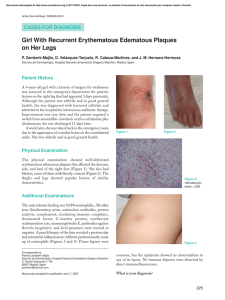
25/3/2019 DynaMed Plus: Erysipelas Erysipelas Overview and Recommendations Background Erysipelas is an acute bacterial infection of the dermis and hypodermis which may also involve cutaneous lymphatics. Definitions of erysipelas vary among experts and geographic regions: Classically, erysipelas is defined as an infection limited to the upper dermis with prominent superficial lymphatic involvement (in contrast to cellulitis, which involves deeper dermis and subcutaneous fat). Alternatively, the term erysipelas can be used to refer to a skin infection occurring only on the face. In Europe, erysipelas is often used as a synonym for cellulitis. In this topic, we will use the classic definition unless otherwise stated. Group A beta-hemolytic Streptococcus is the most common cause but group C or G streptococci and Staphylococcus aureus are also reported causes. Common predisposing conditions include epidermal injury, lymphedema, chronic venous insufficiency, or systemic illnesses such as diabetes and hypertension. Evaluation A diagnosis is made clinically, based on physical examination findings. A typical presentation is a brightly erythematous plaque raised above the level of surrounding skin with a clear line of demarcation between the involved and uninvolved tissue. Skin findings overlap with those of cellulitis and distinguishing the two is not always possible. Features that favor the diagnosis of erysipelas over cellulitis include: area of inflammation raised above surrounding skin distinct demarcation between involved and normal skin Laboratory testing is typically not needed. Management Penicillin has classically been the treatment of choice for erysipelas. Due to a variation of use of the terms cellulitis and erysipelas in practice and in the medical literature, the Infectious Disease Society of America combined treatment recommendations for cellulitis and erysipelas in their 2014 guidelines. For patients with mild infection (for example, without signs of systemic infection), select an antimicrobial agent active against streptococci (Strong recommendation), such as: penicillin V 250-500 mg orally every 6 hours for adults 125-250 mg orally every 6-8 hours for children ≥ 12 years old 25-50 mg/kg/day orally in 3-4 divided doses (maximum 3 g/day) for children < 12 years old a cephalosporin, such as cephalexin cephalexin 500 mg orally every 6 hours for adults 25-50 mg/kg/day orally in equally divided doses in children ≥ 1-14 years old 250 mg every 6 hours or 500 mg every 12 hours for children ≥ 15 years old 1/20 25/3/2019 DynaMed Plus: Erysipelas Treatment modifications should be made for patients with a more severe illness, patients with risk factors for methicillin-resistant Staphylococcus aureus (MRSA), or unusual pathogens and host factors such as immunocompromise. The recommended duration of antimicrobial therapy is 5 days, but treatment should be extended if the infection has not improved within this time period (Strong recommendation). Elevate the affected area to help with drainage and to hasten improvement, and treat predisposing factors and underlying causes, if identified (Strong recommendation). Prevention Erysipelas recurs in approximately 10%-40% of cases. Measures to reduce recurrence of erysipelas include: treating predisposing conditions such as tinea pedis or interdigital maceration reducing peripheral edema with elevation, compressive stockings, pressure pumps, or diuretics if appropriate Consider prophylactic antibiotics in patients with 3-4 episodes of cellulitis per year despite attempts to treat and control predisposing conditions (Weak recommendation), with potential regimens including penicillin or erythromycin orally twice daily for 4-52 weeks or penicillin G benzathine intramuscularly every 2-4 weeks. Related Summaries Cellulitis Group A Streptococcus Staphylococcus aureus Skin and soft tissue infections (SSTI) (list of topics) General Information Description erysipelas refers to a diffuse, superficial spreading infection of the skin(1) Also called superficial cutaneous cellulitis St. Anthony's fire Definitions definitions of skin and soft tissue infections Infection Erysipelas Cellulitis Folliculitis Furuncle Carbuncle Cutaneous abscess Definition Superficial infection of the skin involving lymphatics Acute infection of skin involving deep dermis and subcutaneous fat Superficial infection of hair follicles with purulence in epidermis Hair follicle infection with associated small subcutaneous abscess Cluster of furuncles Localized collection of pus within dermis and deeper skin tissue 2/20 25/3/2019 DynaMed Plus: Erysipelas Pyomyositis Purulent infection of skeletal muscle, often with abscess Superficial infection of skin characterized by Impetigo pustules or vesicles that progress to crusting or bullae Ecthyma Deeper variant of impetigo evolving into ulcer Clostridium myonecrosis (gas gangrene) Necrotizing infection involving muscle Aggressive infection of subcutaneous tissue Necrotizing fasciitis spreading along fascial planes Reference - Ann Intern Med 2018 Feb 6;168(3):ITC17 definitions of erysipelas vary among experts and geographic regions(1, 2, 3) classically, erysipelas is defined as infection limited to the upper dermis with prominent superficial lymphatic involvement alternatively, the term erysipelas can be used to refer to a skin infection occurring only on the face in Europe, erysipelas is often used as a synonym for cellulitis DynaMed commentary – in this topic, we will use the classic definition unless otherwise stated Epidemiology Who is most affected erysipelas may be more common in(3) infants young children older adults Incidence/Prevalence skin and soft tissue infections (SSTIs) are among the most common reasons for ambulatory care visits, emergency department visits, and hospital admission worldwide(1, 3) no validated data is available to determine the precise worldwide incidence of erysipelas or its comparative prevalence to cellulitis variations in use of the terms cellulitis and erysipelas also make quantification difficult in the United States, overall incidence of skin and soft tissue infections approximately 4.8 cases per 100 person-years (BMC Infect Dis 2015 Aug 21;15:362) United Kingdom hospital incidence data reported 69,576 episodes of cellulitis and 516 episodes of erysipelas in 2004-2005 (BMJ Clin Evid 2008 Jan 2;2008 full-text) Risk factors any disruption in skin barrier, such as(1, 2, 3) trauma or surgery skin conditions such as fungal infection including tinea pedis (athlete's foot) inflammatory dermatoses including eczema or psoriasis impetigo or ecthyma herpes simplex infection toe web intertrigo (cutaneous inflammation on opposing skin surfaces) fissured toe webs from maceration previous cutaneous damage such as abrasion insect bite 3/20 25/3/2019 DynaMed Plus: Erysipelas puncture wound ulcer postvaccination exposure of neonatal umbilical stump (rare) any disruption of venous or lymphatic flow, such as(1) venous insufficiency lymphedema lymphatic damage due to prior episodes of erysipelas and/or cellulitis obesity Etiology and Pathogenesis Pathogen group A beta-hemolytic streptococci (GABHS) believed to be the most common pathogens(1, 3) groups C or G streptococci or Staphylococcus aureus are less commonly reported causes(1, 3) GABHS most commonly identified organisms by direct immunofluorescence of skin biopsy specimens based on 2 cohort studies prospective cohort of 27 patients with clinically diagnosed erysipelas erysipelas distinguished from cellulitis based on presence of well-defined, sharp, elevated margin of skin lesions streptococcal species detected in skin biopsies of 18 patients with erysipelas group A streptococci detected in 13 patients group G streptococci detected in 4 patients group C streptococci detected in 1 patient an additional 8 patients had serologic evidence of streptococcal infection no causative organism was identified in 1 patient Reference - Arch Dermatol 1989 Jun;125(6):779 cohort of 12 patients with erysipelas group A streptococci detected by immunofluorescence in skin specimens of 11 of 12 patients with erysipelas group A streptococci isolated from blood in 1 of those 11 patients 7 of 11 patients had serologic evidence of group A streptococcal infection etiology in 1 patient unclear, but S. aureus isolated from superficial wound culture Reference - J Invest Dermatol 2010 May;130(5):1365 full-text streptococci most commonly isolated organisms from blood in bacteremic patients with erysipelas and cellulitis based on systematic review 607 patients with erysipelas or cellulitis identified in systematic review 4.6% had positive blood cultures 75% of positive cultures due to streptococcal species S. pyogenes in 46% other groups of beta-hemolytic streptococci in 29% S. aureus in 14% gram-negative organisms (Citrobacter diversus, Escherichia coli, Proteus morganii) in 11% Reference - J Infect 2012 Feb;64(2):148 Pathogenesis any breach in the skin barrier can serve as portal of entry for bacteria and establishment of infection(1, 3) in classic erysipelas(3) group A beta-hemolytic streptococci (GABHS) is the most common infecting agent infection is typically limited to the upper dermis 4/20 25/3/2019 DynaMed Plus: Erysipelas lymphatic involvement is prominent lower extremities are most often involved(3) any breach in skin of the lower extremities can serve as a portal of entry dermatophyte infections, such as athlete's foot, may additionally serve as a reservoir for GABHS, augmenting risk of infection when erysipelas involves the face(3) preceding streptococcal sore throat is common precise mode of spread from throat to skin is unclear History and Physical Clinical presentation legs most common site of erysipelas involvement based on retrospective cohort study 981 patients (median age 61 years) with 1,142 episodes of erysipelas were evaluated 567 episodes treated in outpatient setting 575 episodes treated in hospital setting recurrent erysipelas in 35% sites of involvement leg in 68% arm or hand in 12% face in 9% Reference - BMC Infect Dis 2015 Sep 30;15:402 full-text lower extremities most commonly involved in patients hospitalized with erysipelas, and fever present in majority of patients based on retrospective cohort study 223 patients (median age 61 years) hospitalized with erysipelas in Poland were evaluated recurrent erysipelas in 22% site of involvement lower extremity in 80.3% face in 13% upper extremity in 6.7% systemic manifestations included fever in 80.7% chills in 46.6% risk factors or portal of entry identified in 72% mechanical trauma in 19.7% tinea pedis and nails in 16.5% venous insufficiency in 15.2% leg ulcers in 12.5% surgery in 8% comparing men vs. women risk factor or portal of entry mechanical trauma in 28.2% vs. 12.5% (p = 0.03) venous insufficiency in 5.8% 23.3% (p = 0.001) leg ulcers in 5.8% vs. 18.3% (p = 0.004) blood testing parameters mean white blood cells 10.5 cells/mm3 vs. 9 cells/mm3 (p = 0.007) mean C-reactive protein 129.5 mg/L vs. 91.2 mg/L (p = 0.04) Reference - Przegl Epidemiol 2016;70(4):575 lower extremities appear to be most common site of involvement in patients hospitalized with erysipelas, with cutaneous fungal infections most frequently identified portals of entry based on retrospective cohort study 99 adults (median age 54 years) with erysipelas admitted to a single hospital in Greece were evaluated 5/20 25/3/2019 DynaMed Plus: Erysipelas diagnosis of erysipelas was made clinically based on classic presentation reported sites of involvement lower extremity in 66% face in 17% upper extremities in 6% associated symptoms fever present in 25% regional lymphadenopathy in 22% risk factors or portal of entry identified in 89% cutaneous fungal infection/tinea pedis in 32% venous insufficiency in 23% dermatologic diseases in 22% skin trauma in 20% insect bite in 13% surgery in 4% lymphedema in 3% Reference - Int J Dermatol 2010 Sep;49(9):1012 History Chief concern (CC) bright, red, tender, warm, painful skin lesion with well-demarcated and raised borders(1, 2, 3) History of present illness (HPI) skin lesions seen with erysipelas expand rapidly, usually over a period of several days(1, 3) affected area is typically bright, red, and raised margins of the affected area are sharp, with clear delineation from surrounding normal skin central resolution of erythema may occur as the outer margins of the lesion continue to expand edema often accompanies erysipelas, and can be significant(1, 3) blebs and bullae may form eyes may be swollen shut in facial erysipelas regional lymph nodes may also swell and become tender(1) systemic symptoms, such as fever and chills, occur in a minority and are typically mild(1, 3) Past medical history (PMH) ask about skin conditions or infections which may serve as portals of entry such as(1, 2, 3) tinea pedis (athlete's foot) impetigo eczema ulcerations toe web intertrigo fissured toe webs from maceration herpes simplex ask about recent injuries such as(1, 3) skin trauma (often undetectable on examination) local surgery for patients with facial erysipelas, ask about recent strep throat(3) an association between strep throat and facial erysipelas is reported mechanism of spread to skin is unclear Physical 6/20 25/3/2019 DynaMed Plus: Erysipelas General physical systemic symptoms are typically mild, when present, but can vary and may include(1) fever tachycardia confusion Skin look for a bright, erythematous lesions with well-defined borders(1, 2, 3) affected skin may be warm and tender to touch lesion is typically raised borders of the lesions clearly delimit involved and uninvolved tissue central clearing may be present note that associated edema can be significant(1, 3) skin may resemble an orange peel (peau d'orange), with dimpling due to swelling around hair follicles vesicles, blebs, or bullae may form palpate regional lymph nodes, which may be tender, warm, or swollen(1) photograph of erysipelas can be found in Acta Dermatovenerol Alp Panonica Adriat 2007 Sep;16(3):123 HEENT when erysipelas affects the face(2) both sides of the face may be involved, in a butterfly distribution associated swelling may be significant, and eyes may be shut unilateral distribution of rash that does not cross midline may suggest herpes zoster Extremities look for any potential portal of entry, such as(1, 2, 3) tinea pedis or other fungal infection affecting the skin abrasions dermatologic diseases, such as psoriasis toe web fissures or maceration intertrigo examine any known breach in the skin, such as a surgical incision or site of trauma carefully(1, 3) Diagnosis Making the diagnosis erysipelas is a clinical diagnosis based on the presence of spreading inflammation of the skin, characterized by(1, 2, 3) a brightly erythematous lesion with well demarcated borders an area of inflammation raised above surrounding skin regional lymphadenopathy clinical features of erysipelas and cellulitis overlap, and distinguishing between them is not always possible or necessary as treatment strategies are similar(1, 2, 3) Differential diagnosis 7/20 25/3/2019 DynaMed Plus: Erysipelas cellulitis erysipelas classically involves more superficial layers of the skin and cutaneous lymphatics, whereas cellulitis extends more deeply into subcutaneous tissues features that favor diagnosis of erysipelas include area of inflammation raised above surrounding skin distinct demarcation between involved and normal skin in cellulitis, no clear distinction between infected and uninfected skin is present both erysipelas and cellulitis may present with signs of local inflammation (warmth, erythema, and pain) as well as lymphangitis and lymphadenitis References - (1, 3), N Engl J Med 1996 Jan 25;334(4):240 additional considerations on the differential diagnosis include(3) contact dermatitis urticaria early herpes zoster erysipeloid infection caused by gram-positive Erysipelothrix rhusiopathiae typically found in meat and fish handlers characterized by red maculopapular lesion on hands and fingers gout superficial thrombophlebitis deep vein thrombosis (DVT) inflammatory breast cancer systemic disorders such as systemic lupus erythematosus (SLE) Sweet syndrome familial Mediterranean fever (FMF) in increasingly ill patients, or if presence of widespread petechiae and ecchymoses indicating systemic toxicity, consider(1, 2) necrotizing fasciitis - within hours or days becomes dusky with bullae formation myositis or myonecrosis toxic shock syndrome Testing overview erysipelas is typically a clinical diagnosis and testing is not usually needed(1, 2, 3) in patients with signs of systemic toxicity, consider(1, 2) blood tests, including complete blood count with differential creatinine bicarbonate creatine phosphokinase C-reactive protein (CRP) blood cultures positive in ≤ 5% of cases overall may be helpful in immunocompromised patients or when atypical pathogen is suspected cultures of aspirate, swab, or skin biopsy generally reserved for atypical cases or cases not responding to empiric therapy(1, 2) imaging not needed for diagnosis but may be helpful in identifying complications or other diagnoses(1) ultrasound may be useful in identifying subcutaneous accumulation or pus or abscess x-ray or magnetic resonance imaging (MRI) may aid in the detection of underlying osteomyelitis MRI may help differentiate cellulitis from necrotizing fasciitis, but definitive diagnosis requires urgent surgical exploration see the Diagnosis subsection in Cellulitis for additional information Treatment 8/20 25/3/2019 DynaMed Plus: Erysipelas Treatment overview penicillin has classically been the treatment of choice for erysipelas(1, 3) due to variation of use of the terms cellulitis and erysipelas in practice and in the medical literature, the Infectious Disease Society of America (IDSA) combined treatment recommendations for cellulitis and erysipelas in 2014 guidelines(1) for patients with mild infection (for example, without signs of systemic infection), select antimicrobial agent active against streptococci, such as (IDSA Strong recommendation, Moderate-quality evidence) penicillin V 250-500 mg orally every 6 hours for adults 125-250 mg orally every 6-8 hours for children ≥ 12 years old 25-50 mg/kg/day orally in 3-4 divided doses (maximum 3 g/day) for children < 12 years old a cephalosporin, such as cephalexin 500 mg orally every 6 hours for adults 25-50 mg/kg/day orally in equally divided doses in children ≥ 1 to 14 years old 250 mg every 6 hours or 500 mg every 12 hours for children ≥ 15 years old dicloxacillin adult dosage 500 mg orally 4 times daily pediatric dosage 25-50 mg/kg/day orally in 4 divided doses clindamycin adult dosage 300-450 mg orally 4 times daily pediatric dosage 25-30 mg/kg/day orally in 3 divided doses for patients with moderate infection (for example, with signs of systemic infection), select an IV antimicrobial agent active against streptococci, such as penicillin G adults dosage 2-4 million units IV every 4-6 hours pediatric dosage 60-100,000 units/kg/dose IV every 6 hours ceftriaxone adult dosage 1-2 g IV once daily pediatric dosage 50-75 mg/kg IV intramuscularly once daily or in divided doses every 12 hours (maximum 2 g/day) cefazolin adult dosage 1 g IV every 8 hours pediatric dosage 50 mg/kg/day in 3 divided doses clindamycin adult dosage 600 mg IV every 8 hours pediatric dosage 25-40 mg/kg/day IV in 3 divided doses need for treatment of methicillin-sensitive Staphylococcus aureus (MSSA) in addition to streptococci controversial many experts include coverage against MSSA (IDSA Weak recommendation, Lowquality evidence) recommended options for treatment of both streptococci and MSSA include cefazolin and clindamycin for patients with severe infection (for example, with signs of systemic infection suggesting sepsis), treat broadly until a pathogen is identified, with a regimen that targets both streptococci and methicillin-resistant Staphylococcus aureus, such as vancomycin plus piperacillintazobactam (IDSA Strong recommendation, Moderate-quality evidence) vancomycin adult dosage 30 mg/kg/day IV in 2 divided doses pediatric dosage 40 mg/kg/day IV in 4 divided doses piperacillin-tazobactam adult dosage 3.375 g IV every 6 hours or 4.5 g IV every 8 hours pediatric dosage 60–75 mg/kg/dose of the piperacillin component IV every 6 hours modifications may be made based on severity of illness, risk factors for methicillin-resistant S. aureus (MRSA), or unusual pathogens and host factors such as immunocompromise 9/20 25/3/2019 DynaMed Plus: Erysipelas see the Treatment section of Cellulitis for additional information recommended duration of antimicrobial therapy is 5 days, but treatment should be extended if the infection has not improved within this time period (IDSA Strong recommendation, High-quality evidence)(1) elevation of the affected area may help drainage and hasten improvement(3) underlying causes, such as tinea pedis, should be treated if identified(3) Treatment setting 2014 Infectious Disease Society of America (IDSA) guidelines on management of skin and soft tissue infections recommendations for hospitalization(1) outpatient therapy recommended for patients who do not have systemic inflammatory response syndrome (SIRS), altered mental status, or hemodynamic instability (IDSA Strong recommendation, Moderate-quality evidence) hospitalization recommended if concern for a deeper or necrotizing infection, for patients with poor adherence to therapy, for infection in a severely immunocompromised patient, or if outpatient treatment is failing (IDSA Strong recommendation, Moderate-quality evidence) Medications 2014 Infectious Diseases Society of America (IDSA) guideline on diagnosis and management of skin and soft tissue infection mild cellulitis without signs of systemic infection antimicrobial agent active against streptococci recommended for non-purulent cellulitis (IDSA Strong recommendation, Moderate-quality evidence) antibiotic options penicillin V 250-500 mg orally every 6 hours for adults a cephalosporin, such as cephalexin 500 mg orally every 6 hours for adults dicloxacillin adult dosage 500 mg orally 4 times daily pediatric dosage 25-50 mg/kg/day orally in 4 divided doses clindamycin adult dosage 300-450 mg orally 4 times daily pediatric dosage 25-30 mg/kg/day orally in 3 divided doses consider regimen active against methicillin-resistant Staphylococcus aureus (MRSA) or both streptococci and MRSA for patients who do not respond to initial therapy or patients with purulent abscess, MRSA risk factors, including penetrating trauma (especially due to injection drug use) or MRSA colonization or infection antibiotic options clindamycin (dosed as above) DynaMed commentary – MRSA has variable resistance to clindamycin, consider local susceptibilities co-trimoxazole (TMP-SMX) with or without amoxicillin or other beta-lactam antibiotic if streptococci still suspected co-trimoxazole dosing adult dosage 1-2 double-strength tablets orally twice daily pediatric dosage 8-12 mg/kg/day (based on trimethoprim component) orally in 2 divided doses amoxicillin dosing adult dosage typically 500 mg every 12 hours or 250 mg every 8 hours pediatric dosage typically 25 mg/kg/day in divided doses every 12 hours or 20 mg/kg/day in divided doses every 8 hours doxycycline with or without amoxicillin or other beta-lactam antibiotic if streptococci still suspected doxycycline dosage 100 mg orally twice daily in patients ≥ 8 years old 10/20 25/3/2019 DynaMed Plus: Erysipelas amoxicillin dosed as above linezolid adult dosage 600 mg orally twice daily pediatric dosage 10 mg/kg orally every 12 hours for children aged 5-11 years 10 mg/kg orally every 8 hours for children < 5 years old cellulitis with signs of systemic infection (moderate nonpurulent infection) IV antibiotics active against streptococci are indicated penicillin G adult dosage 2-4 million units IV every 4-6 hours pediatric dosage 60-100,000 units/kg/dose every 6 hours ceftriaxone typical adult dosage 1-2 g IV once daily cefazolin adult dosage 1 g IV every 8 hours pediatric dosage 50 mg/kg/day in 3 divided doses clindamycin adult dosage 600 mg IV every 8 hours pediatric dosage 25-40 mg/kg/day IV in 3 divided doses need to treat for methicillin-sensitive S. aureus (MSSA) in addition to streptococci controversial many experts include coverage against MSSA (IDSA Weak recommendation, Low-quality evidence) options for treatment of both streptococci and MSSA include cefazolin and clindamycin for patients with risk factors for MRSA infection or purulent cellulitis vancomycin, daptomycin or other antimicrobial effective against both streptococci and MRSA recommended (IDSA Strong recommendation, Moderate-quality evidence) antibiotic options vancomycin adult dosage 30 mg/kg/day IV in 2 divided doses pediatric dosage 40 mg/kg/day IV in 4 divided doses other options with activity against both streptococci and MRSA linezolid adult dosage 600 mg orally twice daily pediatric dosage 10 mg/kg orally every 12 hours for children aged 5-11 years 10 mg/kg orally every 8 hours for children < 5 years old telavancin - typical dose 10 mg/kg IV every 24 hours daptomycin - adult dosage 4 mg/kg IV every 24 hours newer agents ceftaroline, dalbavancin, oritavancin, delafloxacin, and tedizolid are also now available for patients with severe infection (for example, concern for sepsis) concern for deeper infection such as bullae or skin sloughing, hypotension, or evidence of organ dysfunction, or with severe immunocompromise (malignancy on chemotherapy, neutropenia, severe cellmediated immunodeficiency) consider broad-spectrum antibiotic regimen such as (IDSA Strong recommendation, Moderate-quality evidence) vancomycin adult dosage 30 mg/kg/day IV in 2 divided doses pediatric dosage 40 mg/kg/day IV in 4 divided doses plus any of piperacillin-tazobactam 4.5 g IV every 8 hours (antipseudomonal dosing) imipenem-cilastatin standard dose 500-750 mg IV every 6 hours meropenem standard dose 0.5-1 g every 8 hours DynaMed commentary -- combination of vancomycin and piperacillintazobactam has been associated with increased renal toxicity. duration of therapy recommended duration of antimicrobial therapy is 5 days, but treatment should be extended if the infection has not improved within this time period (IDSA Strong recommendation, High11/20 25/3/2019 DynaMed Plus: Erysipelas quality evidence) for patient with febrile neutropenia, 7-14 days recommended for most bacterial skin and soft tissue infections (IDSA Strong recommendation, Moderate-quality evidence) Reference - Clin Infect Dis 2014 Jul 15;59(2):e10 full-text, correction can be found in Clin Infect Dis 2015 May 1;60(9):1448, executive summary can be found in Clin Infect Dis 2014 Jul 15;59(2):147 full-text Antibiotics limited evidence comparing penicillins to other antibiotics for reducing or eliminating symptoms of cellulitis and erysipelas based on Cochrane review with limited evidence systematic review of 25 randomized trials evaluating interventions for nonsurgically acquired cellulitis and erysipelas in 2,488 patients 17 trials evaluated treatment for skin and skin structure infections, 5 trials evaluated cellulitis, and 3 studies specifically evaluated erysipelas for symptom reduction or elimination oral pristinamycin (a macrolide) appeared better than IV penicillin (level 2 [mid-level] evidence) (65% vs. 53%, p < 0.05, NNT 9) in 1 trial with 288 hospitalized patients with erysipelas and fever ceftriaxone (a third-generation cephalosporin) appeared better than flucloxacillin (level 2 [mid-level] evidence) (87.5% vs. 60.9%, NNT 4, p = 0.049) in 1 trial with 47 patients with cellulitis, fever, and lymphadenitis macrolides appeared better than penicillins in 2 trials, but neither trial was statistically significant no significant differences comparing first-generation cephalosporin vs. penicillin in analysis of 2 trials with 41 patients different generations of cephalosporins in analysis of 6 trials with 538 patients insufficient evidence to evaluate varying duration of treatment, different routes, addition of corticosteroids, or vibration therapy Reference - Cochrane Database Syst Rev 2010 Jun 16;(6):CD004299 DynaMed commentary – skin and skin structure infections included wide range of infections including abscess, impetigo, folliculitis (inflammation of hair follicles), furunculosis (boils), and wound infection; minority of trials were focused solely on cellulitis or erysipelas Corticosteroids 2014 Infectious Disease Society of America (IDSA) guidelines on diagnosis and management of skin and soft tissue infection(1) consider systemic corticosteroids in patients with cellulitis or erysipelas in absence of diabetes (IDSA Weak recommendation, Moderate-quality evidence) addition of prednisolone to antibiotic regimen associated with faster time to resolution for patients with erysipelas (level 2 [mid-level] evidence) based on randomized trial with unclear reporting of allocation concealment 112 patients hospitalized with erysipelas were randomized to prednisolone 5-30 mg/day tapering dose plus antibiotic vs. placebo plus antibiotic for 8 days comparing prednisolone vs. placebo at 3 weeks median healing time 5 days vs. 6 days (p < 0.01) 90% healed by 10 days vs. 14.6 days hospital length of stay 5 days vs. 6 days (p < 0.01) median duration of IV antibiotics 3 days vs. 4 days (p < 0.05) relapse in 13 patients (12%) overall, 54% of whom were in placebo group no significant difference in side effects Reference - Scand J Infect Dis 1997;29(4):377 nonsignificant trend toward fewer relapses in follow-up study at 12 months based on follow-up of 103 patients from above trial followed for 12 months after cure 12/20 25/3/2019 DynaMed Plus: Erysipelas relapse occurred in 11.5% of prednisone group vs. 25.5% of placebo group (relative risk 2.2, 95% CI 0.9-5.4) Reference - Scand J Infect Dis 1998;30(2):206 DynaMed commentary -- the term erysipelas may encompass cellulitis in these studies Other management elevation of the affected area and treatment of predisposing factors, such as edema or underlying cutaneous disorders, are recommended (IDSA Strong recommendation, Moderate-quality evidence) Complications and Prognosis Complications most complications are local and include bullae abscess hemorrhagic lesions necrotic lesions lymphedema Reference - Int J Dermatol. 2010 Sep;49(9):1012-7, Am J Clin Dermatol. 2003;4(3):157-63 less frequent but more severe complications include sepsis meningitis endocarditis necrotizing fasciitis streptococcal toxic shock syndrome Reference - (2), Acta Dermatovenerol Alp Panonica Adriat 2007 Sep;16(3):123 abscess and thrombosis more common in men hospitalized for erysipelas compared to women based on retrospective cohort study 223 patients (median age 61 years) hospitalized for erysipelas were evaluated recurrent erysipelas in 22% complications observed in 22%, including abscess in 8% ulcers in 6.3% phelgmon in 4.5% thrombosis in 3.1% comparing men vs. women abscess in 14.6% vs. 2.5% (p = 0.001) thrombosis in 5.8% vs. 0.83% (p = 0.039) Reference - Przegl Epidemiol 2016;70(4):575 Prognosis prognosis is excellent with early diagnosis and treatment(3) recurrence recurrence of erysipelas is common, with 1 report estimating 40% (J Dtsch Dermatol Ges 2004 Feb;2(2):89) recurrent erysipelas may occur more often in patients who are overweight and patients with venous insufficiency, lymphedema, and tinea pedis based on retrospective cohort study 574 patients (mean age 58 years) hospitalized for initial or recurrent erysipelas were assessed recurrent erysipelas in 46.2% recurrent erysipelas occurred more often in lower leg than single-episode cases comparing patients with single episode vs. recurrent episodes 13/20 25/3/2019 DynaMed Plus: Erysipelas overweight in 9.5% vs. 20.2% (p < 0.001) venous insufficiency in 7.9% vs. 16.7% (p < 0.003) lymphedema in 3.6% vs. 8.2% (p < 0.04) tinea pedis in 37.2% vs. 50.2% (p = 0.003) trauma in 12.5% vs. 6% (p < 0.02) Reference - J Dtsch Dermatol Ges 2004 Feb;2(2):89 lymphedema, venous insufficiency, and skin disease associated with recurrence of erysipelas based on retrospective cohort study 502 adults (median age 60 years) with 573 episodes of erysipelas were evaluated 142 patients (29%) had recurrent erysipelas factors associated with recurrent erysipelas in multivariable analysis lymphedema (odds ratio [OR] 4.3, 95% CI 1.3-14) venous insufficiency (OR 2.3, 95% CI 1-5.2) skin disease (OR 1.9, 95% CI 1-3.7) Reference - BMC Infect Dis 2014 May 18;14:270 full-text recurrent and treatment-resistant erysipelas of cheek reported in patient with homozygous mannose binding lectin (MBL) deficiency (Lancet 2012 Apr 21;379(9825):1560) Prevention and Screening Prevention Infectious Diseases Society of America (IDSA) recommendations for prevention of recurrence as part of routine patient care, and during acute stage of cellulitis and erysipelas, identify and treat predisposing conditions (IDSA Strong recommendation, Moderate-quality evidence), such as edema obesity eczema venous insufficiency toe web abnormalities carefully examine interdigital toe spaces treatment of fissures, scaling, or maceration may reduce recurrence (IDSA Strong recommendation, Moderate-quality evidence) prophylactic antibiotics consider prophylactic antibiotics in patients with 3-4 episodes of cellulitis per year despite attempts to treat and control predisposing conditions (IDSA Weak recommendation, Moderate-quality evidence) continue prophylactic program as long as predisposing factors persist (IDSA Strong recommendation, Moderate-quality evidence) regimens include penicillin or erythromycin orally twice daily for 4-52 weeks penicillin G benzathine intramuscularly every 2-4 weeks Reference - Clin Infect Dis 2014 Jul 15;59(2):e10 full-text, correction can be found in Clin Infect Dis 2015 May 1;60(9):1448, executive summary can be found in Clin Infect Dis 2014 Jul 15;59(2):147 full-text prophylactic antibiotics reduce risk of recurrent cellulitis during treatment but may not reduce risk after completion of treatment (level 2 [mid-level] evidence) based on Cochrane review with wide confidence interval for recurrent cellulitis after completion of treatment systematic review of 6 randomized trials evaluating interventions for preventing recurrent cellulitis in 573 adults inclusion criteria for minimum number of previous episodes of cellulitis varied across trials; all previous episodes occurred ≤ 3 years before trial entry 5 trials evaluated antibiotics in patients with previous episode of leg cellulitis 14/20 25/3/2019 DynaMed Plus: Erysipelas 4 trials evaluated penicillin (largest trials below) and 1 trial evaluated erythromycin treatment duration ranged from 6 to 18 months comparing antibiotics to placebo/no treatment at end of treatment phase antibiotics associated with decrease in recurrence of cellulitis in analysis of 5 trials with 513 adults risk ratio 0.31 (95% CI 0.13-0.72) NNT 4-12 with recurrence of cellulitis in 32% of control group no significant differences in hospitalization in analysis of 3 trials with 429 adults any adverse reactions in analysis of 4 trials with 469 adults comparing antibiotics to placebo after completion of treatment, no significant difference in recurrence of cellulitis (risk ratio 0.88, 95% CI 0.59-1.31) in analysis of 2 trials with 287 adults, but CI includes possibility of benefit or harm Reference - Cochrane Database Syst Rev 2017 Jun 20;(6):CD009758 prophylactic penicillin prophylactic penicillin reduces risk for recurrent leg cellulitis during treatment (level 1 [likely reliable] evidence) but may not reduce risk after completion of treatment (level 2 [mid-level] evidence) based on randomized trial with wide confidence interval for recurrence during follow-up 274 patients (mean age 58 years) with ≥ 2 episodes of leg cellulitis in past 3 years randomized to penicillin V 250 mg orally twice daily vs. placebo for 1 year and followed up to 3 years all patients began trial medication following treatment of index cellulitis episode and had recurrent episode within past 24 weeks at baseline median follow-up 25 months 90% completed 18 months of follow-up and all patients were included in intention-to-treat analyses comparing penicillin vs. placebo recurrence during treatment in 22% vs. 37% (hazard ratio 0.55, 95% CI 0.35-0.86, p = 0.01, NNT 4-9) recurrence during follow-up in 27% vs. 27% (hazard ratio 1.08, 95% CI 0.61-1.93), not significant but CI includes possibility of benefit or harm median time to first recurrence of cellulitis 626 days vs. 532 days (no p value reported) total recurrences during treatment 76 vs. 122 (p = 0.03) total recurrences during follow-up 43 vs. 42 (not significant) adverse events in 27% vs. 35% (not significant) mean length of hospital stay 10 days vs. 9.2 days (no p value reported) no significant differences in new edema or ulceration during treatment or follow-up Reference - PATCH I trial (N Engl J Med 2013 May 2;368(18):1695), commentary can be found in Br J Dermatol 2014 Dec;171(6):1300, N Engl J Med 2013 Aug 29;369(9):880, N Engl J Med 2013 Aug 29;369(9):881 prophylactic penicillin associated with nonsignificant decrease in recurrence of cellulitis or erysipelas (level 2 [mid-level] evidence) based on randomized trial with inadequate statistical power 123 patients recently treated for cellulitis or erysipelas of the leg (79% with initial infection) randomized to penicillin V 250 mg orally twice daily vs. placebo for 6 months and followed for 2.5 years only 31% of target sample size recruited due to slow recruitment and changes in hospitalization policy full adherence in 79% (defined as ≥ 75% of prescribed dose) cellulitis or erysipelas recurred in 20% of penicillin group vs. 33% of placebo group (p = 0.07) Reference - PATCH II trial (Br J Dermatol 2012 Jan;166(1):169 full-text) penicillin prophylaxis may reduce recurrence of cellulitis in patients without predisposing factors for cellulitis (level 2 [mid-level] evidence) based on prospective cohort study 115 patients with definite or presumptive streptococcus cellulitis evaluated 15/20 25/3/2019 DynaMed Plus: Erysipelas 31 patients had prophylaxis with benzathine penicillin G 1.2 million units per month for 1 year 84 patients had no or incomplete prophylaxis (control group) 49.6% of patients had predisposing factors for infection percentage higher in prophylactic group (64.5%) vs. control group (44%) factors included diabetes mellitus, impaired venous drainage, deep-venous thrombosis, stasis dermatitis, and coronary artery bypass graft comparing prophylaxis vs. control group recurrence 12.9% vs. 19% (not significant) recurrence in patients with predisposing factors 21.6% vs. 17% (no p value reported) recurrence in patients without predisposing factors 0% vs. 20% (no p value reported) Reference - Clin Infect Dis 1997 Sep;25(3):685 Guidelines and Resources Guidelines International guidelines Italian Society of Infectious and Tropical Diseases (Societa Italiana di Malattie Infettive e Tropicali)/International Society of Chemotherapy (SIMIT/ISC) consensus statement on diagnosis and management of skin and soft-tissue infections can be found in J Chemother 2011 Oct;23(5):251, SIMIT update can be found in J Chemother 2017 Aug;29(4):197 PDF United States guidelines Infectious Diseases Society of America (IDSA) clinical practice guideline on treatment of methicillinresistant Staphylococcus aureus infections in adults and children can be found in Clin Infect Dis 2011 Feb 1;52(3):e18, correction can be found in Clin Infect Dis 2011 Aug 1;53(3):319, commentary can be found in Clin Infect Dis 2015 Apr 15;60(8):1290 Infectious Diseases Society of America (IDSA) practice guideline on diagnosis and management of skin and soft tissue infections: 2014 update can be found at IDSA 2014 Jun or in Clin Infect Dis 2014 Jul 15;59(2):e10 full-text, correction can be found in Clin Infect Dis 2015 May 1;60(9):1448, executive summary can be found in Clin Infect Dis 2014 Jul 15;59(2):147 full-text Surgical Infection Society (SIS) expert consensus guideline on treatment of complicated skin and soft tissue infections can be found in Surg Infect (Larchmt) 2009 Oct;10(5):467 European guidelines Finnish updated current care guideline on bacterial skin infections can be found in Duodecim 2010;126(24):2883 [Finnish] Asian guidelines Korean Society of Infectious Diseases/Korean Society for Chemotherapy/Korean Orthopaedic Association/Korean Dermatological Association (KSID/KSC/KOA/KDA) clinical practice guideline on antibiotic treatment for community-acquired and soft tissue infections can be found in Infect Chemother 2017 Dec;49(4):301 full-text Review articles 16/20 25/3/2019 DynaMed Plus: Erysipelas review of skin and soft tissue infections can be found in Am Fam Physician 2015 Sep 15;92(6):474 full-text review of bacterial skin infections can be found in Prim Care 2015 Dec;42(4):485 review of impetigo, erysipelas, and cellulitis can be found in Ferretti JJ, Stevens DL, Fischetti VA, ed. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City, OK: University of Oklahoma Health Sciences Center; 2016 review of diagnosis and management of cellulitis and erysipelas can be found in Br J Hosp Med (Lond) 2015 Aug;76(8):C114 review of skin and soft-tissue infections and role of telavancin in their treatment can be found in Clin Infect Dis 2015 Sep 15;61 Suppl 2:S69 full-text review of cellulitis and erysipelas prevention can be found in BMJ Clin Evid 2015 Nov 18;2015: fulltext review of group A streptococcal skin infection and rheumatic heart disease with focus on resource limited settings can be found in Curr Opin Infect Dis 2012 Apr;25(2):145 reviews of group A streptococcal biology can be found in Curr Opin Infect Dis 2011 Jun;24(3):196 Nat Rev Microbiol 2011 Sep 16;9(10):724 review of the skin microbiome can be found in Trends Microbiol 2013 Dec;21(12):660 full-text review of cellulitis can be found in N Engl J Med 2004 Feb 26;350(9):904 discussion of antibiotic stewardship in skin and soft-tissue infections can be found in Clin Infect Dis 2010 Oct 15;51(8):895 full-text review of deep vein thrombosis in patients with erysipelas and cellulitis can be found in Int J Dermatol 2013 Mar;52(3):279 MEDLINE search to search MEDLINE for (Erysipelas) with targeted search (Clinical Queries), click therapy, diagnosis, or prognosis Patient Information handout on cellulitis and erysipelas from British Association of Dermatologists handout on erysipelas and cellulitis from Patient UK handout on cellulitis from KidsHealth ICD Codes ICD-10 codes A46 erysipelas References General references used 1. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52 full-text, executive summary can be found in Clin Infect Dis 2014 Jul 15;59(2):147 full-text 2. Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005 Nov 15;41(10):1373-406 full-text, correction can be found in Clin Infect Dis 2005 Dec 15;41(12):1830, Clin Infect Dis 2006 Apr 15;42(8):1219 17/20 25/3/2019 DynaMed Plus: Erysipelas 3. Stevens DL, Bryant AE. Impetigo, Erysipelas and Cellulitis. In: Ferretti JJ, Stevens DL, Fischetti VA, ed. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City, OK: University of Oklahoma Health Sciences Center; 2016 Recommendation grading systems used Infectious Diseases Society of America (IDSA) 2014 uses Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system strength of recommendation Strong recommendation - desirable effects clearly outweigh undesirable effects, or vice versa Weak recommendation - desirable effects closely balanced with undesirable effects, or (with low- or very low-quality evidence) uncertainty in estimates of desirable effects, harms, and burden so they may be closely balanced quality of evidence High-quality evidence - consistent evidence from well-performed randomized controlled trials (RCTs) or exceptionally strong evidence from unbiased observational studies Moderate-quality evidence - evidence from RCTs with important limitations (inconsistent results, methodologic flaws, indirect, or imprecise) or exceptionally strong evidence from unbiased observational studies Low-quality evidence - evidence for ≥ 1 critical outcome from observational studies, RCTs with serious flaws, or indirect evidence Very low-quality evidence - evidence for ≥ 1 critical outcome from unsystematic clinical observations or very indirect evidence Reference - IDSA practice guideline on diagnosis and management of skin and soft tissue infections: 2014 update can be found in Clin Infect Dis 2014 Jul 15;59(2):e10 full-text Infectious Diseases Society of America (IDSA) grading system for recommendations strength of recommendation grades Grade A - good evidence to support recommendation for or against use Grade B - moderate evidence to support recommendation for or against use Grade C - poor evidence to support recommendation quality of evidence ratings I - evidence from ≥ 1 properly randomized, controlled trial II - evidence from ≥ 1 well-designed nonrandomized clinical trial; from cohort or casecontrolled analytic studies (preferably from > 1 center); from multiple time series; or from dramatic results from uncontrolled studies III - evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees Reference - IDSA clinical practice guideline on treatment of methicillin-resistant Staphylococcus aureus infections in adults and children (Clin Infect Dis 2011 Feb;52(3):e18), correction can be found in Clin Infect Dis 2011 Aug 1;53(3):319, commentary can be found in Clin Infect Dis 2015 Apr 15;60(8):1290 Synthesized Recommendation Grading System for DynaMed Plus DynaMed systematically monitors clinical evidence to continuously provide a synthesis of the most valid relevant evidence to support clinical decision-making (see 7-Step Evidence-Based Methodology). Guideline recommendations summarized in the body of a DynaMed topic are provided with the recommendation grading system used in the original guideline(s), and allow DynaMed users to quickly see where guidelines agree and where guidelines differ from each other and from the current evidence. In DynaMed Plus (DMP), we synthesize the current evidence, current guidelines from leading authorities, and clinical expertise to provide recommendations to support clinical decision-making in the Overview & Recommendations section. We use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) to classify synthesized recommendations as Strong or Weak. 18/20 25/3/2019 DynaMed Plus: Erysipelas Strong recommendations are used when, based on the available evidence, clinicians (without conflicts of interest) consistently have a high degree of confidence that the desirable consequences (health benefits, decreased costs and burdens) outweigh the undesirable consequences (harms, costs, burdens). Weak recommendations are used when, based on the available evidence, clinicians believe that desirable and undesirable consequences are finely balanced, or appreciable uncertainty exists about the magnitude of expected consequences (benefits and harms). Weak recommendations are used when clinicians disagree in judgments of relative benefit and harm, or have limited confidence in their judgments. Weak recommendations are also used when the range of patient values and preferences suggests that informed patients are likely to make different choices. DynaMed Plus (DMP) synthesized recommendations (in the Overview & Recommendations section) are determined with a systematic methodology: Recommendations are initially drafted by clinical editors (including ≥ 1 with methodological expertise and ≥ 1 with content domain expertise) aware of the best current evidence for benefits and harms, and the recommendations from guidelines. Recommendations are phrased to match the strength of recommendation. Strong recommendations use "should do" phrasing, or phrasing implying an expectation to perform the recommended action for most patients. Weak recommendations use "consider" or "suggested" phrasing. Recommendations are explicitly labeled as Strong recommendations or Weak recommendations when a qualified group has explicitly deliberated on making such a recommendation. Group deliberation may occur during guideline development. When group deliberation occurs through DynaMed-initiated groups: Clinical questions will be formulated using the PICO (Population, Intervention, Comparison, Outcome) framework for all outcomes of interest specific to the recommendation to be developed. Systematic searches will be conducted for any clinical questions where systematic searches were not already completed through DynaMed content development. Evidence will be summarized for recommendation panel review including for each outcome, the relative importance of the outcome, the estimated effects comparing intervention and comparison, the sample size, and the overall quality rating for the body of evidence. Recommendation panel members will be selected to include at least 3 members that together have sufficient clinical expertise for the subject(s) pertinent to the recommendation, methodological expertise for the evidence being considered, and experience with guideline development. All recommendation panel members must disclose any potential conflicts of interest (professional, intellectual, and financial), and will not be included for the specific panel if a significant conflict exists for the recommendation in question. Panel members will make Strong recommendations if and only if there is consistent agreement in a high confidence in the likelihood that desirable consequences outweigh undesirable consequences across the majority of expected patient values and preferences. Panel members will make Weak recommendationsif there is limited confidence (or inconsistent assessment or dissenting opinions) that desirable consequences outweigh undesirable consequences across the majority of expected patient values and preferences. No recommendation will be made if there is insufficient confidence to make a recommendation. All steps in this process (including evidence summaries which were shared with the panel, and identification of panel members) will be transparent and accessible in support of the recommendation. Recommendations are verified by ≥ 1 editor with methodological expertise, not involved in recommendation drafting or development, with explicit confirmation that Strong recommendations are adequately supported. Recommendations are published only after consensus is established with agreement in phrasing and strength of recommendation by all editors. If consensus cannot be reached then the recommendation can be published with a notation of "dissenting commentary" and the dissenting commentary is included in the topic details. 19/20 25/3/2019 DynaMed Plus: Erysipelas If recommendations are questioned during peer review or post publication by a qualified individual, or reevaluation is warranted based on new information detected through systematic literature surveillance, the recommendation is subject to additional internal review. DynaMed Editorial Process DynaMed topics are created and maintained by the DynaMed Editorial Team and Process. All editorial team members and reviewers have declared that they have no financial or other competing interests related to this topic, unless otherwise indicated. DynaMed provides Practice-Changing DynaMed Updates, with support from our partners, McMaster University and F1000. Special acknowledgements Renee Ridzon, MD (Adjunct Associate Professor, Boston University School of Public Health; Massachusetts, United States) Dr. Ridzon declares no relevant financial conflicts of interest. Esther Jolanda van Zuuren, MD (Head of Allergy, Dermatology, and Venereology, Leiden University Medical Centre; Netherlands; Editor, Cochrane Skin Group) Dr. van Zuuren declares no relevant financial conflicts of interest. Vito Iacoviello, MD, FIDSA (Deputy Editor of Infectious Diseases, Immunology, and Rheumatology; Assistant Professor of Medicine, Harvard Medical School; Chief of the Division of Infectious Diseases, Mount Auburn Hospital; Massachusetts, United States) Dr. Iacoviello declares no relevant financial conflicts of interest. DynaMed Plus topics are written and edited through the collaborative efforts of the above individuals. Deputy Editors, Section Editors, and Topic Editors are active in clinical or academic medical practice. Recommendations Editors are actively involved in development and/or evaluation of guidelines. Editorial Team role definitions Topic Editors define the scope and focus of each topic by formulating a set of clinical questions and suggesting important guidelines, clinical trials, and other data to be addressed within each topic. Topic Editors also serve as consultants for the internal DynaMed Plus Editorial Team during the writing and editing process, and review the final topic drafts prior to publication. Section Editors have similar responsibilities to Topic Editors but have a broader role that includes the review of multiple topics, oversight of Topic Editors, and systematic surveillance of the medical literature. Recommendations Editors provide explicit review of DynaMed Plus Overview and Recommendations sections to ensure that all recommendations are sound, supported, and evidencebased. This process is described in "Synthesized Recommendation Grading." Deputy Editors are employees of DynaMed and oversee DynaMed Plus internal publishing groups. Each is responsible for all content published within that group, including supervising topic development at all stages of the writing and editing process, final review of all topics prior to publication, and direction of an internal team. How to cite National Library of Medicine, or "Vancouver style" (International Committee of Medical Journal Editors): DynaMed Plus [Internet]. Ipswich (MA): EBSCO Information Services. 1995 - . Record No. T115431, Erysipelas; [updated 2018 Dec 04, cited place cited date here]. Available from https://www.dynamed.com/topics/dmp~AN~T115431. Registration and login required. 20/20