
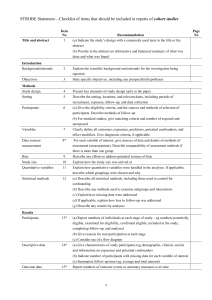
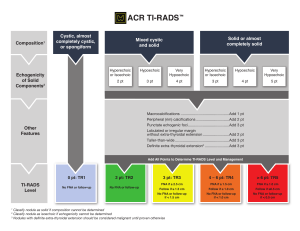
ORIGINAL ARTICLE Pain Physiology Education Improves Health Status and Endogenous Pain Inhibition in Fibromyalgia A Double-Blind Randomized Controlled Trial Jessica Van Oosterwijck, PT, PhD,*wz Mira Meeus, PT, PhD,*w Lorna Paul, PT, PhD,y Mieke De Schryver, PT, MSc,* Aurelie Pascal, PT, MSc,* Luc Lambrecht, MD, PhD,8 and Jo Nijs, PT, PhD*wz Objectives: There is evidence that education on pain physiology can have positive effects on pain, disability, and catastrophization in patients with chronic musculoskeletal pain disorders. A doubleblind randomized controlled trial (RCT) was performed to examine whether intensive pain physiology education is also effective in fibromyalgia (FM) patients, and whether it is able to influence the impaired endogenous pain inhibition of these patients. Methods: Thirty FM patients were randomly allocated to either the experimental (receiving pain physiology education) or the control group (receiving pacing self-management education). The primary outcome was the efficacy of the pain inhibitory mechanisms, which was evaluated by spatially accumulating thermal nociceptive stimuli. Secondary outcome measures included pressure pain threshold measurements and questionnaires assessing pain cognitions, behavior, and health status. Assessments were performed at baseline, 2 weeks, and 3 months follow-up. Repeated measures ANOVAS were used to reveal possible therapy effects and effect sizes were calculated. Results: After the intervention the experimental group had improved knowledge of pain neurophysiology (P < 0.001). Patients from this group worried less about their pain in the short term (P = 0.004). Long-term improvements in physical functioning (P = 0.046), vitality (P = 0.047), mental health (P < 0.001), and general health perceptions (P < 0.001) were observed. In addition, the intervention group reported lower pain scores and showed improved endogenous pain inhibition (P = 0.041) compared with the control group. Discussion: These results suggest that FM patients are able to understand and remember the complex material about pain Received for publication May 25, 2012; revised October 28, 2012; accepted November 4, 2012. From the *Department of Human Physiology, Faculty of Physical Education & Physiotherapy, Vrije Universiteit Brussel; zDepartment of Physical Medicine and Physiotherapy, University Hospital Brussels, Brussels; wDepartment of Health Care Sciences, Division of Musculoskeletal Physiotherapy, Artesis University College Antwerp, Antwerp; 8Private Practice for Internal Medicine, Gent/Aalst, Belgium; and yNursing and Health Care, School of Medicine, University of Glasgow, Glasgow, UK. J.V.O. is financially supported by grant no. OZR1596 from the research council of the Vrije Universiteit Brussel, Brussels, Belgium. M.M. is a postdoctoral research fellow of the Research Foundation Flanders (FWO). The remaining authors declare no conflict of interest. Reprints: Jo Nijs, PT, PhD, Department of Human Physiology, Faculty of Physical Education & Physiotherapy, Vrije Universiteit Brussel, Building L-3rd floor, Pleinlaan 2, Brussels BE-1050, Belgium (e-mail: jo.nijs@vub.ac.be). Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.clinicalpain.com. Copyright r 2013 by Lippincott Williams & Wilkins Clin J Pain Volume 29, Number 10, October 2013 physiology. Pain physiology education seems to be a useful component in the treatment of FM patients as it improves health status and endogenous pain inhibition in the long term. Key Words: patient education, conditioned pain modulation, spatial summation, rehabilitation, central sensitization. (Clin J Pain 2013;29:873–882) F ibromyalgia (FM) is characterized by the presence of chronic widespread pain1,2 and studies have demonstrated exaggerated pain responses after sensory stimulation of healthy tissues.3 This has led to the hypothesis that the central nervous system is hyperexcitable in these patients and that pain could be perceived in the absence of a nociceptive input, due to induction of irreversible plasticity changes in the central nervous system (CNS).4 Staud et al5,6 found evidence for this hypothesis in FM patients and demonstrated that hypersensitivity of the CNS or central sensitization is a characteristic of FM. Sensitivity to pain results from the outcome of the balance between pain facilatory and inhibitory pathways, which either potentiate (descending facilitation) or suppress (descending inhibition) the passage of nociceptive messages to the brain.7 Malfunctioning of these central inhibitory pathways seems to be responsible for abnormal spatial summation and contributes to central sensitization in people with chronic pain.8–10 In FM patients a disturbed and/or impaired descending nociceptive inhibition has been observed.10 It has been shown that pain inhibitory systems are not optimally recruited by spatial summation procedures (SSP) using noxious conditioning stimuli in FM patients, in contrast to chronic low back pain patients and pain-free individuals.10,11 Although some researchers have observed normal spatial summation responses,12 efficient activation of the pain modulatory systems in FM12–14 has only been established when short and/or innocuous stimuli are utilized. The dysfunctional descending nociceptive inhibitory mechanism as seen in FM is primarily biological, but is influenced by negative and maladaptive thoughts, emotions, cognitions, and behaviors like catastrophizing, hypervigilance, avoidance behavior, and somatization.3 These negative cognitions can develop when FM patients do not understand the origin of their generalized musculoskeletal chronic pain and can facilitate pain. Therefore they need to be addressed during therapy, for instance, through education.15,16 Most of the educational models have limited efficacy in decreasing pain and disability in patients with chronic musculoskeletal pain, and may even increase patients’ fears www.clinicalpain.com | 873 Van Oosterwijck et al and pain.17 However, clinical studies have shown that education concerning pain physiology is able to influence negative, maladaptive cognitions about pain, improve pain behavior and pain-free movement performance, and reduce pain in chronic musculoskeletal disorders.17 Although this type of education has been shown to reduce pain, we were interested to know if pain physiology education is able to directly influence endogenous pain inhibition. Although pain physiology education has been studied in patients with chronic low back pain,18–20 chronic whiplash,21 and chronic fatigue syndrome in combination with widespread pain,22 studies in FM are lacking. In addition, previous studies in patients with chronic musculoskeletal pain have only studied immediate posttreatment effects. The present study examined whether reconceptualisation of pain, by pain physiology education, was able to influence (1) pain cognitions, (2) health status, and (3) endogenous nociceptive processing in FM patients. In addition, long-term effects of pain physiology education were examined. MATERIALS AND METHODS Setting and Patients Patients with FM were referred for study participation from a private practise for internal medicine. To be included into the study, participants had to (1) have FM as defined by the criteria of the 1990 ACR,2 (2) be between 18 and 65 years of age, and (3) have Dutch as their native language. A power analysis was performed based on the outcomes of the perceived pain scores during a SSP procedure (similar to the SSP procedure used in this study) from a previous study, which examined the efficacy of pain inhibition in chronic pain patients.23 The analysis revealed that 15 participants per group were required to obtain a power between 0.865 and 0.985, and with a significance level of 0.01. This double-blind, randomized controlled clinical trial (ClinicalTrials.gov identifier NCT00857740) was conducted between March 2009 and June 2010 at the Department of Human Physiology, Vrije Universiteit Brussel, Belgium and the Division of Musculoskeletal Physiotherapy, Artesis University College Antwerp, Belgium. Because of the long study duration (3 mo follow-up) patients were able to continue with their medication, but were asked to abstain from analgesics and antidepressants at least 24 hours before the assessments and not to commence any new treatments during the study. Ethical approval was obtained from the Ethics Committee of the University Hospital Brussels/Vrije Universiteit Brussel. Patients provided written informed consent. Measurements At the first day of the study baseline assessments were performed, that is, a battery of questionnaires was filled out and pain was assessed using pressure algometry and a SSP. After the initial education session, patients from both groups were asked to fill out the Neurophysiology of Pain Test and undergo pressure pain threshold (PPT) measurements once again. The patients underwent the same clinical assessments as in baseline at 2 weeks and after 3 months follow-up, conducted by the same blinded assessor. The procedure of the study and the CONSORT diagram for the patient flow through the study are presented in Figure 1. 874 | www.clinicalpain.com Clin J Pain Volume 29, Number 10, October 2013 Primary Outcome Measures The SSP was chosen as the primary outcome measure. The examiner marked the patients dominant arm at the following locations: (1) proximal interphalangeal joints, (2) mid-palm, (3) wrist, (4) distal-third of the forearm, (5) middle-third of the forearm, (6) elbow, (7) mid-biceps, and (8) shoulder, dividing the limb into 8 segments as shown in Figure 2. The dominant arm was gradually immersed into warm circulating water (461C) starting with the fingertips (first segment) until the whole arm was immersed. Each segment was immersed for 2 minutes. The participants were asked to rate their pain on a score of 100, every 15 seconds of the 2-minute immersion period. The 2-minute immersions were alternated with 5 minutes rest where the arm was withdrawn from the water and wrapped in a towel. This procedure was described by Marchand and Arsenault24 who used this protocol to investigate inhibitory mechanisms and pain perception in pain-free people. The same procedure has been used with noxious cold or hot water to examine the efficacy of the pain inhibitory mechanisms in patients with FM,10 chronic low back pain,10 and chronic fatigue syndrome with chronic widespread pain.23 Secondary Outcome Measures Self-reported questionnaires include the Fibromyalgia Impact Questionnaire (FIQ) (scores range from 0 to 100, average FM patients score about 50 and severely affected patients score >70),25 the Medical Outcomes Short Form 36 Health Status Survey (SF-36) (8 separate subscales with scores ranging from 0 to 100, with improvement as scores increase),26 the Pain Coping Inventory (PCI) (consisting of 3 scales representing active coping strategies, ie, transformation, distraction, reducing demands, and 3 scales representing passive coping strategies; ie, ruminating, retreating, resting; with higher scores indicating more frequent application of that specific coping strategy),27 the Pain Catastrophizing Scale (PCS) (consisting of a general score and 3 subscales, ie, helplessness, magnification, rumination; higher scores indicating more severe catastrophic thoughts about pain),28,29 the Pain Vigilance and Awareness Questionnaire (PVAQ) (scores range from 0 to 80 and high scores indicate hypervigilance for pain),30 the Tampa Scale Kinesiophobia (TSK) (scores range from 17 to 68, with scores >37 indicating high fear of movement),31,32 and the Neurophysiology of Pain Test—patient version (scores ranging from 0 to 19, with high scores representing a better knowledge regarding pain neurophysiology).19,33 As patients were Dutch speaking, we used the Dutch translations of the above mentioned questionnaires. Clinical assessment comprised pressure algometry to measure PPTs bilaterally at the spine (5 cm left and right of the spinous process of T8 and L3),34 the upper trapezius muscle (pars descendens midway, between the seventh cervical vertebra and the tip of the acromion),35 the proximal third of the calf, and the web between index finger and thumb36 using an analog Wagner algometer (Wagner Instruments, Greenwich, CT). The threshold was determined as the mean of the 2 last values out of 3 consecutive measurements (10 s in between).37 Randomization Thirty patients participated in this study and were randomly allocated to either the experimental group (n = 15) or the control group (n = 15) using a simple randomization procedure.38,39 Therefore patients drew r 2013 Lippincott Williams & Wilkins Clin J Pain Volume 29, Number 10, October 2013 Intensive Pain Physiology Education for FM Patients numbered lots that were marked either 1 (control group) or 2 (experimental group) from a bag. Neither the patients, nor the researcher who performed the assessments (J.V.O.) were informed about the allocation (double-blind design). The patients were aware of participating in an experiment in which 2 educational sessions were compared and that the drawn lot determined the allocation, but the design and the interventions were not explained in terms of control or experimental groups. The researcher who delivered both the control and the experimental intervention was blinded to the outcomes of the measurements. However, this researcher was aware of the study hypothesis. At the end of the trial, the success of the blinding procedure was examined, both among the patients and the assessor, by asking them whether they thought the participant received the experimental or control intervention. ENROLLMENT Intervention The patients in the experimental group received 2 oneon-one educational sessions about the neurophysiology of pain, whereas the patients of the control group received 2 one-on-one educational sessions about activity self-management techniques. The sessions were delivered by a bachelor in physiotherapy (M.D.S. educated 19 patients, March to May 2009; A.P. educated 11 patients, November 2009 to May 2012) who received training from 2 qualified physiotherapists (J.V.O. and J.N.) with experience in providing education. A standard PowerPoint presentation was used by the therapist to guide the patient through the first educational session, but the therapist tailored the education session to the patients’ specific experiences. Pictures, examples, and metaphors were used to maximize the intelligibility. The session had a duration of 30 minutes. FM-patients assessed for eligibility (n=115) Excluded (n=85) • • Not meeting inclusion criteria (n=25) Declined to participate (n=60) Measure 1 - Baseline: • Questionnaires Pressure algometry • • Spatial summation procedure ALLOCATION Randomized (n=30) Experimental group (n=15) session 1: Education about pain neurophysiology D A Y Control group (n=15) session 1: Education about activity management 1 Measure 2: • Questionnaire: the Neurophysiology of Pain Test • Pressure algometry Information brochure about pain neurophysiology to read at home Information brochure about activity management to read at home Session 2: Education about pain neurophysiology (by telephone) Session 2: Education about activity management (by telephone) D A Y 7 FOLLOW-UP Lost to follow-up (n=3): • Familial reason • Lost the ability to drive the car • Hospitalized because of a blood cloth D A Y Measure 3: Questionnaires • Pressure algometry • Spatial summation procedure • Measure 4: Questionnaires • Pressure algometry • • Spatial summation procedure • Effectiveness of blinding 14 M O N T H Lost to follow-up (n=1): • Relocation 3 ANALYSIS Experimental group analysed (n=15) • Intention-to-treat analysis by last observation-carried-forward approach (n=3) Control group analysed (n=15) • Intention-to-treat analysis by last observation-carried-forward approach (n=1) FIGURE 1. CONSORT diagram for the patient flow through the study protocol. r 2013 Lippincott Williams & Wilkins www.clinicalpain.com | 875 Van Oosterwijck et al Clin J Pain Volume 29, Number 10, October 2013 were. Patients were motivated and coached to apply their new insights into their daily life. Statistical Analysis FIGURE 2. Effect of intervention on the mean pain score slopes from the spatial summation procedure at 3 months follow-up. Estimated marginal means covarying for sex and medication use (analgesics or antidepressants). Error bars represent SE of the mean. Interaction effects between group and slope of the mean pain scores during the spatial summation procedures were examined using repeated measures ANOVA (P = 0.041). The experimental group received education about pain neurophysiology, which covers the physiology of the nervous system in general and of the pain system in particular. Pain physiology education focuses on informing the patient on the difference between “nociception” and “pain,” and teaches patients that the CNS has the ability to increase or decrease its sensitivity (neuroplasticity) to help them cope with persistent pain.17 The content of the education sessions and the pictures were based on the book “Explain Pain” by Butler and Moseley40 and an overview can be found in Table S1 (Supplemental Digital Content 1 http://links. lww.com/CJP/A56). The control group received education about pacing self-management techniques, teaching FM patients how to manage their daily activities with respect to their symptoms. This activity management strategy is used to encourage patients to achieve an appropriate balance between activity and rest to avoid exacerbation and to set realistic goals for increasing activity.41 The oral education was completed by a leaflet containing written information about either pain neurophysiology or activity management. Patients were asked to take the brochure home and to read it several times. One week after the initial education session, the researcher that provided the education contacted the patients for a second educational intervention session delivered by telephone. Patients were asked whether they had read and understood the brochure and if they had any questions that could be answered by the researcher. In addition the questions on the Neurophysiology of Pain Test, which were incorrectly answered after the first educational session were explained once again to the interventional group. Patients from both the experimental and the control groups were asked if they had tried to apply what they had learned during the education in their daily life and what their experiences 876 | www.clinicalpain.com All data were analyzed using IBM SPSS Statistics 19.0 for Windows (IBM Corporation, Somers, NY). Normality of the variables was tested with the Kolmogorov-Smirnov test and appropriate descriptive statistics were calculated. Comparability of the groups before the intervention was studied with the Fisher exact test and the independent samples t test. Possible changes in the outcomes of the questionnaires and the PPTs in response to the intervention were examined between the 2 groups using repeated measures analysis of variance. Intervention (education about pain physiology vs. education about pacing self-management techniques) served as the between-subjects factor and time (baseline vs. the follow-up treatment scores) served as the within-subjects factor. An 8-factor repeated measures analysis of variance on the data of the SSP (segments group interaction) was used to identify differences between the 2 groups in pain intensity evolution or slope during the immersion procedure. Confounding effects of sex might play an important role in the findings of impaired pain inhibitory system activation in patients with chronic pain, and differences in pain modulatory responses between male and female FM patients have been reported.8,42,43 Many participants indicated that they did not comply with the request to avoid analgesics and antidepressants for at least 24 hours before testing. For these reasons sex and medication (analgesics and antidepressants) use were entered as covariates in the repeated measures analysis of the SSP. Four FM patients did not attend the 3-month followup assessment (experimental group, n = 3; control group, n = 1). The reason for their absence and the patient flow throughout the study are represented in Figure 1. We therefore conducted intention-to-treat analysis using the last observation-carried-forward approach. The significance level was set at 0.05 for all statistical tests and effect sizes were obtained by calculating Cohen’s d.44 The Cohen’s d effect size was defined as the mean baseline value minus the mean follow-up value divided by the pooled SD of both. A d value around 0.20 is described as small, around 0.50 as medium (moderate), and near 0.80 as large.44 RESULTS As shown in Table 1 there were no significant differences for demographic and baseline characteristics between the experimental group and the control group. Primary Outcome Measures Efficacy of the endogenous pain inhibitory mechanisms was assessed using the SSP and results showed no significant differences between the experimental and the control groups in baseline (P = 0.960) or after 2 weeks (P = 0.521), but there was a significant difference after 3 months (P = 0.041). At 3 months follow-up, the mean pain scores in the experimental group were lower than those in the control group. Regarding the slope of the mean scores, pain scores increased as the immersed surface increased in the control group, whereas pain scores decreased slightly in the intervention group (Fig. 2). r 2013 Lippincott Williams & Wilkins Clin J Pain Volume 29, Number 10, October 2013 Intensive Pain Physiology Education for FM Patients TABLE 1. Patient Demographic and Baseline Characteristics Age (y) (mean [SD]) Sex (n [%]) Male Female Employment status (n [%]) Full time Part time Unemployed or on disability Onset symptom (mo) (mean [SD]) Patients using analgesics (n [%]) Patients using antidepressants (n [%]) Received academic education about neurophysiology before study participation (n [%]) Experimental Group (n = 15) Control Group (n = 15) 45.8 (9.5) 45.9 (11.5) 3 (20) 12 (80) 1 (6.7) 14 (93.3) BetweenGroup Comparison (P) 0.973 0.598 0.697 2 (13.3) 3 (20) 10 (66.7) 156 (96) 2 (13.3) 5 (33.3) 8 (53.3) 116 (46) 0.162 12 (80) 1.000 11 (73.3) 6 (40) 0 (0) 7 (46.7) 0 (0) 1.000 1.000 Secondary Outcome Measures The experimental group and the control group were comparable in baseline regarding the secondary outcome measures (P < 0.05). After the intervention no significant differences in PPTs were found (P-values between 0.323 and 0.889) between the 2 groups. However, we found significant changes in some of the self-reported outcome measures in response to the treatment. Scores on the Neurophysiology of Pain Test significantly increased in response to the experimental intervention (P < 0.001), whereas no significant effects were established in response to the control intervention (P = 0.150). When the 2 groups were compared this resulted in a significant difference (P < 0.001) between the 2 groups, and the effect size was large (d = 1.97). In addition, the experimental and the control groups showed some significant changes in health status (SF-36). Although scores for physical functioning (P = 0.046) improved from baseline to 3 months follow-up in the experimental group, scores declined in the control group. Scores on the general health perceptions scale (P < 0.001) increased between baseline and 3 months follow-up in the experimental group, in the control group scores increased in response to the intervention but declined at 3 months follow-up. A large effect size was established (d = 0.98). In response to the intervention, scores on the vitality scale (P = 0.047) slightly decreased 2 weeks posttreatment in the experimental group, but increased at 3 months follow-up. In the control group, a linear decrease was established and the effect size was small. Mental health scores (P < 0.001) increased from baseline to 2 weeks and 3 months follow-up in the experimental group, whereas they decreased in the control group resulting in a large effect size. A significant change in the PCI subscales transformation (P = 0.027) and worrying (P = 0.004) was established 2 weeks after treatment. However, at 3 months follow-up the changes r 2013 Lippincott Williams & Wilkins were no longer significant (transformation, P = 0.079; worrying, P = 0.141). Although the mean scores of the remaining self-reported outcome measures, that is, PCS, PVAQ, FIQ, and TSK decreased the changes were not significant (P-values between .064 and .929). The mean scores and effect sizes of the secondary outcome measures are represented in Table 2. Efficacy of Blinding Procedure At the end of the study, 2 of the 13 FM patients from the experimental group thought that they had received the experimental intervention and 11 thought that they had received the control intervention. Six of the 14 FM patients from the control group thought that they had received the experimental intervention and 8 thought that they had received the control intervention. The assessor’s assumption regarding the allocation was right in 9 from the 15 cases in the experimental group and in 8 from the 15 cases in the control group. DISCUSSION The results of this study suggest that education about pain physiology can be a useful component in the treatment of FM patients, as it can be used to improve health status and endogenous pain inhibition in the long term. Influencing Descending Nociceptive Processing The results of our study suggest that pain physiology education had a positive effect on descending nociceptive processing at 3 months follow-up. In pain-free individuals, the mean pain intensity scores over the different segments vary between 26 and 45 during the SSP, whereas in FM patients scores between 49 and 77 have been observed.10 In our study, FM patients from the experimental group showed lower pain scores (varying between 52 and 58), than patients from the control group (varying between 59 and 68), 3 months after the intervention. In the control group, pain scores tended to augment as the immersed surface increased, which has been observed before in FM patients10 and indicates a deficit of the endogenous pain inhibitory systems. In the experimental group, pain perception was not related to the stimulated surface area, which indicates that the inhibitory efferents counterbalance the facilitatory afferents efficiently as seen in healthy controls.24 Could it be possible that pain physiology education is able to improve descending nociceptive processing? As descending facilitatory pathways depart from brain areas that are involved in the regulation of cognitive and emotional reactions, such as the limbic system, these cognitions and emotions can modulate the activity in the descending pathways.45 Negative cognitions and emotions, which can develop when patients do not understand their condition or the cause of experiencing pain, can facilitate pain through these descending pathways. Thus on theoretical basis, one can assume that altering maladaptive thoughts and cognitions could result in less top down pain facilitation and an improved descending nociceptive inhibition of the CNS, which in turn can lead to less pain and improvements in movement performance. Indeed, the current study findings can confirm this theory to certain extent. If pain physiology education can be used to improve endogenous pain inhibition, as suggested by our findings, this could be a feasible mechanism to explain previously reported improvements in pain18,21 and (pain-free) movement performance15,21 after pain physiology education. www.clinicalpain.com | 877 Clin J Pain Van Oosterwijck et al Volume 29, Number 10, October 2013 TABLE 2. Changes in Secondary Outcome Measures After the Intervention Experimental Group (n = 15) Mean (SD) Neurophysiology of pain test Measurement 1: baseline Measurement 2: postintervention Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up FIQ Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 physical functioning Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 role limitations due to physical pain Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 bodily pain Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 general health perceptions Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 vitality Measurement 1: Baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 social functioning Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 role limitations due to emotional problems Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up SF-36 mental health Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCI transformation Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCI distraction Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCI reducing demands Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCI worrying Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCI retreating Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCI resting Measurement 1: baseline 5.5 10.9 11.4 11.3 (2.9) (3.4) (2.3) (3.0) Control Group (n = 15) Mean (SD) 5.9 7.1 6.8 7.2 Within-Group Comparison (F; P) Cohen’s d ES 10.3; <0.001* 1.97w 3.3; 0.079 0.36 3.3; 0.046* 0.27 1.0; 0.388 0.29 0.5; 0.602 0.28 9.2; <0.001* 0.98w 3.2; 0.047* 0.19 3.1; 0.064 0.20 1.7; 0.189 0.24 9.2; <0.001* 0.89w 2.7; 0.079 0.36 0.7; 0.929 0.14 0.2; 0.861 0.00 2.0; 0.141 0.36 0.5; 0.624 0.00 1.3; 0.269 0.16 (3.8) (3.7) (4.3) (4.2) 38.7 (10.7) 34.9 (10.1) 36.5 (9.9) 59.4 (12.9) 58.7 (15.4) 60.1 (10.5) 47.7 (22.7) 51.0 (21.6) 53.7 (21.8) 49.7 (17.9) 45.7 (17.1) 45.3 (12.3) 18.3 (34.7) 25.0 (35.4) 28.3 (35.2) 13.3 (22.9) 5.0 (10.4) 15.0 (26.4) 37.1 (19.2) 45.8 (25.8) 42.5 (19.9) 40.3 (15.8) 49.2 (20.2) 52.4 (21.5) 24.7 (10.6) 32.8 (15.5) 37.7 (15.5) 31.47 (12.8) 33.3 (14.0) 28.6 (12.8) 36.3 (17.8) 35.7 (18.5) 40.0 (21.0) 42.2 (14.3) 38.5 (13.1) 35.3 (13.7) 63.1 (21.5) 63.8 (27.0) 58.1 (27.4) 48.4 (17.2) 54.9 (20.2) 61.2 (15.9) 71.1 (45.2) 60.5 (43.1) 59.9 (47.5) 42.2 (14.3) 38.5 (13.1) 35.3 (13.7) 60.8 (17.3) 61.9 (22.4) 66.7 (17.5) 62.0 (19.5) 60.1 (20.8) 48.5 (18.3) 2.1 (0.5) 1.8 (0.6) 1.9 (0.6) 1.7 (0.6) 1.9 (0.7) 1.9 (0.7) 2.2 (0.6) 2.1 (0.8) 2.1 (0.8) 2.1 (0.6) 1.9 (0.6) 2.0 (0.7) 2.2 (0.6) 2.3 (0.8) 2.2 (0.9) 2.0 (0.8) 2.0 (0.9) 2.1 (0.8) 1.7 (0.6) 1.5 (0.5) 1.5 (0.5) 1.7 (0.6) 1.7 (0.7) 1.6 (0.5) 1.7 (0.7) 1.6 (0.6) 1.7 (0.6) 1.9 (0.6) 1.9 (0.7) 1.8 (0.6) 2.1 (0.4) 1.9 (0.7) (Continued ) 878 | www.clinicalpain.com r 2013 Lippincott Williams & Wilkins Clin J Pain Volume 29, Number 10, October 2013 Intensive Pain Physiology Education for FM Patients TABLE 2. (continued) Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCS helplessness Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCS magnification Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCS rumination Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PCS total Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PVAQ Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up TSK Measurement 1: baseline Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PPT thoracic spine Measurement 1: baseline Measurement 2: postintervention Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PPT lumbar spine Measurement 1: baseline Measurement 2: postintervention Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PPT trapezius Measurement 1: baseline Measurement 2: postintervention Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PPT calf Measurement 1: baseline Measurement 2: postintervention Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up PPT hand Measurement 1: baseline Measurement 2: postintervention Measurement 3: 14 d follow-up Measurement 4: 3 mo follow-up Experimental Group (n = 15) Mean (SD) Control Group (n = 15) Mean (SD) 1.9 (0.6) 2.0 (0.8) 1.9 (0.7) 2.1 (0.6) 9.5 (5.3) 6.7 (6.0) 6.7 (5.8) 11.1 (5.8) 10.5 (5.6) 9.6 (5.5) 2.8 (2.4) 2.2 (2.5) 1.7 (2.1) 3.0 (2.5) 3.2 (2.1) 3.3 (2.5) 7.1 (5.0) 6.1 (3.8) 5.1 (4.2) 7.7 (2.7) 7.5 (3.3) 7.5 (4.0) 19.5 (11.8) 14.9 (11.6) 13.3 (11.6) 21.9 (9.9) 20.5 (10.2) 20.4 (12.3) 35.3 (14.5) 34.4 (11.7) 32.2 (13.7) 39.7 (12.6) 42.6 (15.1) 40.3 (14.4) 38.7 (10.7) 34.9 (10.1) 36.5 (9.9) 40.7 (8.4) 39.8 (7.1) 39.9 (8.2) 2.4 2.7 2.8 2.4 (1.5) (1.4) (1.7) (1.5) 2.3 2.1 2.2 2.2 (1.8) (2.1) (2.2) (2.5) 2.8 2.9 3.1 3.1 (1.8) (1.7) (1.8) (2.0) 2.6 2.8 2.7 2.7 (2.3) (2.6) (2.7) (2.6) 1.7 1.5 1.6 1.5 (1.2) (0.9) (1.1) (1.0) 2.7 1.1 1.1 1.3 (6.0) (1.2) (1.3) (1.5) 2.7 2.7 2.7 2.9 (1.5) (1.5) (1.7) (1.9) 1.9 2.1 1.8 2.1 (1.0) (1.0) (1.1) (1.8) 2.9 2.1 2.8 2.4 (1.6) (1.3) (1.9) (1.5) 2.3 1.9 1.9 1.9 (1.4) (1.7) (1.7) (1.9) Within-Group Comparison (F; P) Cohen’s d ES 1.3; 0.265 0.53 2.3; 0.109 0.50 1.6; 0.219 0.49 1.9; 0.158 0.53 1.3; 0.279 0.21 1.0; 0.360 0.21 1.3; 0.286 0.00 0.4; 0.667 0.16 0.7; 0.409 0.18 0.1; 0.895 0.12 1.9; 0.157 0.32 Statistically significant results are marked with “*” and large ES are marked with “w”. ES indicates effect size; FIQ, Fibromyalgia Impact Questionnaire; PCI, Pain Coping Inventory; PCS, Pain Catastrophizing Scale; PPT, pressure pain threshold; PVAQ, Pain Vigilance and Awareness Questionnaire; SF-36, Short Form 36 Health Status Survey; TSK, Tampa Scale Kinesiophobia. However, we did not find any evidence to support that pain inhibition can be improved by altering negative cognitions through pain physiology education, as the observed reductions were not significantly relevant. It is possible that short-term alterations in pain cognitions occurred as seen in other studies, which could have led to an improved pain inhibition. Although it is clear that the central nervous r 2013 Lippincott Williams & Wilkins system shows remarkable plasticity, we do not know how long it takes for changes to appear. In this study, the changes in the pain inhibitory systems could be detected after 3 months. This indicates that changes of the CNS need time to occur and that when examining the influence of an intervention on descending nociceptive processing, a sufficient long follow-up is required. In contrast, we cannot www.clinicalpain.com | 879 Van Oosterwijck et al exclude the possibility that the pain physiology intervention influenced other cognitions then the ones that were measured in this study and that this contributed to the positive treatment effects. It has been previously shown that pain physiology education will result in a reduction of brain activity in the cortical pain matrix.20 This observation, together with the current findings regarding the positive effects on descending nociceptive inhibition, suggests that this type of education has an influence on central pain processing. However, these observations need to be confirmed, and future studies using objective and sophisticated measures of pain and nociceptive processing are required to test this hypothesis. In addition, further analysis of data from existing studies needs to be performed to identify mediators, moderators, and predictors of the established treatment effects, and will provide us with insights on how pain physiology education exactly influences central pain processing. Understanding Pain Physiology FM patients were able to understand and remember the complex material about pain physiology, as shown with the scores on the Neurophysiology of Pain Test, which increased immediately after patients received a one-to-one educational session about pain physiology. After the second educational session, a further small increase in the Neurophysiology of Pain Test scores was observed. Although it has been shown that using only written education on pain physiology is insufficient to change illness perceptions and health status in FM,46 the results of this study indicate that giving FM patients written information to read at home in addition to a one-on-one session is a valuable part of educational therapy. This is especially important for those patients with memory or concentration problems. In addition, patients were encouraged to apply what was learned in daily life, and had the chance to discuss questions and experiences over the telephone with the therapist. The new acquired knowledge about pain physiology was maintained, even after 3 months. Influencing Cognitions, Behavior, and Health Patients reported to ruminate or worry less in response to their pain at 2 weeks follow-up. However, we were not able to establish any long-term effects on pain cognitions or pain behavior. Although Meeus et al22 and Moseley15 showed improvements in pain catastrophizing due to pain physiology education in chronic fatigue syndrome and chronic low back pain patients, respectively, those studies reported immediate posttreatment effects and did not examine longer term effects. Because of the length of time required for baseline measures (± 105 min) combined with the educational session (± 30 min), we did not subject the patients to immediate posttreatment measures except for algometry and the Neurophysiology of Pain Test. It is possible that education about pain physiology has an influence on pain coping and catastrophizing in FM, but that these effects are only present at short term, and that they will lead to improvements in health status and endogenous pain inhibition in the long term. Future studies should try to asses immediate posttreatment effects as well as include several follow-up assessments to study both short-term and long-term effects. Although pain physiology education did not have any long-term effects on cognitions or behavior linked to pain, there was an effect on general health perceptions, mental health, and behavior. FM patients from the experimental 880 | www.clinicalpain.com Clin J Pain Volume 29, Number 10, October 2013 group showed improvements in their general health perceptions and mental health with a large effect size. These patients also reported an improved physical functioning and more vitality. We can conclude that education about pain physiology improves the health status of FM patients. Strengths and Limitations Although this study is limited by a relatively small sample, the sample size was determined based on an a priori power analysis. In addition, Cohen’s d effect sizes from significantly changed outcome measures were presented and varied from 0.19 to 1.97, representing values for the 58th to the 98th percentile, indicating that the average person in the experimental group scored higher than 58% to 98% of the control group. It could seem a concern that the study sample only included 4 males (13.3%); however, this is in line with prevalence of FM, which is a disorder that is predominant in females. It has been reported that 13% of the FM patients are male, whereas 87% are female.47 One of the strengths of this study is the control intervention, that is, education about activity self-management techniques. Not only was the format from the control intervention similar then the format of the experimental intervention, it allowed us to provide a useful intervention for all study participants. It is a limitation that because of practical issues it was impossible to withhold the study hypothesis from the researcher/clinician who delivered the interventions. However, it must be clarified that the educator was aware that the control intervention, that is, pacing activity management, has positive benefits and is often included in rehabilitation programs for FM patients, thus delivering both interventions with the same enthusiasm. FM patients were recruited using the 1990 ACR criteria,2 which have been used for 20 years by physicians to diagnose FM, and these criteria have recently been adapted.1 This was done to make the diagnosis more practical for physicians. Besides widespread pain, the new 2010 criteria considers other important somatic symptoms often experienced by FM patients and allow differentiation among patients according to the level of FM symptoms.1 The new criteria were published in May 2010. The recruitment for this study took place between March 2009 and March 2010, therefore patients were diagnosed using the 1990 criteria. Because the 1990 criteria were more stringent than the 2010 criteria, we believe that the conclusions of this study also apply to FM patients diagnosed according to the 2010 criteria. CONCLUSIONS FM patients are able to understand and remember the complex material about pain physiology. Education about pain physiology in FM will lead to less worrying in the short term, and long-term improvements in vitality, physical functioning, mental health, and general health perceptions. No significant changes were established in pain catastrophizing, hypervigilance, or kinesiophobia. Although PPTs remained the same, a positive effect was found on endogenous pain inhibition at 3 months follow-up. Education of pain physiology seems to be a useful component in the treatment of FM patients. Future studies should further examine the underlying, explanatory mechanisms of the efficacy of pain physiology education in chronic pain patients, and the effect of pain physiology education in conjunction with other treatment components or therapies. r 2013 Lippincott Williams & Wilkins Clin J Pain Volume 29, Number 10, October 2013 REFERENCES 1. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. 2. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. 3. Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007;26:465–473. 4. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. 5. Staud R, Price DD, Robinson ME, et al. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–696. 6. Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. 7. Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. 8. Granot M, Weissman-Fogel I, Crispel Y, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus, painfulness, gender and personality variables matter? Pain. 2008;136:142–149. 9. Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–7692. 10. Julien N, Goffaux P, Arsenault P, et al. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. 11. de Souza JB, Potvin S, Goffaux P, et al. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain. 2009;25:123–127. 12. Staud R, Vierck CJ, Robinson ME, et al. Spatial summation of heat pain within and across dermatomes in fibromyalgia patients and pain-free subjects. Pain. 2004;111:342–350. 13. Staud R, Koo E, Robinson ME, et al. Spatial summation of mechanically evoked muscle pain and painful after sensations in normal subjects and fibromyalgia patients. Pain. 2007;130: 177–187. 14. Staud R, Robinson ME, Goldman CT, et al. Attenuation of experimental pain by vibro-tactile stimulation in patients with chronic local or widespread musculoskeletal pain. Eur J Pain. 2011;15:836–842. 15. Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain. 2004; 8:39–45. 16. Nijs J, Van Houdenhove B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: application of pain neurophysiology in manual therapy practice. Man Ther. 2009;14:3–12. 17. Louw A, Diener I, Butler DS, et al. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil. 2011;92: 2041–2056. 18. Moseley GL. Combined physiotherapy and education is efficacious for chronic low back pain. A randomised controlled trial. Aust J Physiother. 2002;48:297–302. 19. Moseley GL. Unraveling the barriers to reconceptualization of the problem in chronic pain: the actual and perceived ability of patients and health professionals to understand the neurophysiology. J Pain. 2003;4:184–189. 20. Moseley GL. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: FMRI evaluation of a single patient with chronic low back pain. Aust J Physiother. 2005;51:49–52. r 2013 Lippincott Williams & Wilkins Intensive Pain Physiology Education for FM Patients 21. Van Oosterwijck J, Nijs J, Meeus M, et al. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. J Rehabil Res Dev. 2011;48:43–58. 22. Meeus M, Nijs J, Van Oosterwijck J, et al. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared to pacing and self-management education: a double-blind randomized controlled trial. Arch Phys Med Rehabil. 2010;91:1153–1159. 23. Meeus M, Nijs J, Van de Wouwer N, et al. Diffuse noxious inhibitory control is delayed in chronic fatigue syndrome: an experimental study. Pain. 2008;139:439–448. 24. Marchand S, Arsenault P. Spatial summation for pain perception: interaction of inhibitory and excitatory mechanisms. Pain. 2002;95:201–206. 25. Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol. 1991;18:728–734. 26. Ware J, Snow K, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. 27. Kraaimaat FW, Bakker AH, Evers AW. Pain coping strategies in chronic pain patients: the development of the Pain Coping Inventory (PCI). Gedragstherapie. 1997;22:267–277. 28. Crombez G, Vlaeyen JW, Heuts PH, et al. Pain-related fear is more disabling than pain itself: evidence on the role of painrelated fear in chronic back pain disability. Pain. 1999;80: 329–339. 29. Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7: 524–532. 30. McCracken LM. “Attention” to pain in persons with chronic pain: a behavioural approach. Behav Ther. 1997;28:271–284. 31. Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behaviour. Pain Manag. 1990;3:35–43. 32. Vlaeyen JW, Kole-Snijders AM, Boeren RG, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. 33. Meeus M, Nijs J, Elsemans KS, et al. Development and properties of the Dutch neurophysiology of pain test in patients with chronic fatigue syndrome. J Musculoskelet Pain. 2010;18:58–65. 34. Farasyn A, Meeusen R. The influence of non-specific low back pain on pressure pain thresholds and disability. Eur J Pain. 2005;9:375–381. 35. Johnston V, Jimmieson NL, Jull G, et al. Quantitative sensory measures distinguish office workers with varying levels of neck pain and disability. Pain. 2008;137:257–265. 36. Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109: 497–499. 37. Farasyn A, Meeusen R. Pressure pain thresholds in healthy subjects: influence of physical activity, history of lower back pain factors and the use of endermology as a placebo-like treatment. J Bodyw Mov Ther. 2003;7:53–61. 38. Daniel J. Sampling Essentials: Practical Guidelines for Making Sampling Choices. Thousand Oaks: SAGE; 2011. 39. Singh G. Randomization made easy for small size controlled clinical trial. JIAMSE. 2006;16:75–78. 40. Butler D, Moseley GL. Explain Pain. Adelaide: NOI Group Publishing; 2003. 41. Nijs J, Paul L, Wallman K. Chronic fatigue syndrome: an approach combining self management with graded exercise to avoid exacerbations. J Rehabil Med. 2008;40:241–247. 42. Staud R, Robinson ME, Vierck CJ, et al. Diffuse noxious inhibitory control (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or in fibromyalgia patients. Pain. 2003;101:167–174. 43. van Wijk G, Velhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–419. www.clinicalpain.com | 881 Van Oosterwijck et al 44. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale NJ: Lawrence Earlbaum Associates; 1988. 45. Zusman M. Forebrain-mediated sensitization of central pain pathways: “non-specific” pain and a new image for MT. Man Ther. 2002;7:80–88. 46. van Ittersum MW, van Wilgen CP, Groothoff JW, et al. Is appreciation of written education about pain neurophysiology 882 | www.clinicalpain.com Clin J Pain Volume 29, Number 10, October 2013 related to changes in illness perceptions and health status in patients with fibromyalgia? Patient Educ Couns. 2011;85: 269–274. 47. Bazelmans E, Vercoulen JH, Galama JM, et al. Prevalence of chronic fatigue syndrome and primary fibromyalgia syndrome in THE Netherlands. Ned Tijdschr Geneeskd. 1997;141: 1520–1523. r 2013 Lippincott Williams & Wilkins






![[Myoung-jae Lee] Micro-Econometrics for Policy, Pr(BookFi)](http://s2.studylib.es/store/data/009050455_1-b46867032a881a39b32fae19b7a60af1-300x300.png)