, while those derived from phenylalanine, such as guaiacol, MeSA, and eugenol, also contribute to the aroma of tomato fruits. 1055 Postharvest Physiology of Edible Plant Tissues 16.6.9 Vitamins and Health-Promoting Substances The importance of vitamins in edible plant tissues in promoting good health and preventing or alleviating disease has long been known. The nutritional factors provided in significant amounts to human diets by edible plant tissues are water-soluble vitamins B1 (thiamine), B2 (riboflavin), B6 (pyridoxine), B12, niacin, biotin, folic acid, pantothenic acid, and vitamin C (ascorbic acid). All of these are not produced by humans (except some niacin) and therefore plant-based diets are essential for maintaining human health. In addition, there has been increasing emphasis of other antioxidants such as tocopherols (nutritionally required), flavonoids, carotenoids, and glucosinolates. Edible plant foods are also the exclusive source of dietary fiber for humans (see Section 3.4 for a detailed account). Fruits and vegetables have received recognition as “chemopreventors” and “functional foods.” 16.6.9.1 Ascorbic Acid (Ascorbate, Vitamin C) l-Ascorbic acid is a water-soluble antioxidant that is structurally related to C6 sugars (C6H8O6), being an aldono-1,4-lactone of a hexonic acid (Figure 16.22) [68]. It is one of the most important nutritional quality factors in many horticultural crops and has many biological functions in the human body. It is widely accepted that the predominant pathway for the biosynthesis of ascorbic acid is via GDP-mannose and l-galactose. d-Galacturonic acid, d-glucuronic acid, and GDP-lgulose could be minor ascorbate precursors [69]. However, humans and some other vertebrae are unable to synthesize ascorbic acid due to the lack of the l-glucono-1,4-lactone oxidase enzyme. Around 90% of vitamin C in the human diet is derived from fresh vegetables and fruits to meet needs such as maintenance of cartilage, bones, gums, skin, and teeth, and as powerful reducing HO HO H HO O O OH HO O O Dehydroascorbic acid Structures of ascorbic acid and dehydroascorbic acid. Ascorbate (ASC) H2O2 O H Ascorbic acid FIGURE 16.22 O HO Oxidized glutathione (GSSH) NADPH NAD(P)+ APX MDAR DHAR GR NAD(P)H H2O Monodehydroascorbate (MDA) Dehydroascorbate (DHA) Glutathione (GSH) NADP+ FIGURE 16.23 The ascorbate–glutathione cycle. Hydrogen peroxide (H2O2) is reduced to H2O by ascorbate peroxidase (APX) activity using ascorbate (ASC) as the electron donor to produce monodehydroascorbate (MDA). ASC is regenerated from MDA by monodehydroascorbate reductase (MDAR) activity using NAD(P)H. Any MDA that is not reduced disproportionates to ASC plus dehydroascorbate (DHA). DHA is reduced to ASC by dehydroascorbate reductase (DHAR) activity at the expense of glutathione (GSH), yielding oxidized glutathione (GSSG). Finally GSSG is reduced by glutathione reductase (GR) using NADPH as the electron donor. 1056 Fennema’s Food Chemistry agent against oxidative stress–related diseases including cancers, cardiovascular disease, aging, and cataract formation [68]. In plants, ascorbic acid functions as an enzyme cofactor, a radical scavenger, and a donor/­ acceptor in electron transport either at the plasma membrane or in the chloroplasts, and it serves as the substrate for oxalate and tartrate biosynthesis in species such as grapes. The primary function of ascorbic acid is to detoxify hydrogen peroxide (H2O2) produced by metabolism during photosynthesis and especially in stress conditions. An intrinsic feature during fruit ripening is the increased production of reactive oxygen species (ROS). Recent studies also reveal that ROS are central players in the complex signaling network of cells, and acquire dynamic and specific roles in signaling. ROS homeostasis is maintained by a system of redox system enzymes. Ascorbic acid contributes ROS detoxification via a four-step biochemical pathway, known as the ascorbate–glutathione cycle or the Foyer, Halliwell, Ashada cycle (Figure 16.23) [70]. Several enzymes are involved in the cycle, such as ascorbate peroxidase oxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR). Under normal metabolic conditions and in response to survivable stresses, these reactions maintain a high redox state of ascorbate (ASA/DHA) and glutathione (GSH/GSSG). Other critical antioxidant enzymes include peroxidases, superoxide dismutase, and catalase. Concentrations of ascorbic acid in grain staples are low, especially in comparison with fruits and vegetables. Ascorbic acid concentrations in edible plant tissues can be influenced by various factors such as genotypic differences, preharvest climatic conditions and cultural practices, maturity and harvesting methods, and postharvest handling procedures [71]. In general, postharvest treatments that maintain quality, such as low storage temperatures and avoidance of water loss, slow down the loss of ascorbic acid. 16.6.9.2 Vitamin A Vitamin A is the generic descriptor for lipid-soluble compounds with the qualitative biological activity of retinol (retinols and some carotenoids). Vitamin A is an isoprenoid with a 6-carbon cyclic ring and an 11-carbon side chain (see Section 8.7.1 for more details). It is extremely important to human nutrition, as its synthesis is dependent on plant-based sources of carotene. Vitamin A is formed in the intestinal mucosa by the cleavage of carotene, and because retinol is not present per se in plants, the provitamin A carotenoids are considered to be the vitamin A functional group recognized as international units (IU) of α- and β-carotene, 1.2 and 0.9 µg, respectively [8,72]. Deficiency of this vitamin is common, afflicting millions of children each year with xerophthalmia, blindness, or death. Genetic engineering of the β-carotene biosynthetic pathway in rice endosperm to develop Golden Rice has the potential to greatly improve human health but is the subject of regulatory concerns (see Section 16.8.2). Leafy vegetables and fruits average 5000 IU and 100–500 IU vitamin A per 100 g fresh weight, respectively, but mango and papaya contribute greater levels (3000 and 2500 IU per 100 g fresh weight, respectively) [8]. With the exception of Golden Rice and sweet potato (2500 IU per 100 g fresh weight), staple crops have negligible levels. The stability of carotenoids is generally greater than ascorbic acid [72] during postharvest storage, but can be affected by genotype, prestorage conditions, and nonoptimal storage temperature and humidity. 16.6.9.3 Phytochemicals Phenolic compounds are strong antioxidants (Section 16.6.4), and they are recognized for their health-promoting activity for antioxidant and other biological effects. In fruits such as the apple, the contribution of ascorbic acid to overall antioxidant activity is small (0.4%). It has been suggested that most of the antioxidant activity of some fruits and vegetables may come from phenolics and flavonoids and that the additive and synergistic effects of phytochemicals in fruit and vegetables are responsible for their potent antioxidant and anticancer activities [73]. In general, phenolic compounds are stable during storage and processing, unless PPO activity results in their oxidation. Postharvest Physiology of Edible Plant Tissues 1057 16.6.9.4 Sulfur Compounds Onions and other Allium vegetables have flavor precursor compounds that are secondary metabolites involved in the biosynthesis and metabolism of cysteine and glutathione in essential pathways for the uptake of sulfur and detoxification. The major compounds are γ-glutamyl-S-alk(en)yl-lcysteines and S-alk(en)yl-l-cysteine sulfoxides (ACSOs). Hydrolysis of the cytoplasmic-located ACSOs with vacuolar alliinase activity during mastication or homogenization produces pungent sulfur compounds and the by-products pyruvate and ammonia [10]. Cruciferous vegetables (Brassicaceae, syn. Cruciferae) contain glucosinolates that are transformed to isothiocyanates and indoles, which are thought to be health-promoting compounds. The active compounds are not present in intact products, but mastication or homogenization results in hydrolysis by myrosinase. ACSOs and glucosinolates may increase during storage, but generally decrease as the product quality deteriorates [6]. 16.7 POSTHARVEST TECHNOLOGIES 16.7.1 Storage Temperature The most fundamental postharvest tool available to maintain quality of edible plant tissues is temperature control. Temperature has a profound effect on the rates of biological reactions [11]. Therefore, for each edible plant tissue, it is critical to cool these products as quickly as possible after harvest to slow down metabolic activity. Benefits of lower temperatures include reduced respiration rates, reduced water loss, reduced sensitivity to ethylene, and decreased susceptibility of products to physiological and microbial decay. A major exception to low storage temperatures is curing, which is used for onions to dry the neck and outer scales, and for potatoes to develop wound periderm over damaged surfaces. Curing is usually carried out at ambient conditions in the field or at temperatures from 7°C to 16°C in curing rooms. Low storage temperature can maintain the appearance of edible plant tissues, including the stability of nutritionally important compounds such as anthocyanins, carotenoids, and ascorbic acid, as well as preserve color and retard the rate of undesirable softening and texture changes [6,74]. Flavor and aroma compounds are often higher in freshly eaten products rather than cold-stored (e.g., tomato) [75], but the interplay of flavor compounds with sugars, acids, and tissue matrices is complex and results in varied responses to storage. Cooling methods are customized to the product type and scale of operation. These methods include room or passive cooling, forced-air cooling, hydro-cooling, package icing, and vacuum cooling. Full details of each are available from many books and web-based sources [76,77] and will not be described here. However, regardless of cooling method, the cooling rate of products follows standard laws of physics in that it becomes progressively slower to cool products down over time as the temperature differential between product and cooling medium diminishes. The typical cooling curve for an edible plant tissue in a cold storage room (Figure 16.24) illustrates the concept of “half-cooling” or “seven-eighths-cooling” times. Half-cooling refers to the time taken to reduce the initial product temperature from that when first placed in the cold room halfway to the set temperature in the room. For example, if the product temperature was 20°C when placed in the room and air temperature in the room was set at 0°C or 10°C, half cooling would refer to the time that it took for the product temperature to reach 10°C and 15°C, respectively. It will then take the same time period to reduce the product temperature by half again, and so on. So, seven-eighths cooling is three times as long as half-cooling. Decreasing temperatures over the physiological range (0°C–30°C) of most crops causes an exponential decrease in respiration. The van’t Hoff Rule states that the velocity of a biological reaction decreases two- to threefold for every 10°C decrease in temperature. The temperature quotient for a 10°C interval is called the Q10, which is calculated by dividing the reaction rate at a higher temperature by the rate at a 10°C lower temperature: Q10 = R2/R1. The temperature quotient is useful 1058 Fennema’s Food Chemistry Initial product temperature Product temperature (°C) 20 Air temperature 0°C 15 Average product temperature 1/2 cool 10 3/4 cool 5 0 7/8 cool 0 24 48 72 15/16 cool 96 Hours of cooling FIGURE 16.24 A typical cooling pattern for an edible plant tissue in a cold room. In this example, a product at 20°C is placed in a cold room with air temperature of 0°C. The time to reduce the temperature of the product by 50% (i.e., to 10°C) is known as the “half cooling.” because it allows us to estimate potential shelf-life gains by the extent of cooling based on reduction in respiration rates. However, the respiration rate does not follow ideal behavior, and the Q10 is usually smaller at higher than at lower temperature ranges. Typical values for Q10 at various temperatures range 2.5–4.0 from 0°C to 10°C; 2.0–2.5 from 10°C to 20°C; 1.5–2.0 from 20°C to 30°C, and 1.0–1.5 from 30°C to 40°C [11]. The Q10 values can be used to demonstrate the effects of different temperatures on the rates of respiration or deterioration and relative shelf-life of a typical perishable commodity (Table 16.11). This table shows that a product with a storage life of 100 days at 0°C has a storage life of 13 days at 20°C, and only 4 days at 40°C. TABLE 16.11 Effect of Temperature on Respiration Rate and Relative Storage Life of Edible Plant Tissues Based on Q10 Temperature (°C) Assumed Q10 0 Relative Velocity of Respiration Relative Storage Life 1.0 100 3.0 33 7.5 13 15.0 7 22.5 4 3.0 10 2.5 20 2.0 30 1.5 40 Source: Kader, A.A. and Saltveit, M.E., Respiration and gas exchange, in Postharvest Physiology and Pathology of Vegetables, Bartz, J.A. and Brecht, J.K., Eds, Marcel Dekker, New York, 2003, pp. 7–30. 1059 Postharvest Physiology of Edible Plant Tissues TABLE 16.12 Edible Plant Tissues Classified according to Sensitivity to Chilling Injury Non-Chilling-Sensitive Apples* Apricots Asparagus Beans, Lima Beets Blackberries Blueberries Broccoli Cauliflower Celery Corn, sweet Cherries Currants Garlic Grapes Mushrooms Onions Parsley Peaches* Raspberries Spinach Strawberries Turnips Chilling-Sensitive Avocado Banana Bean, snap Cantaloupe Cranberry Cucumber Eggplant Lemon Lime Mango Muskmelons Orange Papaya Pepper Pineapple Potatoes Pumpkins Squash Sweet potatoes Tomatoes Watermelons Yams Zucchini Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002. * Some cultivars are chilling sensitive. In general, the lower the storage temperature, the longer the storage life of a given commodity, as long as the freezing temperature for the product is not reached. However, the lowest safe storage temperature is not the same for all products, as many of them are sensitive to low temperatures and develop chilling injuries. Products can be categorized as chilling-sensitive or chilling-­insensitive (Table 16.12). Chilling-sensitive products are often subtropical and tropical in origin, but the specific product, maturity and degree of ripeness at harvest, and length of exposure to specific low temperatures affect responses of different products to cold temperatures. Examples of differences among product types include bananas, which are extremely chilling-sensitive if stored below 12.5°C for a few days, while honeydew melons require weeks to show chilling symptoms at 5°C. Maturity and the degree of ripeness are also important factors in conferring chilling sensitivity in fruits such as avocados, honeydew melons, and tomatoes. Riper fruits are less sensitive to chilling injury, especially if a symptom of damage is failure to ripen. Also, damage may occur in a short time if the temperatures are considerably below the threshold level, but take a longer time to express if the product is just under the minimum safe temperature. For example, it is common for tomatoes to be kept in the refrigerator despite their chilling sensitivity, but unless the time period 1060 Fennema’s Food Chemistry TABLE 16.13 Selected Edible Plant Tissues Susceptible to Chilling Injury When Stored at Low but Nonfreezing Temperatures Lowest Safe Temperature (°C) Edible Plant Tissue Apple—certain cultivars Asparagus Avocado Bananas Bean (lima) Bean (snap) Cranberries Cucumbers Eggplant Guavas Grapefruit Lemons Limes Lychee Mango Pineapple Potato Pumpkins and hardshell squash Sweet potato Tomato—ripe Tomato—mature green Watermelon Injury Symptoms 2–3 Internal browning, brown core, soggy breakdown 0–2 4.5–13 11.5–13 1–4.5 7 2 7 7 4.5 10 11–13 7–9 3 10–13 7–10 3 10 13 7–10 13 4.5 Dull, gray-green, limp tips Grayish-brown discoloration of flesh Dull color when ripened Rusty brown specks, spots or areas Pitting and russeting Rubbery texture, red flesh Pitting, water-soaked spots, decay Surface scald, Alternaria rot, blackening of seeds Pulp injury, decay Scald, pitting, watery breakdown Pitting, membranous staining, red blotch Pitting, turning tan with time Skin browning Grayish scald-like discoloration of skin, uneven ripening Dull green when ripe, internal browning Mahogany browning, sweetening Decay, especially Alternaria rot Decay, pitting, internal discoloration, hardcore when cooked Water soaking and softening, decay Poor color when ripe, Alternaria rot Pitting, objectionable flavor Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002. 80 Non-chilling sensitive 70 Chilling sensitive Storage life (days) 60 50 40 30 20 10 0 0 5 10 15 20 Temperature (°C) 25 FIGURE 16.25 Relative storage lives of chilling and non-chilling sensitive products. 30 1061 Postharvest Physiology of Edible Plant Tissues Primary response Physical change in membrane lipids Time of exposure to chilling temperatures Reversible damage Secondary responses, e.g., increased membrane permeability, reduced ATP levels, accumulation of toxic compounds, failure of essential reactions Manifestation of chilling injury (see examples in Table 16.13) Irreversible damage FIGURE 16.26 A simplified scheme of the responses of chilling sensitive plant tissues to chilling stress. is extended, chilling injury is not detected. Also, storage recommendations can be complex; for example, fruits such as peach develop chilling injuries at a slower rate at 0°C than at temperatures between 4°C and 10°C, and therefore the lower storage temperature is recommended. Postharvest strategies that can alleviate chilling injury of edible plant tissues include modified and controlled atmosphere storage, heat treatments, temperature preconditioning, and intermittent warming [78]. The optimum temperature for storage is higher for chilling-sensitive than for non-chilling products. Examples of safe storage temperatures for several fruits and vegetables are provided in Table 16.13, as well as a range of chilling injury symptoms. Injury symptoms can be manifested in many ways, including irregular ripening, failure to ripen, water-soaked appearance, skin discoloration, mealiness, pits on the skin surface, and increased susceptibility to decay. Figure 16.25 illustrates the differences between the responses of non-chilling-sensitive and chilling-sensitive fruits and vegetables to storage temperature. In non-chilling-sensitive products, the longest storage life is associated with the lowest nonfreezing storage temperature. However, for chilling-sensitive products, the storage life increases with decreasing storage temperature to reach a maximum from 7°C to 18°C depending on the product. At lower temperatures, the storage life decreases because of susceptibility to chilling injuries. The physiological basis for chilling injury development has long centered on physical changes in membranes in which the molecular ordering of membrane lipids is altered (liquid to gel phase transition for some lipid species) in the temperature range where chilling effects become apparent. Subsequent secondary events occur and, if the chilling event is limited in intensity and exposure, the plant tissues can recover (Figure 16.26). Support for this hypothesis includes evidence that tropical species tend to have lipids with a higher proportion of saturated fatty acids (e.g., palmitic acid, which lacks double bonds and therefore have higher melting points), while cool-climate plants tend to have more unsaturated fatty acids such as oleic, linoleic, and linolenic acids. However, a consistent pattern of differences in lipid membrane composition between chilling-susceptible and chilling-resistant plants has yet to emerge, and no single physiological factor has been linked with plant susceptibility to chilling injury [79]. Mechanisms that may be involved in greater resistance to chilling injury, especially those resulting from postharvest strategies such as heat treatments, include enhancement of membrane integrity by the increase of unsaturated fatty acid/saturated fatty acid ratio; enhancement of heat shock protein gene expression and accumulation; enhancement of the antioxidant system activity; enhancement of the arginine pathways which lead to the accumulation of signaling molecules with pivotal roles in 1062 Fennema’s Food Chemistry improving chilling tolerance such as polyamines, nitric oxide, and proline; alteration in phenylalanine ammonia-lyase and polyphenol oxidase enzyme activities; and enhancement of sugar metabolism [80]. Another form of damage that can occur is freezing injury, which occurs if edible plants are exposed (usually inadvertently) to temperatures below the freezing point. Freezing injury results from the formation of ice crystals and the destruction of cell integrity during thawing. As a consequence, injury is expressed most commonly as a water-soaked appearance, associated with loss of cellular structure and turgor. The susceptibility of different fruits and vegetables to freezing injury varies widely [81]. The exact temperature below 0°C at which the product will freeze depends on the amount of sugars or other solutes present and the corresponding freezing point depression. For example, lettuce with a low sugar content may freeze at −0.2°C, while plums with a high sugar content may freeze at −1.7°C or lower. Some commodities may be frozen and thawed a number of times with little or no injury, whereas others are permanently injured by even a slight freezing. All fruits and vegetables can be categorized into three groups based on their sensitivity to freezing: most susceptible—likely to be injured by even one light freezing; moderately susceptible—will recover from one or two light freezing periods; and least susceptible—those that can be lightly frozen several times without serious damage. The nature of freezing damage and the effects on metabolism and chemistry of edible plant tissues resemble outcomes conferred by other types of physical damage, which are not very important in the context of this chapter. However, slow warming of frozen tissues can sometimes result in partial recovery of the product, though typically the subsequent storage life of the products will be compromised. 16.7.2 Relative Humidity Relative humidity (RH) is defined as the ratio of water vapor pressure in the air to the saturation vapor pressure at the same temperature. Water loss during postharvest handling and storage is a function of product permeability, temperature, and the vapor pressure deficit between the internal tissues of the product and the surrounding atmosphere [74]. The RH directly affects the storage quality of products. Cereal grains should be maintained under low humidity conditions, but for most products excessive water loss results in wilting, shriveling, flaccidness, soft texture, and loss of nutritional value as well as saleable weight. The RH around most fruits and vegetables should be kept high, and while RH approaching 100% can encourage growth of microorganisms and splitting of skin surfaces, it is usually more of a problem to maintain sufficiently high humidity than the opposite. Nevertheless, interactions between RH and temperature mean that recommended regimens for storage of edible plant tissues can represent compromises among their physical, physiological, and pathological responses. 16.7.3 Modified and Controlled Atmosphere Storage Modified atmosphere (MA) storage refers to a change in the atmosphere around the product, typically a reduction of O2 levels from 21% in ambient air, and an increase in CO2 from 0.04% in ambient air. MA can be developed passively by product respiration or by active means where the desired gas composition is injected into a headspace, often in a package (MA packaging: MAP). Subsequently, the atmosphere in the bags is then a function of factors such as product type and temperature, which affect the respiration rates, permeability of the plastic film to oxygen and carbon dioxide diffusion, and the ratio of product mass to the bag volume. Controlled atmosphere (CA) is a subset of MA, but as the name suggests, the atmosphere around the product is continuously controlled. Control is conferred by use of equipment such as nitrogen generators and carbon dioxide scrubbers to maintain the desired gas composition. Based on the net chemical changes for respiration (Section 16.4) it has long been assumed that low O2 and high CO2 would inhibit respiration and thereby increase storage life. However, the interaction between the gases and metabolism of edible plant tissues is more complex, especially in relation to ethylene perception and production. Ethylene action is inhibited by low O2, 2.8% being the concentration 1063 Postharvest Physiology of Edible Plant Tissues TABLE 16.14 O2 Limits below Which Injury Can Occur for Selected Horticultural Crops Held at Typical Storage Temperatures Minimum O2 Concentration Tolerated (%) 0.5 1 2 3 5 Commodities Tree nuts, dried fruits and vegetables Some cultivars of apples and pears, broccoli, mushrooms, garlic, onion, most cut or sliced (minimally processed) fruits and vegetables Most cultivars of apples and pears, kiwifruit, apricot, cherry, nectarine, peach, plum, strawberry, papaya, pineapple, olive, cantaloupe, sweet corn, green bean, celery, lettuce, cabbage, cauliflower, Brussels sprouts Avocado, persimmon, tomato, peppers, cucumber, artichoke Citrus fruits, green pea, asparagus, potato, sweet potato Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002. TABLE 16.15 CO2 Limits above Which Injury Can Occur for Selected Horticultural Crops Held at Typical Storage Temperatures Maximum CO2 Concentration Tolerated (%) 2 5 10 15 Edible Plant Tissue Asian pear, European pear, apricot, grape, olive, tomato, pepper (sweet), lettuce, endive, Chinese cabbage, celery, artichoke, sweet potato Apple (most cultivars), peach, nectarine, plum, orange, avocado, banana, mango, papaya, kiwifruit, cranberry, pea, pepper (chili), eggplant, cauliflower, cabbage, Brussels sprouts, radish, carrot Grapefruit, lemon, lime, persimmon, pineapple, cucumber, summer squash, snap bean, okra, asparagus, broccoli, parsley, leek, green onion, dry onion, garlic, potato Strawberry, raspberry, blackberry, blueberry, cherry, fig, cantaloupe, sweet corn, mushroom, spinach, kale, Swiss chard Source: Modified from Kader, A.A., Postharvest Technology of Horticultural Crops, Regents of the University of California, Division of Agricultural and Natural Resources, Oakland, CA, 2002. at which ethylene action is halved [82], and by elevated CO2 concentrations. The effects of the two gases can be interactive, and together they may slow down respiration to a greater extent than either alone. Reducing O2 concentrations around fruits and vegetables slows down respiration rates until the ACP is reached (Figure 16.7). This transition in respiration is associated with fermentation and injurious accumulations of acetaldehyde and ethanol, which result in damage. Each type of edible plant tissue has tolerances to low O2 and high CO2 (Tables 16.14 and 16.15), although there is considerable variation conferred by variety, growing condition, and length of exposure to each gas. The safe concentration range of O2 and CO2 for most edible plant tissues during storage at optimal temperatures have been identified [83], and are well above the ACP to account for variability of product responses. Atmospheres obtained within MA systems such as MAP are a function of respiration rates, which is affected by product weight and storage temperature, permeability of the films to O2 and CO2, and volume of the film bag. Applications of MAP are still relatively restricted because of the 1064 Fennema’s Food Chemistry TABLE 16.16 Overview of the General Effects of O2 Levels below 5% and CO2 Levels above 5% on Metabolism of Edible Plant Tissues General Effects on Metabolism Respiration 1. Rate 2. Shift from aerobic to anaerobic 3. Energy produced Ethylene biosynthesis and action 1. Methionine to S-AdoMet 2. Synthesis of ACC synthase 3. ACC synthase activity 4. Synthesis of ACC oxidase 5. ACC oxidase activity 6. Ethylene action Compositional changes 1. Pigments a. Chlorophyll degradation b. Anthocyanin development c. Carotenoids biosynthesis 2. Phenolics a. Phenylalanine ammonia lyase activity b. Total phenolics c. Polyphenol oxidase activity 3. Cell wall components a. Polygalacturonase activity b. Soluble polyuronides 4. Starch to sugar conversion 5. Organic and amino acids a. Loss of acidity b. Succinic acid c. Malic acid d. Aspartic and glutamic acid e. ϒ-Amino butyric acid 6. Volatile compounds a. Characteristic volatile aroma compounds b. Off-flavors (fermentation products) 7. Vitamins a. Provitamin A (carotene) loss b. Ascorbic acid loss Reduced O2 Elevated CO2 ↓ ↑(<1%) ↓ ↓, NE, or ↑ ↑ (>20%) NE ↓ NE ↓ ↓ ↓ ? ↓ ↓ ↓ ↓ or ↑ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↑ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↓ ↑ ? ? ↓ ↑ ↓ ↓ ↓ ↓ ↑(<1%) ↓ ↑(>20%) ↓ ↓ ↓ ↓ Source: Modified from Kader, A.A. and Saltveit, M.E., Atmosphere modification, in Postharvest Physiology and Pathology of Vegetables, Bartz, J.A. and Brecht, J.K., Eds, Marcel Dekker, New York, 2003, pp. 229–246. ↓ = decrease or inhibit, NE = no effect, ↑ = stimulate or increase, ? = inadequate data for conclusion. Postharvest Physiology of Edible Plant Tissues 1065 limited availability of suitable films that are cost effective, and difficulties in maintaining uniform temperatures through the entire marketing chain. The major exception is the wide use of MAP for fresh-cut produce (Section 16.9.3). Beneficial effects at the optimal CA conditions include retardation of senescence (including ripening) and associated biochemical and physiological changes, particularly slowing down rates of respiration, ethylene production, softening, and compositional changes. An overview of the general effects of O2 and CO2 concentrations on metabolic processes is shown in Table 16.16. The specific concentrations at which these general responses occur and their magnitude differ among plant types and cultivars, maturity and ripening stages, storage temperatures and durations, and in some cases ethylene concentrations [84]. Stressful CA conditions decrease pH and ATP levels and reduce pyruvate dehydrogenase activity while activating pyruvate decarboxylase and alcohol dehydrogenase activities with the production of the fermentation products acetaldehyde and ethanol (Figure 16.6). Commercial use of CA storage is greatest for preserving apples and pears, less on cabbages, sweet onions, kiwifruits, avocados, persimmons, pomegranates, nuts, dried fruits, and vegetables. Atmospheric modification during long-distance transport is used with apples, asparagus, avocados, bananas, broccoli, cane berries, cherries, figs, kiwifruits, mangos, melons, nectarines, peaches, pears, plums, and strawberries. Continued technological developments in the future to provide CA during transport and storage at a reasonable cost (positive benefit/cost ratio) are essential to greater applications on fresh horticultural commodities and their products. CA is limited for many products because it requires significant capital investment. Structures must be air-tight and refrigerated, with precise temperature control and equipment to modify the atmospheres. The volume of these storage rooms is also large to maximize the value of the equipment. Therefore, the return on investment requires long-lived commodities that are stored for months, not days or weeks. The economic importance and long-term storage potential of apples have been drivers for the development of new CA technologies. Standard CA, in which O2 and CO2 concentrations are maintained in the 2%–3% range, has increasingly become replaced by ultralow O2 (ULO). Some cultivars in certain growing regions can be routinely stored in low O2 concentrations between 1% and 1.5% if high-quality storage rooms and computerized monitoring and maintenance of gas levels are available. A new CA technology, known as dynamic CA (DCA), has been developed and commercialized. Rather than establishing a predetermined low O2 concentration, fruit quality in storage can further be maximized by storing them at concentrations that are closer to the ACP (Section 16.4; Figure 16.7). O2 concentrations in the storage room are lowered to the point where stress signals are measurable, and then the concentration is increased by about 0.2% above the O2 concentration at which stress is observed to ensure that fermentation and damage to the product are avoided. It is possible, therefore, to follow fruit responses in real time and ensure that rates of fruit metabolism are maintained as low as possible throughout storage. Three methods to determine stress in the fruit are ethanol accumulation, RQ, and chlorophyll fluorescence [85,86]. DCA can, in principle, be applied to any fruit or vegetable tissue, but most use of this technology has been on apples. Other atmosphere-based technologies such as hypobaric storage, in which commodities are stored at low atmospheric pressures, provide potential for long-term storage, but practical costaffordable systems are not yet available. 16.7.4 Edible Coatings Edible coatings are thin layers of edible material that are applied to surfaces of plant tissues in addition to or as a replacement for natural protective waxy coatings. Application to products can be by dipping, spraying, or brushing. Edible coatings can act in a similar manner as MAP in that internal gas concentrations may be modified as a result of coating applications. An ideal coating is defined as one that can extend the storage life of whole or minimally processed fresh fruits and vegetables without causing anaerobiosis or negatively affecting any desirable quality attribute. 1066 Fennema’s Food Chemistry The main reasons for use of edible coatings are improved appearance, reduced water loss, delayed ripening, and reduced incidence of decay and physiological disorders. Shellac and carnauba wax are the two most common coating materials because they are used alone or in combination on apples and oranges. Synthetic coatings have been used for decades; most consumers are probably aware that apples are waxed, but they may not be as aware for other products such as cucumbers, oranges, mangoes, papayas, and peppers. Nevertheless, recent consumer interest in nutrition, food safety, and environmental concerns have revitalized efforts in edible coating research [87–89]. There is increasing use of edible coatings of fresh-cut fruits and vegetables to reduce deterioration rates associated with cutting and processing. Edible coatings may also be used on nonfleshy products such as peanuts and roasted almonds. Another area of interest is the addition of active ingredients such as antioxidants, antimicrobials, and neutraceuticals to edible coatings [88]. The requirements of edible coatings vary according to product and purpose. However, they must not result in low O2 or high CO2 concentrations that would result in off-flavor development and deterioration, or interfere with product quality. Desirable characteristics of coatings include improved appearance, maintenance of structural integrity, improved mechanical handling properties, or carry active agents such as antioxidants and vitamins; water resistance, so that they remain intact and cover the product; reduced water permeability; melt above 40°C without decomposition; be easily emulsifiable, nonsticky or tacky, and have efficient drying performance; have low viscosity and be economical, and be translucent to opaque, and capable of tolerating slight pressure [88,89]. Materials used for coatings include lipids, polysaccharides, proteins, and combinations of these materials or composite coatings [90]. Any Generally Recognized As Safe (GRAS) materials that are approved for use in coatings without restriction are considered by the FDA to be “edible.” Lipids are used primarily because of their hydroscopic properties, which makes them good barriers to water loss [88]. Commonly used lipid coatings include the following: • Wax- and oil-based coatings. These can be animal derived (e.g., shellac wax, bees wax), vegetable-derived (e.g., carnauba wax and candelilla wax), or mineral and synthetic waxes (e.g., paraffin wax). • Fatty acids and monoglycerides. These are mainly used as emulsifiers and dispersing agents (fatty acids are extracted from vegetable oils and monoglycerides prepared by transesterification of glycerol and triacylglycerols). • Resins and rosins. Shellac resin, composed of aleuritic and shelloic acids, is a secretion of the insect Laccifer lacca, while resins are obtained from the oleoresins of pine trees, residues that are left after distillation of volatiles from the crude resin. • Emulsions (derivatives of glycerol and fatty acids, e.g., polyglycerols-polystearates). Polysaccharides are hydrophilic and do not function well as physical moisture barriers. They can still interact with water and retard loss of moisture to the atmosphere. However, they can have excellent gas barrier properties, and the linear structure of many polysaccharides renders their films tough, flexible, and transparent [88]. Commonly used polysaccharides include the following: • Cellulose and derivatives. Polymer chains of anhydroglucose are tightly packed, resulting in a highly crystalline structure (cellulose; Section 16.6.2.1), which requires treatment with alkali to increase its water solubility, followed by treatment with chloroacetic acid, methyl chloride, or propylene oxide to produce carboxymethyl cellulose (CMC), methyl cellulose (MC), hydroxypropyl methyl cellulose (HPMC), or hydroxypropyl cellulose (HPC). These coatings are water-soluble and transparent, and exhibit higher barrier capabilities to moisture and O2 transmission than cellulose. • Starch and derivatives. Amylose and amylopectin (Section 16.6.2.3) have been used to produce biodegradable films. However, starch typically needs to be treated either with plasticizers (e.g., glycerol, polyethylene glycol, mannitol, sorbitol) blended with other materials Postharvest Physiology of Edible Plant Tissues • • • • 1067 and/or chemically modified to form films with good mechanical properties such as high percentage elongation as well as tensile and flexural strength. Chitin and chitosans. Chitin, structurally analogous to cellulose, is found in exoskeletons of crustaceans, fungal cell walls, and other biological materials. Chitosan is derived from chitin by deacetylation in the presence of alkali. It also has antimicrobial activity against a wide range of pathogenic and spoilage microorganisms, including Gram-positive and Gram-negative bacteria, and has been widely used in antimicrobial films [89]. Alginates and carrageenans. Alginates, extracted from seaweeds, are salts of alginic acid, which is a linear copolymer of d-mannuronic and l-guluronic monomers. Alginate reacts with gelling agents such as calcium and magnesium to form coating materials. Carrageenan is a complex mixture of at least five water-soluble galactose polymers. These films are poor moisture barriers as they are hydrophilic films, although incorporation of calcium can reduce their water vapor permeability. Pectin (Section 16.6.2.1). High methoxy pectin forms excellent films, and plasticized blends of citrus pectin and high amylose starch give strong, flexible films. Aloe gel. Aloe vera gel, obtained from the parenchyma cells of perennial Aloe spp. ­succulent plants, has been identified as a novel skin coating [89]. Aloe gels also have antifungal activity. Protein. A variety of protein sources can be used as edible coatings, but protein coatings are the least developed materials [88]. However, they have received greater attention because of their abundance as agricultural by-products and food-processing residuals. The presence of reactive amino acid residues enables proteins to be modified and cross-linked through physical and chemical treatments to produce novel polymeric structures [91]. Proteins are generally hydrophilic and susceptible to moisture absorption, and therefore affected by temperature and RH. The most common edible coatings derived from proteins are the following: • Gelatins. Gelatins are obtained by controlled hydrolysis from the fibrous insoluble protein, collagen, a major constituent of skin, bones, and connective tissues. Gelatins characteristically have high glycine, proline, and hydroxyproline contents, and contain mixtures of single and double unfolded chains of hydrophilic character. • Corn zein. Corn zein is a prolamine protein of corn endosperm, dissolves in ethanol, and has excellent film-forming characteristics. • Wheat gluten is a general term for water-insoluble proteins of wheat flour, and contains gliadin and glutenin. Gliadin is soluble in 70% ethanol, while glutenin is not, and edible films can be made by drying aqueous ethanol solutions of wheat gluten. • Soy protein. Most of the protein in soybean is insoluble in water but soluble in dilute neutral salt solutions. Soy protein consists of two major protein fractions; 7S (conglycin, 35%) and 11S (glycinin, 52%), each containing cysteine residues leading to disulfide bridge formation. Edible coatings can be formed by surface film formation from heated soymilk or film formation from solutions of soy protein isolates [91]. • Casein. Casein is a milk-derived protein that is easily processable due to its random coil structure to produce materials that range from stiff and brittle to flexible and tough [88]. • Keratin. Keratin is extracted from waste materials such as hair, nails, and feathers, but is difficult to process due to its high cystine (disulfide) content and low aqueous solubility. After processing, a fully biodegradable, water-insoluble plastic is produced, but its mechanical properties are still poor compared with those of other proteins. Blending or lamination is required to overcome insensitivity to RH [88]. 1068 Fennema’s Food Chemistry • Whey. Whey protein is a by-product of cheese and yogurt production, and can be processed to produce flexible but brittle films. Whey protein is hydrophilic, and lipids are added to film-forming solutions to reduce moisture migration. The effects of edible coatings have been tested extensively on a range of whole and minimally processed fruits and vegetables [88–90]. While commonly used on apples, bell pepper, lemon, oranges, cucumbers, and other products, the actual commercial use of coatings is not easily quantified. Minimally processed products where edible coatings may be applied include apple, cantaloupe, carrot, lettuce, muskmelon, pear, peach, and potato. However, use of coatings requires labeling on packages, which might be perceived as detracting from the fresh image of their products [87]. The primary effects of edible coatings on product quality are exerted through modification of water loss and internal atmospheres. Fruits and vegetables lose water to the surrounding environment as a result of transpiration, and therefore storage under high RH conditions is often desirable. Loss of water from products can be aggravated by damage to the natural protective coatings by washing and other handling steps, and can be especially so in minimally processed products. In general, lipid materials (wax and oil) offer the most effective barrier to water vapor, followed by shellac, with carbohydrates and proteins being the least effective due to their hydrophilic ­characteristics [90]. Edible coatings can modify the internal atmosphere of treated products by creating a barrier to gas exchange. O2 concentrations decrease while CO2 concentrations increase in treated products, resulting in effects similar to those obtained using MAP; if concentrations of the respective gases are below 8% or higher than 5%, decreased respiration rates and ethylene production can result in slower ripening or senescence. High gas-permeable materials, such as polyethylene and carnauba wax, control water loss but do not cause much modification of internal atmospheres and the progress of ripening, while resins have low gas permeability and can control ripening more effectively. However, resin use has greater potential for development of injurious anaerobic gas concentrations under abusive temperature conditions that can occur commercially. Carbohydrate and protein coatings are generally hydrophilic but can modify internal atmospheres. Quality characteristics affected by the inhibition of ripening and senescence are as follows: • Appearance. High gloss and shine and reduced water loss (shriveling) result in better product appearance for consumers. Other O2-dependent processes that can be inhibited by edible coatings include sprouting and chlorophyll and solanine synthesis in potatoes, degreening of limes and lemons, and white blush (haze) formation of baby carrots. • Physical factors. Rates of loss of product firmness and acidity and increases of soluble solids concentrations are commonly reduced by application of edible coatings, but the magnitude of the effects is greatly dependent on product type and specific coating used [89]. • Flavor and nutrition. Coatings can affect flavor of products by influencing the metabolism of volatile biosynthesis and/or their entrapment in the treated product. Positive effects have been reported, but others may be negative, especially if ethanol production is increased due to anaerobiosis. Losses of phenolic concentrations and total antioxidant activities can be reduced by edible coatings, but this is also affected by product type and coating [87,89]. Higher carotenoid and ascorbic acid have been found in coated compared to uncoated peeled carrots and peppers, respectively. • Browning. Browning and PPO activity can be reduced in longan, fresh-cut mushroom, pumpkin, and peach by the use of edible coatings [92]. • Decay. Edible coatings can reduce wounding (surface injury, scarring, abrasion), which can result in infection by opportunistic pathogens. Coatings carrying acidulants or preservatives can reduce decay in citrus, cucumber, cut potato, and strawberry [87]. 1069 Postharvest Physiology of Edible Plant Tissues 16.7.5 Ethylene Exposure to ethylene can be beneficial or detrimental, not only depending on the specific fruit or vegetable but also when the exposure occurs [93]. Responses of commodities to ethylene can be affected by the species, cultivar, cultural practices, prior exposure to hormones, and levels of past and current stresses. There is no set standard for ethylene concentrations at which detrimental effects will occur, and there are important differences in ethylene sensitivity among fruit and vegetable types (Table 16.17). Climacteric fruits such as apples and pears have high ethylene production and high sensitivity, while other products (e.g., broccoli, cabbage, carrots, and strawberries) can have low rates of ethylene production but are highly sensitive to ethylene. Most non-climacteric fruits, such as cherry, grape, berries and pepper, have low ethylene production and low sensitivity to ethylene. Ethylene, released from a liquid chemical formulation ethephon (2-chloroethane phosphonic acid), can be used for preharvest treatment of apples and tomatoes to stimulate red color development. After harvest, ethylene is applied commercially to accelerate chlorophyll loss and ensure even ripening of bananas, and sometimes as part of “ready-to-eat” protocols for avocados and pears. Consumers can also use ethylene-producing fruits such as apples to ripen other fruit types by enclosing both fruits in a paper bag. Detrimental effects of ethylene are often of more concern to growers and marketers. Exposure of unripe climacteric fruits to ethylene can cause earlier than desirable ripening, as well as undesirable yellowing of green vegetables such as cucumbers, parsley, and broccoli, and many other negative effects (Table 16.18). Exposure of vegetables and non-climacteric fruits also increases their respiration rates, meaning that the carbohydrate reserves are used more rapidly, as well as increasing water loss and hastening the onset of senescence. Typically, exposure occurs from mixing ethylene-producing and ethylene-sensitive commodities in the same storage room. The same response to ethylene, though biochemically identical for different commodities, can be beneficial or detrimental. Acceleration of chlorophyll loss, promotion of ripening, and stimulation of phenolics production can be beneficial or detrimental depending on the product (Table 16.19). TABLE 16.17 Ethylene Production and Sensitivity of Selected Edible Plant Tissues Commodity Ethylene Production Ethylene Sensitivity Climacteric fruit Apple, kiwifruit, pear, cherimoya Avocado, cantaloupe melon, passion fruit Apricot, banana, mango Nectarine, papaya, peach, plum, tomato High High Medium Medium High (0.03–0.1 ppm) Medium (>0.4 ppm) High (0.03–0.1 ppm) Medium (>0.4 ppm) Vegetables and non-climacteric fruits Broccoli, Brussels sprouts, cabbage, carrot, Cauliflower, cucumber, lettuce, persimmon Potato, spinach, strawberry Asparagus, bean, celery, citrus, eggplant Artichoke, berries, cherry, grape, pineapple Pepper Low Low Low Low Low Low High (0.01–0.02 ppm) High (0.01–0.02 ppm) High (0.01–0.02 ppm) Medium (0.04–0.2 ppm) Low (>0.2 ppm) Low (>0.2 ppm) Source: Modified from Martinez-Romero, D. et al., Crit. Rev. Food Sci. Nutr., 47, 543, 2007. 1070 Fennema’s Food Chemistry TABLE 16.18 Summary of Detrimental Effects of Ethylene on Quality of Edible Plant Tissues Ethylene Effect Physiological disorders Abscission Bitterness Toughness Off-flavors Sprouting Color Discoloration Softening Symptom or Affected Organ Commodity Chilling injury Russet spotting Superficial scald Internal browning Bunch Stalk Calyx Isocoumarin Lignification Volatiles Tubercle, bulb Yellowing Stem browning Mesocarp Firmness Persimmon, avocado Lettuce Pear, apple Pear, peach Cherry tomato Muskmelon Persimmon Carrot, lettuce Asparagus Banana Potato, onion Broccoli, parsley, cucumber Sweet cherry Avocado Avocado, mango, apple, Strawberry, kiwifruit, melon Source: Modified from Martinez-Romero, D. et al., Crit. Rev. Food Sci. Nutr., 47, 543, 2007. TABLE 16.19 Examples of How the Same Physiological or Biochemical Response to Ethylene Can Be Beneficial in One System and Detrimental in Another Ethylene Response Accelerates chlorophyll loss Promotes ripening Stimulates phenylpropanoid metabolism Example of Benefit Example of Detriment Degreening of citrus Ripening of climacteric fruit Defense against pathogens Yellowing of green vegetables Overly soft and mealy fruit Browning and bitter taste Source: Saltveit, M.E., Postharvest Biol. Tec., 15, 279, 1999. 16.7.5.1 Ethylene Avoidance Avoidance of exposure to ethylene begins with careful harvesting, grading, and packing to minimize damage to the commodities. In the case of climacteric products, it is difficult to reduce the internal levels of ethylene once autocatalytic production has started. Products should be cooled rapidly to their lowest safe temperature to reduce naturally occurring ethylene production and to decrease sensitivity to ethylene. Use of internal combustion engines around ethylene-sensitive commodities should be avoided by using electric forklifts or isolating vehicles from handling and storage areas. Natural sources of ethylene such as overripe and decaying produce should be removed from storage and handling areas. Ethylene-producing and ethylene-sensitive commodities should not be stored together for long periods. Retail displays should avoid placement of ethylene-producing fruits such as apples and tomatoes close to commodities such as lettuce and cucumbers, although good ventilation in such areas probably reduces the severity of ethylene exposure. 1071 Postharvest Physiology of Edible Plant Tissues Ethylene concentrations in the storage environment can be reduced by ventilation with clean, fresh air. However, the fresh air has to be cooled, and increasing ventilation is therefore ­energy-intensive. Higher ventilation rates will also reduce the ability to maintain high RH in the cold room. Ventilation is also not suitable for CA storages or even packaged produce within normal storage environments because the atmospheres are tightly controlled. 16.7.5.2 Ethylene Adsorbers, Oxidation, and Catalytic Decay Ethylene in storage rooms can be lowered by adsorption or oxidation [94]. Adsorbers (“scrubbers”) such as activated carbon and zeolites (microporous aluminosilicate minerals) have been available for many years. Zeolites incorporated into plastic films can maintain sensory quality and reduce microbial storage. Ethylene can be oxidized using a number of strategies. Potassium permanganate (KMnO4) is available in sachets, films, and filters, but its direct contact with edible products must be avoided because of its toxicity. Studies show effectiveness with some products, but effectiveness with high ethylene-producing products is commercially questionable. Because ethylene is absorbed by the potassium permanganate, its effectiveness is based on the presence of a large surface area, although systems have been developed where room air is drawn though the scrubber to increase efficiency. Ozone (O3) will also oxidize ethylene, and its use in slowing down ripening and as a disinfectant that lowers mold and bacterial contamination, has been documented. However, commodities vary in sensitivity to ozone exposure. Also, ozone is unstable, and therefore maintaining stable concentrations in storage can be difficult. Catalytic decay of ethylene can be separated into two types. In the first, pure metallic elements can be used to increase the rate of chemical reactions, and in the case of ethylene, effectively oxidize it to CO2 and water. Most work on ethylene removal has centered on Pd (palladium) and TiO2 (titanium dioxide), using activated carbon as the catalyst support. Delayed ripening of tomatoes and avocados has been demonstrated using Pd-activated carbon. Another means of removing ethylene is light-activated catalysis (photocatalysis). The main compound used in photocatalysis is TiO2, which is activated by UV light (300–370 nm wavelengths). The advantages of photocatalysis include destruction of ethylene where it is produced; Ti is cheap, photostable, and clean; RH in the storage room is unaffected; and ethylene destruction can be achieved at room temperature [94]. The main disadvantage is that the technology needs permanent UV light, and therefore it cannot be used inside packages. 16.7.5.3 Inhibitors of Ethylene Action MA/CA storage inhibits ethylene perception and production by the action of low O2 and high CO2, as described on Section 2.6, but a powerful method to control ethylene perception has recently become available for fruits and vegetables. 1-Methycyclopropene (1-MCP) is a cyclopropene (Figure 16.27) that is a competitive inhibitor of ethylene perception, which acts by binding practically irreversibly to ethylene-binding sites, thereby preventing ethylene binding and the eliciting of subsequent signal transduction and translation (Section 16.5.1). 1-MCP is extremely active, but unstable in the liquid phase, but a process in which 1-MCP was complexed with α-cyclodextrin to maintain the stability of 1-MCP has been developed. The commercialization of 1-MCP as the SmartFreshSM Quality System led to the rapid adoption of 1-MCP-based technologies for many horticultural industries. 1-MCP has undetectable residues, is a gas at physiological temperatures, CH3 FIGURE 16.27 Chemical structure of 1-methylcyclopropene, an inhibitor of ethylene binding. 1072 Fennema’s Food Chemistry applied for a short time period (≤24 h) and is active at low concentrations (1 ppm). 1-MCP is typically applied to horticultural products as soon as possible after harvest, with the objective of quickly inhibiting ethylene action [95]. By 2016, regulatory approval for use of 1-MCP had been obtained in over 40 countries. 1-MCP is registered for use on a wide variety of fruits and vegetables, including apple, avocado, banana, broccoli, cucumber, date, kiwifruit, mango, melon, nectarine, papaya, peach, pear, pepper, persimmon, pineapple, plantain, plum, squash, and tomato. The specific products that are registered within each country vary greatly and according to the importance of the crop in that country. As is the case for CA storage, most use of 1-MCP technology is for apples [96]. The focus on apples is in large part is due to the large volumes of fruit that are kept in CA storage for periods up to 12 months depending on the cultivar and growing region. In some cases, 1-MCP treatment at harvest can be used as an alternative to CA storage, but usually CA and 1-MCP are used in combination. The advantage of 1-MCP is that it prevents the rapid softening of fruit that can occur after removal from CA storage. Also, apple has been an ideal fruit for 1-MCP because the ideal product in the marketplace is one resembling that at harvest—one with a crisp fracturable texture, and an acid to sugar ratio appropriate to each cultivar. Use of 1-MCP on other products is relatively limited. In contrast to apple, many other climacteric fruits such as the avocado, banana, pear, and tomato require a delay, not an inhibition of ripening, to ensure that the consumer receives high-quality products with the expected characteristics of color, texture, and flavor. Lower 1-MCP concentrations that do not inhibit ripening can be difficult to apply as a gas. However, new aqueous technologies for the application of 1-MCP in the field or as dips continue to be investigated [95]. Another factor that limits 1-MCP use is its cost relative to benefit, where for some products such as vegetables the cost of 1-MCP application may not justify its use. Yellowing of broccoli, which can result from storage and transport under abusive conditions of high temperature and exposure to ethylene, can be controlled by 1-MCP treatment, but such abuses are not common enough to warrant the treatment of a low-cost commodity. Overall, several generalizations can be made about responses of fruits and vegetables to 1-MCP: 1. The primary features of ripening in climacteric fruits such as softening, color development, and volatile production of climacteric fruits are inexorably linked to ethylene production, but the specific effects of 1-MCP treatment are closely linked to the species, cultivars, and maturity. The capacity to interrupt the progression of ripening once initiated varies by the specific fruit and attributes studied. In general, fruits with faster rates of metabolism or at a riper physiological stage are less responsive to 1-MCP; if ethylene production has been initiated, inhibiting ethylene perception is less effective. The ripening of certain fruits such as guava, tomato, and banana can be completely inhibited or abnormal if the fruits are immature. 2. Non-climacteric fruits can be affected by 1-MCP, and this outcome provides insights about the occurrence of ethylene-dependent and ethylene-independent events during ripening including changes of gene expression (up and downregulation). Common benefits of 1-MCP treatment on non-climacteric products include delayed chlorophyll and protein losses. 3. Losses of health-promoting compounds such as vitamin C are usually slower in 1-MCPtreated products, whereas effects on phenolic compounds are often smaller. 4. The quality of treated products, including the levels of health-promoting compounds, is usually close to that of untreated fruit if ripening is delayed but not inhibited by 1-MCP. 5. Physiological disorders that are associated with senescence or induced by ethylene (endogenous and exogenous) are inhibited by 1-MCP treatment, but others such as those associated with elevated CO2 in the storage environment are increased. Chilling injury is increased or decreased depending on whether ethylene production enhances or alleviates this disorder. Postharvest Physiology of Edible Plant Tissues 1073 16.7.6 Heat Treatments The use of heat treatments as a non-chemical means of controlling insect pests, preventing decay, increasing storage life, and preventing development of physiological disorders has been investigated in a number of edible plant tissues [97]. The three methods used to treat these products are (1) hot water treatments either by dips or sprays; (2) vapor heat (water-saturated air); and (3) hot air, either static or forced. Hot water treatments may be supplemented with other treatments such as brushing of the fruit [98]. Each product is treated with these methods at specific temperature and time period combinations (from seconds to days in length), which result in the desired response without injury to the tissues or an inability to recover metabolically from the treatment. The response of a particular fruit or vegetable will result from a combination of factors: preharvest environmental conditions, thermophysiological age of the product, the time and temperature of exposure, and whether the product is transferred from heat to storage or ripening temperature. The responses of many plant products to heat treatments have been investigated (e.g., apples, asparagus, carrots, celery, lettuce, mangoes, peaches, papaya, potatoes, strawberries, and tomatoes), but commercial acceptance of the technology is limited by factors such as high energy costs. Heat treatments can decrease decay by washing spores off products, by inflicting direct lethal effects on decay-causing organisms or pests, or by altering the wax structure and composition. Improved storage quality occurs through inhibition of the metabolic processes involved in ripening and senescence. An important feature of heat treatments on ripening fruits is inhibition of ethylene biosynthesis, largely because ACC oxidase activity is inhibited, but thermal effects may also evoke desensitized ethylene perception and diminished protein synthesis [97]. Respiration rates may initially increase during treatment but then decrease to lower levels than in control fruit. Other ripening factors inhibited by heat treatment include cell-wall disassembly, synthesis of carotenoids such as lycopene in tomato fruit that is mediated by ethylene, and flavor and volatiles evolution. Undesirable degreening of fruit has been observed in apple, cucumber, plantain, and tomato. If fruits are treated with inappropriate temperature/time combinations, then fruit will not recover from ripening inhibition. Heat treatments are associated with a thermal stress response, involving the upregulation of a specific set of genes coding for heat shock proteins (HSPs); this response often corresponds to a downregulation of many ripening genes. Thermotolerance is thought to require transcription and translation of these HSPs, which leads to cellular protection. If a product is treated with incorrect temperature/time combinations, synthesis of cytoprotective proteins may be attenuated, leading to heat damage. However, products can be preconditioned using a moderate heat stress to provide tolerance to higher temperature stresses, and this process may be mediated by induction of HSPs [97]. Other changes to heat-treated products include greater fatty acid saturation in heated than unheated fruits. 16.7.7 Ionizing Radiation Food irradiation involves exposing the products to gamma rays from a radioisotope source or to X-rays or electrons generated from an electron accelerator. The technology is considered safe and effective by the WHO, FAO, and the International Atomic Energy Agency, although some consumer resistance exists [99]. The potential of ionizing radiation is based on the fact that DNA of undesirable microorganisms is damaged, or that desirable physiological responses can be obtained without damaging or reducing the quality of the treated product. Radiation has no residues and can reduce the need for the use of chemicals on edible plant products. Irradiation can protect product quality and reduce postharvest losses in a number of ways, including reducing microbial loads of pathogens such as Escherichia coli and Listeria monocytogenes; inhibition of carrot, onion, and potato spouting; and extending the shelf-life of whole and fresh-cut 1074 Fennema’s Food Chemistry fruits and vegetables. The effects of irradiation on the quality of edible plant products, including those on ethylene production, respiration, appearance, texture, flavor, and nutritional composition, are generally small [99,100]. Products vary in sensitivity, but a limiting factor for the use of irradiation is loss of product quality in the range of 1–2 kGy and above. Also, undesirable effects have been found at doses lower than 1 kGy. These include greater softening, loss of ascorbic acid, and interference with wound healing at doses that prevent potato sprouting. Fresh-cut fruits and vegetables appear less sensitive to irradiation than whole products. The use of hurdle technology, where a combination of methods is used to maintain quality, continues to be investigated to reduce effective dose rates. These additional methods include MAP, hot water treatments, chemical sanitizers, calcium salts, and antioxidants. 16.7.8 Other Technologies Research is continuing to identify new technologies to maintain quality and increase the storage potential of edible plant tissues. These include the following: 1. Polyamines, which decrease during ripening and interact with the ethylene biosynthetic pathway (Sections 16.5.1.1 and 16.5.6.1). Postharvest treatment of fruit with polyamines can increase their endogenous levels, inhibit ethylene production, maintain quality, and protect against mechanical damage [29,94]. 2. Nitric oxide (Section 16.5.6.2), which can delay senescence of several non-climacteric fruit and vegetables, in part by suppressing ethylene generation [16]. NO gas is applied as a fumigant or released from solutions of sodium nitroprusside, S-nitrosothiols, or diazeniumdiolates, and future development of the technology requires a smart carrier/controlled release system for NO [30,101]. An alternative treatment option is to apply compounds such as arginine, a precursor of NO biosynthesis, to stimulate NO production [30]. Application of these treatments and others may continue as modes of their action are better understood and application technologies developed. However, limitations to commercialization are not always solely related to effectiveness. Factors such as limited opportunities for patent control and small markets for many fruits and vegetables result in a lack of financial incentives to bear the cost of meeting the required regulatory approvals. 16.8 TRANSGENIC PLANT PRODUCTS 16.8.1 Genetically Modified Organisms Plant breeding has paralleled human civilization, being the basis of the shift from hunting and gathering to agriculture. Domestication of crops for agricultural production for the human diet has resulted in many of the staples such as rice, wheat, maize, and potatoes, and selection for desirable traits of quality, yield, and disease and pest resistance continues. Whatever the edible plant tissue, farmers usually select cultivars on the basis of marketability (visual qualities specific to the market of choice) and yield, because these factors directly affect economic sustainability. As discussed in Section 16.3.4, desirable characteristics can be in conflict with quality. Breeders have sometimes favored fruit and vegetable selections with better resistance to the handling abuses but yielding cultivars that have tougher skins and sometimes reduced eating quality. Many approaches have been used in plant breeding in addition to simple selection of plants with desirable attributes, including deliberate hybridization and mutation breeding [102]. Many fruit and vegetable crops have been generated by hybridization and selection (e.g., apple, strawberry, tomato, and squash) but the technology is limited by the requirement of two compatible plants in the same or closely related genus/species. Also, the possibility of transfer of undesirable traits Postharvest Physiology of Edible Plant Tissues 1075 along with desired traits is high. Mutation breeding relies on spontaneous variations of species, for example, semi-dwarf cereal crops, and apple strains with red coloration, or by exposure of seeds, cuttings, pollen, or tissue-cultured cells to physical or chemical mutagens. Mutation breeding is a random, nonspecific process, and can produce mutations that revert to the original phenotype and are chimeras.* More recently, transgenic technology, where a gene with desirable traits can be inserted into a host genome, has been used. Commonly known as genetically modified (GM) or genetically modified organisms (GMOs), the technology involves the insertion, or the upregulation and downregulation, of genes with specific functions. Genetic modification can be classified as “transgenic” where genes from other species are introduced into plants, or “cisgenic,” where only genes within the same species or closely related ones are used for transformation. Most commercial application has been on field crop production, especially resistance to herbicides, for example, glyphosate (Roundup), stress, and insect and disease resistance. This technology has also been used for fruits such as papaya, where a gene that resists ringspot disease virus (PRSV) has been inserted into the fruit [103]. The field is moving very rapidly, with new technologies such as “clustered regularly interspaced short palindromic repeats” (CRISPRs) being employed to carry out gene editing with unprecedented precision, efficiency, and flexibility [104]. The technique is in early stages, but can potentially be used to modify metabolic processes of edible plant tissues. Safety of GM food—principally concern about risks to human health, environmental impact, and perceptions of naturalness—has been elevated by groups opposed to its commercial development [105,106]. Relative hostility to GM foods in the EU, and the subsequent legislative barriers for their approval, is greater than in the United States [107]. Factors that affect public attitudes to GM foods include socioeconomic variables, individuals’ knowledge and scientific background, and parents’ education in science and religion [105]. Nevertheless, at least 36 countries have granted regulatory approval for GM crops since 1994, and more than 300 million acres of GM crops are grown by 17 million farmers in more than 25 countries. Safety evaluation of transgenic food is based on the “Principle of Substantial Equivalence,” in which the composition of the transgenic product is compared with that of the traditionally cultivated counterpart [108,109]. The objective of such comparisons is to detect unintended changes resulting from genetic modification. Examples of potential changes are toxicity, allergenicity, possible antibiotic resistance from GM crops, carcinogenicity from consuming GM foods, and alteration of nutritional quality (macro-, micro-, and anti-nutrients) [110]. All comparative studies on nutrients and natural toxicant composition of products such as potato, papaya, red pepper tomato, wheat, corn, and rice have found “substantial equivalence” in typical measurements including sugars, organic acids, carotenoids, alkaloids, VOCs, antioxidants, and minerals [103,110,111]. For edible plant products, however, most focus has been on gene modification that results in increases in nutritional quality or modification of the senescence and ripening processes to improve the maintenance of quality. 16.8.2 Nutritionally Enhanced Food Crops Biofortification of crops can take place by adding appropriate minerals or inorganic compounds to the fertilizer or by conventional plant breeding, but biotechnology allows direct cost-effective and sustainable methods to improve product attributes [103,112]. An example is biofortified rice in which the gene for β-carotene, the precursor molecule for vitamin A, has been inserted to provide higher vitamin A concentrations [113]. GM rice, known as Golden Rice, was the first crop specifically designed to combat malnutrition; vitamin A efficiency causes eye degeneration in three million children each year. Biofortification with β-carotene has been extended to maize and cassava. * Chimera: when cells of more than one genotype (genetic makeup) are found growing adjacent in the tissues of that plant. 1076 Fennema’s Food Chemistry Other GM crops include rice where gene insertions have been carried out to increase iron bioavailability and lower levels of phytic acid (an inhibitor of zinc absorption), and wheat to increase zinc content. A triple-vitamin-fortified maize expresses high amounts of β-carotene, ascorbate, and folate. Levels of celiac-disease-causing gliadins have been lowered in wheat. An interesting area of research is the development of “designer crops” where the levels of bioactive compounds that are important to human health are increased. Examples include increased omega-3 fatty acids in plant seed storage oils, and expression of anthocyanins and resveratrol in tomatoes. 16.8.3 Modification of Ripening and Senescence Processes Genetic modification of edible plant products, especially tomato, is commonplace in many laboratories and has led to increased understanding of ripening and senescence processes. The first GM food available for human consumption was the Flavr Savr tomato. This tomato, produced by Calgene, was genetically engineered by inserting an antisense gene for the cell wall softening enzyme PG (Section 16.6.2.1). While the shelf-life of the fruit was increased, positive effects on firmness were not realized, and production lasted only between 1994 and 1997 [114]. A similar GM tomato with downregulated PG gene expression, produced in England by Zeneca, resulted in tomato paste that was 20% cheaper. This product, labeled as genetically engineered, was popular in the market, but increased anti-GMO sentiment resulted in production being stopped [115]. Recently, transgenic apples and potatoes have received regulatory approval in the United States [116]. Apples with reduced PPO activity and associated low browning, trademarked as Artic apples, and GM “Innate” potatoes, produced by J.R. Simplot Co., are designed to resist blackspot bruising and browning and contain less asparagine. Lower asparagine concentrations reduce the potential for the formation of acrylamide, a possible carcinogen, during the frying of potatoes. 16.9 COMMODITY REQUIREMENTS 16.9.1 Cereals, Nuts, and Seeds Cereals, nuts, and seeds can typically be stored for extended periods provided that there is no insect infestation and water activity is low enough to prevent microbial growth. In contrast with fruits and vegetables, therefore, manipulations of the storage conditions for grains, nuts, and seeds is focused less on the product than on conditions that affect pests and microbial growth. Components of successful storage of these products include the following [117]: 1. Appropriate storage structures. Storages should protect grains, nuts, and seeds from external environmental factors such as rain and groundwater, minimize the effects of environmental temperature and humidity, and exclude insects, rodents, and birds. 2. Temperature control. Temperature does not directly affect the product quality, but affects activity of insects and populations of molds, yeast, and bacteria. 3. Humidity control. Humidity in the intergranular air reaches equilibrium with the moisture of the grains, nuts, and seeds within the storage. RH should be maintained ≤70% to prevent losses due to molds, yeast, and bacteria. Alternatives to synthetic pesticides include manipulation of temperature using forced aeration to modify the grain bulk microclimate to minimize pests and contamination while maintaining product quality; chilling of grain using refrigeration; and heat treatments. The gas composition within grain storages, which comprises about 50% of the volume of the storage structure, has lower O2 Postharvest Physiology of Edible Plant Tissues 1077 and higher CO2 concentrations than air depending on the levels of aeration. These atmospheres can be further modified (decreased O2 and/or elevated CO2 concentrations) to kill insects and inhibit pathogen growth. Inert dusts (e.g., clays, sands, ash, diatomaceous earth, synthetic silica) and mineral dusts (e.g., dolomite, lime), which function as desiccants, can be used to kill insects through abrasion of their cuticles and subsequent water loss. 16.9.2 Whole Fruits and Vegetables Each fruit and vegetable, and sometimes the cultivar within a species, has specific storage requirements that represent an integration of the factors discussed in Sections 16.3 and 16.7. Factors that impact commodity requirements include the following: 1. The maximum storage life that can be obtained, which is usually a function of the genetics of the cultivar and stage of maturity and/or ripening at the time of harvest. For example, tomatoes have much shorter storage potential than apples, but even within each group potential can vary from days to weeks and weeks to months, respectively. 2. The optimum storage temperatures based on sensitivity to chilling and freezing injury. Subtropical and tropical fruits, for example, tend to have higher rates of metabolism and are more susceptible to chilling injury than temperate fruits. 3. RH: Generally high for fruits and vegetables, as moisture loss results in adverse effects on appearance, texture, flavor, and weight. Rates of moisture loss depend on the inherent properties of the product such as cuticular and periderm properties; presence or absence of stomata, lenticels, trichomes, and hairs; and storage temperature, which affects transpiration rates. 4. Tolerances of the product to low O2 and CO2 concentrations. 5. Sensitivity of products to ethylene. Responses of edible plant products to these factors form the basis of published recommendations that are available from many sources including those easily accessible on the web [83]. The degree to which these recommendations are followed will depend on the specific industry involved and the level of sophistication available. A local market retail operation, for example, might store several products together and at temperatures that are inappropriate for some of them. Loss of quality can be negligible because of the limited time periods at these temperatures. In contrast, an apple storage facility that aims to store the fruit for 10 months must pay greater attention to choosing suitable cultivars, ensuring rapid cooling to optimum storage temperatures, utilizing supplementary technologies such as 1-MCP, and rapidly establishing the optimum CAs. 16.9.3 Fresh-Cut (Minimally Processed) Fruits and Vegetables The growth of the market for fresh-cut or minimally processed fruits and vegetables due to the convenience of ready-to-eat products that are perceived as healthy has been an exciting development in recent years. Fresh-cut processing affects food chemistry of edible plant tissues is many ways [118,119]. The most significant difference between fresh-cut and whole products is obviously the extensive cutting of tissues and the associated physiological changes to the former, including wound responses. Cutting of the products removes the natural protection of the epidermis and causes major tissue disruption, which results in the contact between enzymes and substrates and exposes tissue surfaces to microbes. Fresh-cut processing increases respiration rates, wound-induced ethylene, water activity, and surface area per unit volume, the latter of which may accelerate water loss. These physiological changes may be accompanied by flavor loss, cut surface discoloration, color loss, decay, increased rate of vitamin loss, rapid softening, shrinkage, and a shorter storage life. 1078 Fennema’s Food Chemistry Production of fresh-cut products involves a series of processes that are designed to minimize the microbial load of incoming raw materials through efficient preparation in clean temperatureand humidity-controlled environments [119]. Unit operations involved in preparation include the following: 1. Receiving and storage 2. Preliminary washing and sorting of product for appropriate maturity and ripeness stage that is suitable for cutting 3. Precutting and processing treatments 4. Peeling (if necessary) 5. Size reduction and cutting 6. Washing and cooling 7. Dewatering 8. Packaging Of these steps, common factors among whole fruits and vegetables that affect quality are ­cultivar selection appropriate for desired purposes, preharvest crop management, proper postharvest ­temperature and storage regimes, and the balance between harvest timing and quality (see Figure 16.4). A less mature fruit, for example, may be firmer and have better handling, shipping, and storage qualities, but may have lower aroma and flavor attributes. As with whole products, removal from the parent plant limits the energy resources available to continue “normal” postharvest metabolic activity. In contrast to whole products, the application of MAP is a common and often essential feature of quality maintenance in fresh-cut produce [120]. Because of the removal of epidermal barriers that provide resistance to gas diffusion, it is common to find optimal O2 and CO2 concentrations that are lower and higher, respectively, in fresh-cut than whole products. Also, fresh-cut products from chilling-sensitive fruits and vegetables are often stored at lower temperatures than the whole product because the part of the tissue that visually exhibits injury has been removed and/or storage periods are not long enough for CI symptoms to develop. Specific effects of fresh-cut processing that require good management are [118] as follows: 1. Mechanical damage. Sharp knives for cutting of fresh-cut products result in reduced damage and lower respiration rates compared with blunt knives. The smaller the cut product size, the higher the rates of respiration and ethylene production, and the greater the stimulation of PAL activity by ethylene. Wound-induced responses can include production of lignins (fibrous) and coumarins (bitter). Nutritional quality, especially vitamin C, might be decreased by water loss, exposure of tissue to light and air, enzymatic or chemical degradation, and sanitation chemicals such as chlorine. However, stability of vitamins is dependent on commodity type and temperature. The application of MAP, often a critical component of maintaining quality of fresh-cut products, can maintain nutritional compounds, but high CO2 in packages can result in more rapid degradation. Phenolic concentrations and antioxidant capacity of fresh-cut products can increase as a result of wounding, from 26% to 191% and 51% to 442%, respectively [121]. 2. Enzymatic browning. Reactions due to mixing phenolics and PPO activity can result in rapid browning after cutting, especially with products with high concentrations of preformed phenolic compounds (apple, artichokes, peach, pear, potato). Also, synthesis and accumulations of phenolics in products such as lettuce that have low concentrations at time of cutting can be stimulated by injury. Treatments applied to reduce enzymatic browning include ascorbic acid and other acidulants and/or sulfites, and O2 and high CO2 atmospheres (MAP). Postharvest Physiology of Edible Plant Tissues 1079 3. Undesirable changes in coloration. Loss of chlorophyll and exposure of yellow or colorless carotenoids in green vegetables leads to unacceptable yellowing, while pheophytin formation can result in tissue browning. Cut carrots can develop whitening on the surface, which is associated with desiccation and sloughing of the outer cell layers and/or lignin formation. Pink or brown stains (“russet spotting”) on lettuce are associated with the exposure of tissues to ethylene. Depending on the disorder, control measures include low temperature, MAP, humidity control, edible coatings, and antioxidants. 4. Softening. Pectic enzymes released during cutting can cause tissue softening, though mainly in parts of the product in contact with the cut surface. Texture changes also may occur because of dehydration. Control of these disorders can be minimized by appropriate temperature and humidity control. Ethylene production can accelerate softening, and in part can be controlled by MAP. Additional treatments of cut products with calcium salts are frequently employed to maintain firmness. 5. Pithiness. Development of airspaces in cortical* tissues of celery and radishes, known as aerenchyma,† is an undesirable feature. The disorder is controlled by low temperature and MAP. 6. Off-flavors and off-odors. Most typically, undesirable flavors and odors are associated with MAP in which O2 concentrations are too low and or CO2 concentrations are too high for the product. Appropriate selection of packaging films and avoidance of temperature ­fluctuations that result in changes in respiration rates are important control strategies. Another cause of off-flavors and off-odors results from wound-stimulated increases in secondary metabolites, such as chlorogenic acid in grated carrots and sesquiterpenes in fresh-cut pineapple. 7. Translucency. Translucency, a physiological disorder in which liquid accumulates in cellular free spaces, occurs in fresh-cut tomato and melon. A preprocessing factor that causes this defect is calcium deficiency in the tissues, although the disorder can be alleviated by maintenance of low temperature and MAP, and 1-MCP treatment to slow ethylene-­mediated responses. 16.10 CONCLUSIONS Edible plant products in the form of staple crops, fruits, and vegetables provide major sources of energy, proteins, carbohydrates, vitamins, and other health-promoting compounds for the world’s population. This population, about 7.5 billion in 2016, is predicted to reach over 9 billion by 2050. Food availability and security is an important part of political stability across the globe, and represents a huge societal challenge. Increased production of edible plant products is needed for feeding the world population, but at the same time we face diminished utilizable arable land, problems with food distribution, increased use of plant resources for animal production, environmental concerns, and climate change. Furthermore, the more affluent the consumer, the more critical he or she is about wanting products that are blemish-free and of uniform size and color, safe from infectious pathogens and without pesticide residues, and often with increasing emphasis on sustainability. These challenges will primarily be addressed at the field level, with emphasis on plant breeding and production practices that will result in higher yields of uniform products with reduced losses due to cosmetic factors. However, a significant improvement in the world food supply can be obtained by reducing the high rates of product losses after harvest in both developed and developing countries. Many of the staple crops have low perishability, but most edible plant products have relatively short storage potential. The topics covered in this chapter have outlined the underlying food (bio)chemistry * Cortical: relating to cortex, unspecialized cells lying between the epidermis and vascular tissues. † Aerenchyma: soft, spongy tissue containing large intercellular air spaces. 1080 Fennema’s Food Chemistry that affects the quality of edible plant products, both whole and fresh-cut, and the technologies that can be imposed to reduce the rates of metabolism that result in unacceptable product quality for the consumer. Application of these technologies is uneven, sometimes because of basic requirements for electricity. Others, such as genetic modification, have incredible potential to improve the nutritional quality and increase the storage potential of edible plant products but remain controversial. REFERENCES 1. USDA Food Composition Databases, USDA Nutrient Data Laboratory, ndb.nal.usda.gov/ndb/search/list, 2014. Accessed September 30, 2016. 2. Zeeman, S.C., J. Kossmann, and A.M. Smith, Starch: Its metabolism, evolution, and biotechnological modification in plants. Annual Review of Plant Biology, 2010. 61:209–234. 3. Food And Agriculture Organization Of The United Nations, FAOSTAT, faostat.fao.org.proxy.library.­ cornell.edu, 2015. Accessed September 30, 2016. 4. FAO, Global food losses and food waster - Extent, causes and prevention. http://www.fao.org/docrep/014/ mb060e/mb060e00.pdf, 2011. 5. Kader, A.A., Increasing food availability by reducing postharvest losses of fresh produce. Acta Horticulturae, 2005. 682:2169–2175. 6. Watkins, C.B. and J.H. Ekman, How postharvest technologies affect quality, in Environmentally Friendly Technologies for Agricultural Produce Quality, S. Ben-Yoshua, Ed. 2005, CRC Press: Boca Raton, FL, pp. 333–396. 7. Shewfelt, R.L., What is quality? Postharvest Biology and Technology, 1999. 15:197–200. 8. Kays, S.J., Postharvest Physiology of Perishable Plant Products. 1997, Athens, Greece: Exon Press. 9. Brecht, J.K., M.A. Ritenour, N.F. Haard, and G.W. Chism, Postharvest physiology of edible plant tissues, in Fennema’s Food Chemistry, S. Damodaran, K.L. Parkin, and O.R. Fennema, Eds. 2008, pp. 975– 1049, CRC Press: Boca Raton, FL. 10. Sams, C.E. and W.S. Conway, Preharvest nutritional factors affecting postharvest physiology, in Postharvest Physiology and Pathology of Vegetables, J.A. Bartz and J.K. Brecht, Eds. 2003, Marcel Dekker: New York, pp. 161–176. 11. Kader, A.A. and M.E. Saltveit, Respiration and gas exchange, in Postharvest Physiology and Pathology of Vegetables, J.A. Bartz and J.K. Brecht, Eds. 2003, Marcel Dekker: New York, pp. 7–30. 12. Ben-Yehoshua, S., R.M. Beaudry, S. Fishman, J. Jayanty, and N. Mir, Modified atmosphere packaging and controlled atmosphere storage, in Environmentally Friendly Technologies for Agricultural Produce Quality, S. Ben-Yoshua, Ed. 2005, CRC Press: Boca Raton, FL, pp. 61–112. 13. Dillahunty, A.L., T.J. Siebenmorgen, R.W. Buescher, D.E. Smith, and A. Mauromoustakos, Effect of moisture content and temperature on respiration rate of rice. Cereal Chemistry, 2000. 77:541–543. 14. Trewavas, A.J., Growth substances in context: A decade of sensitivity. Biochemical Society Transactions, 1992. 20:102–108. 15. Cherian, S., C.R. Figueroa, and H. Nair, ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. Journal of Experimental Botany, 2014. 65:4705–4722. 16. Manjunatha, G., V. Lokesh, and N. Bhagyalakshmi, Nitric oxide in fruit ripening: Trends and opportunities. Biotechnology Advances, 2010. 28:489–499. 17. Munné-Bosch, S. and M. Müller, Hormonal cross-talk in plant development and stress responses. Frontiers in Plant Science, 2013. 4: 529. 18. Murphy, A., Hormone crosstalk in plants. Journal of Experimental Botany, 2015. 66:4853–4854. 19. Gapper, N.E., R.P. McQuinn, and J.J. Giovannoni, Molecular and genetic regulation of fruit ripening. Plant Molecular Biology, 2013. 82:575–591. 20. Van de Poel, B. and D. Van der Straeten, 1-Aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Frontiers in Plant Science, 2014. 5:640. 21. Cara, B. and J.J. Giovannoni, Molecular biology of ethylene during tomato fruit development and maturation. Plant Science, 2008. 175:106–113. 22. Ju, C. and C. Chang, Mechanistic insights in ethylene perception and signal transduction. Plant Physiology, 2015. 169:85–95.